Research
, Volume: 10( 2) DOI: 10.37532/2320-6756.2022.10(2).262UVA Radiation Studying Effect on Morphological Structure, Biochemical and Antioxidant Properties of Yellow Mustard
- *Correspondence:
- Abu Bakr El-Bediwi Physics Department, Faculty of Science, Mansoura University, Egypt E-mail: baker_elbediwi@yahoo.com
Received date: January 10, 2022, Manuscript No. M- 51501; Editor assigned: January 13, 2022, PreQC No. P-51501; Reviewed: January 25, 2022, QC No. 51501; Revised: February 4, 2022, Manuscript No. R-51501; Published date: February 7, 2022, DOI: 10.37532/2320–6756.2022.10(2).262
Citation:Abu Bakr El-Bediwi. UVA Radiation Studying Effect on Morphological Structure, Biochemical and Antioxidant Properties of Yellow Mustard, J Phys Astron.2022;10(2):262.
Abstract
Plants are living chemical factories for enormous array of the secondary metabolites but the production by plants of compounds useful as medicines or raw materials for manufacture of medicines is influenced by ultraviolet radiation. The aim of research is to investigate the effect of UVA on structure, biochemistry and physiology for yellow mustard. The results show internal structure and chemical composition of yellow mustard changed after exposure to UVA for different times and dissimilar distances. Glutathione and total phenolic in yellow mustard increased but total proline and tocopherol in it decreased after exposed by UVA. Total flavonoids in yellow mustard varied after exposure to UVA for different time and dissimilar distances.
- *Correspondence:
- Abu Bakr El-Bediwi Physics Department, Faculty of Science, Mansoura University, Egypt E-mail: baker_elbediwi@yahoo.com
Received date: January 10, 2022, Manuscript No. M- 51501; Editor assigned: January 13, 2022, PreQC No. P-51501; Reviewed: January 25, 2022, QC No. 51501; Revised: February 4, 2022, Manuscript No. R-51501; Published date: February 7, 2022, DOI: 10.37532/2320–6756.2022.10(2).262
Citation:Abu Bakr El-Bediwi. UVA Radiation Studying Effect on Morphological Structure, Biochemical and Antioxidant Properties of Yellow Mustard, J Phys Astron.2022;10(2):262.
Abstract
Plants are living chemical factories for enormous array of the secondary metabolites but the production by plants of compounds useful as medicines or raw materials for manufacture of medicines is influenced by ultraviolet radiation. The aim of research is to investigate the effect of UVA on structure, biochemistry and physiology for yellow mustard. The results show internal structure and chemical composition of yellow mustard changed after exposure to UVA for different times and dissimilar distances. Glutathione and total phenolic in yellow mustard increased but total proline and tocopherol in it decreased after exposed by UVA. Total flavonoids in yellow mustard varied after exposure to UVA for different time and dissimilar distances.
Keywords
Phenolic, Glutathione, DPPH scavenging activity, Proline, Yellow mustard, UVA
Introduction
Solar radiation is a complex mixture of ultraviolet, visible light and infrared wavelengths. UVA radiation (315 nm-400 nm) is a component of solar radiation. Plants are living chemical factories for the biosynthesis of a huge array of the secondary metabolites. Plants are used medicinally in different countries and are a source of many powerful drugs. The World Health Organization stated that about 80% of the world’s population depends mainly on traditional medicine that mainly includes the use of plant extracts. UV light is an important abiotic elicitor, and had use in phytochemical production in a variety of plant cultures in the past [1]. Exposure to UV light stress causes stimulation of defense mechanisms in plants, thus, producing commercially important secondary compounds [2]. Some reports discussed the effect of low levels of UV radiation on plant growth [3,4]. There are many beneficial uses of radiation that offer few risks when properly employed. The exposure to radiations can have stimulatory effects on specific morphological parameters. The mustard plant belongs to the Cruciferae (Brassicaceae) family, used in medicine is external as a liniment and relieving pain from bruises or a stiff neck and relieving colic and respiratory problems.
Growth behavior, secondary metabolites and vitamins of Nigella Sativa and garden cress changed after exposure by UVC [5,6]. UVs have adequate energy to break the chemical bonds causing photochemical reactions and inducing changes in plant metabolic enzyme, subsequently trigger the production of secondary metabolites [7-9]. Effect of UV is varied with duration and irradiation intensity. The objective of this work is to assess the effect of UVA radiation on structure, non-enzymatic and enzymatic antioxidants for yellow mustard.
Experimental Methods
Structure measurements
Internal structure and molecular structure of yellow mustard are studied by Shimadzu X-ray diffractometer, (Dx-30, Japan), scanning electron microscope (JEOL JSM-6510LV, Japan) and Nicolet™ iS™ 10 FT-IR Spectrometer from USA.
GSH determination
GSH is determined using UV/V spectrophotometer, Jenway, England.
DPPH determination
Concentration ranging from 0.4g/100g to 2g/100g are prepared with methanol from each sample (100 μl) extract and DPPH radical (100 μl, 2Mm) dissolved in methanol. The mixture is stirred and left to stand for 15 min in dark. Then the absorbance is measured at 517 nm against a blank. Percentage scavenging effect is calculated as [(Ao-A1)/Ao]× 100 where Ao is the absorbance of the control (without sample) and A1 is the absorbance in the presence of the sample.
Total phenols determination
Total phenolsare determined colorimetric by Folin-Ciocalteu reagent total phenolic content is calculated from the regression equation of the standard plot (y=3.005x(-993.56),r2 =0.9974) and are expressed as mg Gallic acid equivalent/100g sample.
Flavonoid determination
Aluminum chloride colorimetric method is used to determine flavonoid content. 1 ml of sample extract is mixed with 3 ml of methanol, 0.2 ml of 10% aluminum chloride. 0.2 ml of 1 M potassium acetate and 5.6 ml of distilled water and remains at room temperature for 30 minutes. The absorbance is measured at 420 nm. Rutin is used as standard (1mg/ml). Flavonoid content is calculated from the regression equation of the standard plot [y=16.122x(-340.23),r2=0.9777] and are expressed as (mg Rutin equivalent/100g sample).
Proline determination
The proline concentration is determined after extraction with 3% (W/V) aqueous sulfosalicylic acid from a standard curve using DProline (y=36.738x + 1.2739),r2=0.9777)which give by used UV/V spectrophotometer, Jenway, England.
Results and Discussions
Internal structure
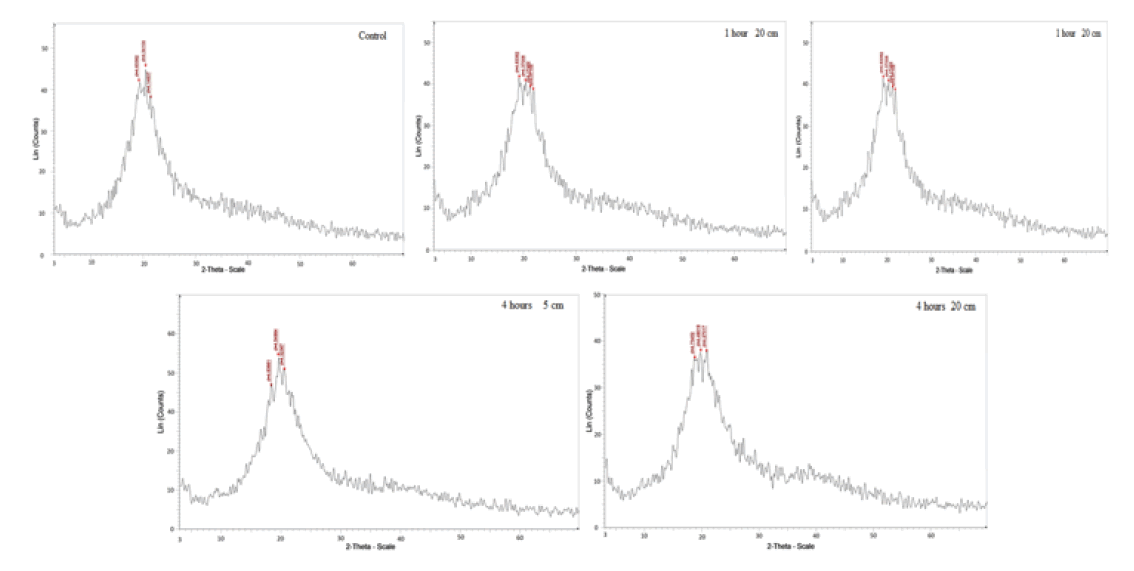
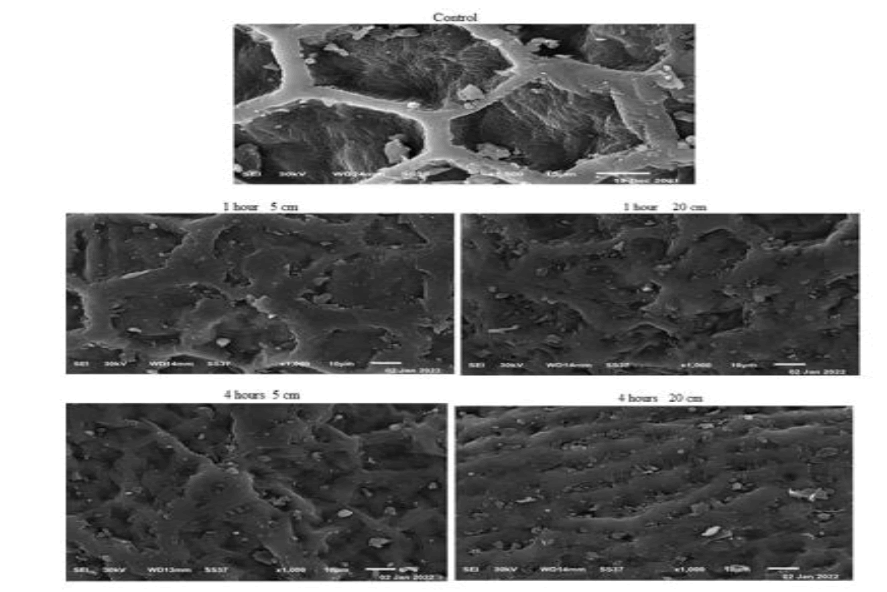
FIG.1 shows x-ray diffraction patterns of normal yellow mustard after exposure to UVA for different period times at dissimilar distances. There is a change in the main peak of yellow mustard, TAB. 1, as intensity, broadness, started base line and area under the peak after exposure to UVA at 5 cm and 20 cm for 1 hour and 4 hours. That is because the interaction of UVA with atoms or molecules break or modify bonds and or make rearrangement it. Also UV radiation stress is caused a change in bond structure. The interaction with atoms or molecules in the cell, particularly water, produce free radicals [10] which can damage or modify important components of plant cells, affected differentially the morphology, anatomy, biochemistry and physiology for plants [11]. Scanning electron micrographs show different features and qualities of the main cell and a round molecules as show in FIG. 2. These changed confirmed the x-ray and IR results.
| Exposure time (hour) | Area under the peak | Width of the peak | ||
|---|---|---|---|---|
| Zero (Control) | 4.61 | |||
| S.No. | Exposure at 5 cm | Exposure at 20 cm | Exposure at 5 cm | Exposure at 20 cm |
| 1 hour | 6.1404938 | 4.5237738 | 7.3240309 | 6.7581327 |
| 4 hours | 4.3627724 | 5.210452 | 6.2214994 | 6.4209859 |
TAB.1. X-ray analysis of yellow mustard before and after exposed to UVA.
Molecular Structure
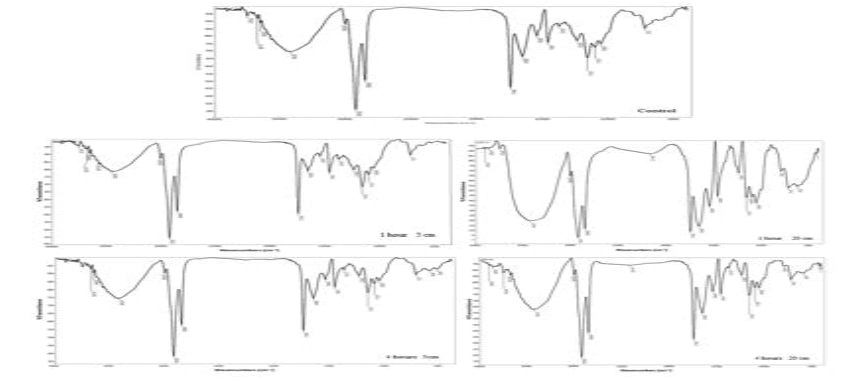
FIG. 3 shows IR spectrum of yellow mustard, a plot of wave number (X-axis) vs. present transmittance (Y-axis). IR analysis of yellow mustard listed in TAB. 2 show transmittance the intensity increased after exposure to UVA at 5 cm for 1 hour and 4 hours but decreased at 20 cm for 1 hour and 4 hours. A significant change occurred in the main peak position, O-H, after exposure to UVA for 1 hour at 5 cm, but a little variation is occurred during other exposure times at 5 cm and 20 cm distance. That is mean, the absorption of UVA in cell, break or modify the position or degraded some molecular bond or switching off of the transcription-translation machinery during radiation exposure [12].
| Control (untreated sample) | ||
|---|---|---|
| Position | Intensity % | Band |
| 1746 | 45.6 | C-O |
| 2925 | 31.04 | C-H |
| 3422 | 69.4 | O-H |
| 1 hour at 5 cm | ||
| 1746 | 50.4 | C-O |
| 2925 | 34.36 | C-H |
| 3450 | 78.4 | O-H |
| 1 hour at 20 cm | ||
| 1746 | 8.32 | C- O |
| 2925 | 2.75 | C-H |
| 3421 | 19.22 | O-H |
| 4 hour at 5 cm | ||
| 1746 | 54.12 | C- O |
| 2925 | 38.11 | C-H |
| 3423 | 74.26 | O-H |
| 4 hour at 20 cm | ||
| 1746 | 32.9 | C- O |
| 2925 | 18.21 | C-H |
| 3421 | 57.37 | O-H |
TAB.2. IR spectrum analysis of yellow mustard after exposure to UVA.
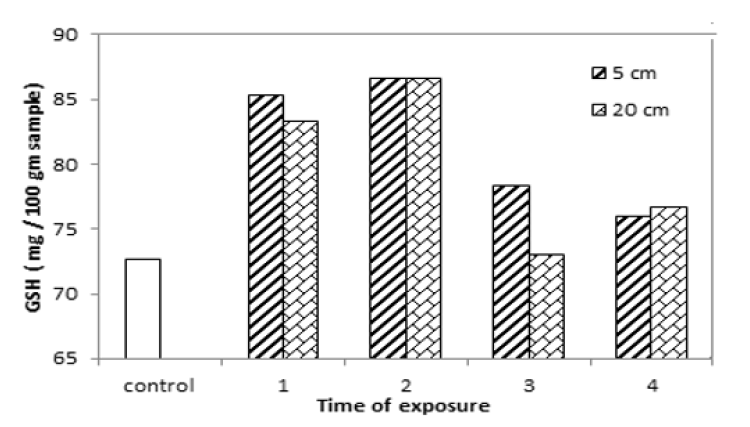
Glutathione Content
Glutathione content for yellow mustard is increased after exposure to UVA at 5 cm and 20 cm for different period of times as seen in TAB.3 and FIG.4. The results show it is increased by 17.44%, 19.3%, 7.8% and 4.58% and, by 14.68%, 19.3%, 0.45% and 5.5% at 5 cm and 20 cm distance far from UVA source for different period times. That is mean, it increased from 0.45% to 19.3% after exposure to UVA, because the glutathione, which one of the most important non-enzymatic antioxidants, is positively affected by radiation as it is a hermetic type of response under applied doses of radiation [13, 14].
| Exposure time (hour) | GSH (mg/ 100g) | |
|---|---|---|
| Zero (Control) | 72.66 | |
| Irradiated at 5 cm | Irradiated at 20 cm | |
| 1 hour | 85.33 | 83.33 |
| 2 hours | 86.66 | 86.66 |
| 3 hours | 78.33 | 72.99 |
| 4 hours | 75.99 | 76.66 |
TAB.3. Glutathione content of yellow mustard after exposed to UVA.
Phenolic Content
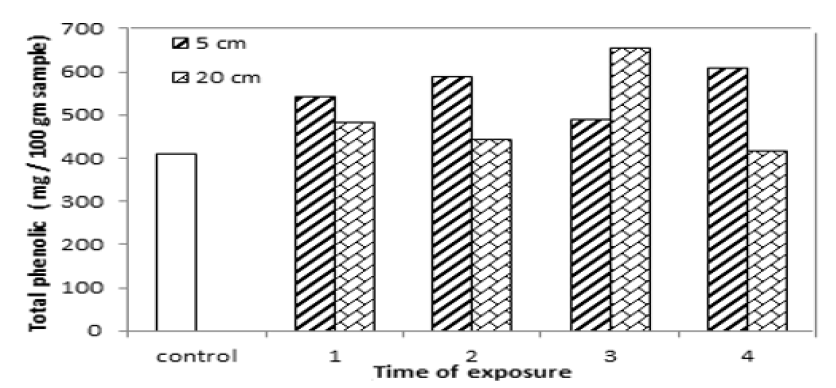
Total phenolic content for yellow mustard increased by 32.57%, 43.5%, 19.5% and 48.35% and by18.17%, 8.31%, 59.71% to 1.82.5% after exposure to UVA at distances 5 cm and 20 cm for 1 hour, 2 hours, 3 hours and 4 hours as shown in TAB.4 and FIG.5. That is because UV radiation increases the accumulation of phenolic compounds along with antioxidant properties. Also phenolic compounds are plant secondary metabolites that hold an aromatic ring bearing at least one hydroxyl groups and IR analysis for yellow mustard show there is a change in the intensity and position of O-H band caused increased in phenolic. The pervious results also shown phenolic compounds is increased or induced after UV radiation exposure during different period times [15-19].
| Exposure time (hour) | Total phenolic (mg/ 100g) | |
|---|---|---|
| Zero (Control) | 409.48 | |
| Irradiated at 5 cm | Irradiated at 20 cm | |
| 1 hour | 542.86 | 483.89 |
| 2 hours | 587.57 | 443.50 |
| 3 hours | 489.33 | 654.21 |
| 4 hours | 607.44 | 416.92 |
TAB.4. Total phenolic content of yellow mustard exposed to UVA.
Flavonoids Content
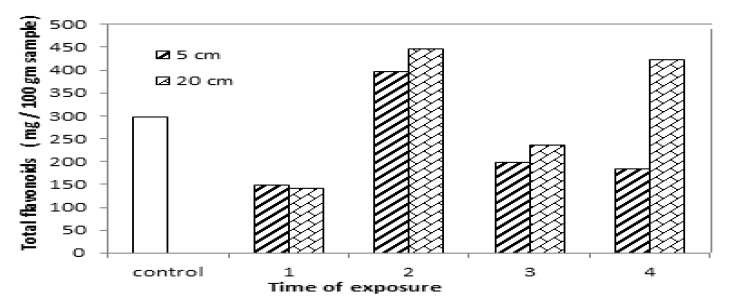
Flavonoid is a term that is a bit ambiguous literally it means flavone-like compound. TAB.5 and FIG.6 show total flavonoids in yellow mustard decrease after exposure for 1 hour, 3 hours and 4 hours and 1 hour and 3 hours at 5 cm and 20 cm from UVA source but it increased after exposure for 2 hours at 5 cm distance and 2 hours and 4 hours at 20 cm. That is mean, flavonoids content in yellow mustard varied after exposed to UVA for different times and distances because flavonoids is plant secondary metabolites that hold an aromatic ring bearing at least one hydroxyl groups, the results, IR analysis, showed a change in hydroxyl group such as intensity and position.
Figure 6: Total flavonoids content in yellow mustard exposed to UVA.
| Exposure time (hour) | Total flavonoids (mg/ 100g) | |
|---|---|---|
| Zero (Control) | 297.6 | |
| Irradiated at 5 cm | Irradiated at 20 cm | |
| 1 hour | 148.45 | 141.95 |
| 2 hours | 395.71 | 446.75 |
| 3 hours | 198.54 | 236.8 |
| 4 hours | 183.07 | 422.64 |
TAB.5. Total flavonoids content of yellow mustard exposed to UVA.
Total Proline Content
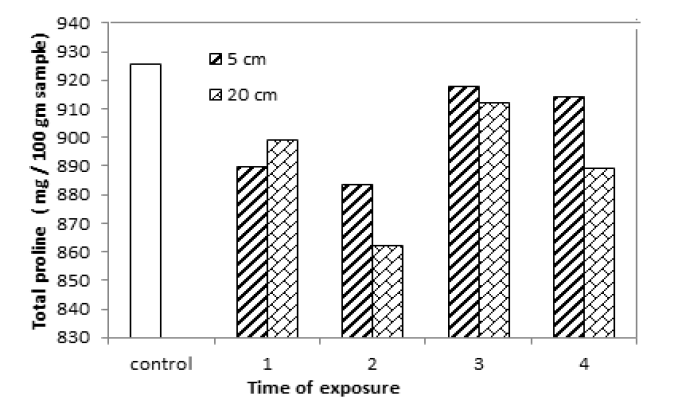
Proline plays important roles in protein synthesis and structure, metabolism and nutrition. Total proline content in yellow mustard decreased after exposure to UVA for different period times and distances as shown in TAB.6 and FIG.7. The chemical composition of yellow mustard such as protein changed after exposure to UVA. That is mean, total proline changed because proline is a substrate for protein synthesis. Also proline accumulation is response to biotic and abiotic stresses caused to the effect of UVA.
| Exposure time (hour) | Total proline (mg/ 100g) | |
|---|---|---|
| Zero (Control) | 925.84 | |
| Irradiated at 5 cm | Irradiated at 20 cm | |
| 1 hour | 889.96 | 899.24 |
| 2 hours | 883.56 | 862.40 |
| 3 hours | 917.54 | 911.96 |
| 4 hours | 914.41 | 888.98 |
TAB.6. Total proline content of yellow mustard exposed to UVA at 5 and 20 cm for different period times.
DPPH Scavenging Activity
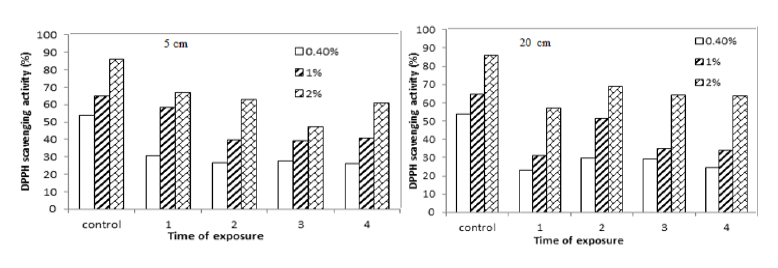
UVR carries higher energy than visible light and its effects on tissues include gene mutations, DNA damage, oxidative stress immunosuppression, and inflammatory responses. Therefore, some compounds increase and others may decrease due to abiotic stress as pathways for secondary metabolite production are interrelated. DPPH scavenging activity (%) for yellow mustard decreased after exposure to UVA for 1 hour, 2 hours, 3 hours and 4 hours at 5 cm and 20 cm as shown in TAB.7 and FIG.8.
| Exposure time (hour) | (A) DPPH scavenging activity (%) 5 cm | ||
|---|---|---|---|
| 0.40% | 1% | 2% | |
| Zero (Control) | 53.9 | 64.89 | 85.82 |
| 1 hour | 30.56 | 58.33 | 67.01 |
| 2 hours | 26.67 | 39.56 | 62.85 |
| 3 hours | 27.8 | 39.03 | 47.43 |
| 4 hours | 26.04 | 40.6 | 60.76 |
| Exposure time (hour) | (B) DPPH scavenging activity (%) 20 cm | ||
| 0.40% | 1% | 2% | |
| Zero (Control) | 53.9 | 64.89 | 85.82 |
| 1 hour | 23.14 | 31.25 | 57.29 |
| 2 hours | 29.51 | 51.39 | 68.75 |
| 3 hours | 29.17 | 35.07 | 64.24 |
| 4 hours | 24.31 | 34.03 | 63.54 |
TAB.7. (A )DPPH scavenging activity (%) of yellow mustard exposed to UVA at 5 and (B) 20 cm for different period times.
Tocopherol Content
TAB.8 and FIG.9 show, tocopherol content in yellow mustard decreased by 10.1%, 6.01%, 47.5% and 42.6% after exposure to UVA at 5 cm for 1, 2, 3 and 4 hours. Also it decreased gradually after exposure for 1, 2, 3 and 4 at 20 cm.
| Exposure time (hour) | Tocopherol (mg/ kg) | |
|---|---|---|
| Zero (Control) | 2029.19 | |
| Irradiated at 5 cm | Irradiated at 20 cm | |
| 1 hour | 1824.13 | 1965.43 |
| 2 hours | 1907.79 | 1726.63 |
| 3 hours | 1065.43 | 1335.00 |
| 4 hours | 1165.43 | 1234.16 |
TAB.8. Tocopherol of yellow mustard exposed to UVA.
Figure 9: Tocopherol content in yellow mustard exposed to UVA.
Conclusion
Variation in radiation resulted in, significant differences in biomass accumulation, protein and total non-structural carbohydrates contents in plants. Exposure to UVA improve/or disprove some antioxidants for yellow mustard. Also UVA caused a change in yellow mustard internal structure.
References
- Xuan TD, Khanh TD, Khang DT, et.al. Changes in chemical composition, total phenolics and antioxidant activity of Alpinia (Alpinia zerumbet) leaves exposed to UV. Int. Lett. Nat. Sci. 2016;55 25-34.
Google Scholar Crossref - Yin X, Singer SD, Qiao H, et.al. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr).“Tonghua-3”. Front. Plant Sci. 2016;7:503.
Google Scholar Crossref - Hideg E, Jansen MA, Strid A. Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Trends Plant Sci. 2013;18:107-115.
Google Scholar Crossref - Bornman JF, Barnes PW, Robinson SA, et.al. Solar ultraviolet radiation and ozone depletion-driven climate change: Effects on terrestrial ecosystems. Photochem Photobiol Sci. 2015;14(1):88-107.
Google Scholar Crossref - El-Bediwi AB, Hasanin S, Abdelrazek A, El-Shora HM. Effect of Ultraviolet on Morphological and Secondary Metabolites Content of Garden Cress. 2018;4:187-194.
Google Scholar Crossref - Zhang WJ, Björn LO. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia. 2009;80(4):207-218.
Google Scholar Crossref - Hectors K, Van Oevelen S, Geuns J, et.al. Dynamic changes in plant secondary metabolites during UV acclimation in Arabidopsis thaliana. Physiologia Plantarum. 2014;152(2):219-230.
Google Scholar Crossref - Ghasemi S, Kumleh HH, Kordrostami M. Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma. 2019;256(1):279-290.
Google Scholar Crossref - Kovacs E, Keresztes A. Effect of gamma and UV-B/C radiation on plant cells. Micron. 2002;33(2):199-210.
Google Scholar Crossref - Ashraf MU, Cheema AA, Rashid MU, et.al. Effect of gamma rays on M~l generation in basmati rice. Pak J Bot. 2004;35:791-796.
Google Scholar Crossref - Sen Raychaudhuri S, Deng XW. The role of superoxide dismutase in combating oxidative stress in higher plants. Bot Rev. 2000;66(1):89-98.
Google Scholar Crossref - Stajner D, Popovic BM, Taski K. Effects of γ-irradiation on antioxidant activity in soybean seeds. Cent Eur J Biol. 2009;4(3):381-386.
Google Scholar Crossref - Chakravarty B, Sen S. Enhancement of regeneration potential and variability by γ-irradiation in cultured cells of Scilla indica. Biol plant. 2001;44(2):189-193.
Google Scholar Crossref - Surjadinata BB, DA Jacobo-Velázquez, L Cisneros-Zevallos. Biophysical methods used to generate tolerance to drought stress in seeds and plants: a review. Molecules. 2017; 22:1-13.
Google Scholar Crossref - Teoh LS, Lasekan O, Adzahan NM, et.al. The effect of ultraviolet treatment on enzymatic activity and total phenolic content of minimally processed potato slices. J Food Sci Tech. 2016;53(7):3035-3042.
Google Scholar Crossref - Ullah MA, Tungmunnithum D, Garros L, et.al. Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum. L Int J Mol Sci. 2019;20(7):1787.
Google Scholar Crossref - Zagoskina NV, Alyavina AK, Gladyshko TO, et.al. Ultraviolet rays promote development of photosystem II photochemical activity and accumulation of phenolic compounds in the tea callus culture (Camellia sinensis). Russ J Plant Physiol. 2005;52(6):731-739.
Google Scholar Crossref - Zagoskina NV, Dubravina GA, Alyavina AK, et.al. Effect of ultraviolet (UV-B) radiation on the formation and localization of phenolic compounds in tea plant callus cultures. Russ J Plant Physiol. 2003;50(2):270-275.
Google Scholar Crossref