Original Article
, Volume: 11( 5)Uranium Extraction from El-Hammamat Sediment, Eastern Desert, Egypt Using Solvent-in-Pulp Method
- *Correspondence:
- Hussein AEM , Nuclear Materials Authority, Cairo, Egypt, Tel: 541176627180; E-mail: ah_mady@yahoo.com
Received: May 19, 2016; Accepted: June 24, 2016; Published: June 29, 2016
Citation: Ali MM, Hussein AEM, El Didamony AM, et al. Uranium Extraction from El-Hammamat Sediment, Eastern Desert, Egypt Using Solvent-in-Pulp Method. Chem Technol Ind J. 2016;11(5):102.
Abstract
The present work deals with uranium recovery from sulfate leach slurry of El-Hammamat sediment GV using solvent-in-pulp technique (SIP), where the solvent (trioctyleamine) mixed with the leaching slurry without prior solid/liquid separation. To achieve this objective, different factors have been studied namely; contact time, solvent concentration, dilution factor, type of surfactant, surfactant/solid ratio. About 90% uranium extraction efficiency was attained by the application of the extraction (solvent-in-pulp) optimum conditions. Also, about 98% of the loaded uranium could be stripped by using sulfuric acid as an effective stripping agent.
Keywords
Uranium; Recovery; Solvent-in-pulp
Introduction
One of the most important uranium occurrences has been discovered by the Nuclear Materials Authority in El-Hammamat sediments occurring at the northern peripheries of Gabal Gattar granite pluton and defined as GV. Gabal Gattar area is located in the northern part of the Eastern Desert of Egypt (at about 35 km to the west of Hurghada City). In this area, several uranium occurrences have been defined in the northern margin of younger granite pluton. However, one occurrence denoted as GV is associated with El Hammamat sediment that is outcropping at the northern contacts of the younger granite. In this mineralization, uranium is mainly found as secondary minerals which are essentially represented by uranophane and beta uranophane [1,2]. The latter mentioned minerals are easily converted to soluble compounds by chemical means (leaching with dilute acids and alkalies) with a high degree of selectivity from its associated gangue and with good recovery in moderate costs. Extractive metallurgy of uranium is a hydro-chemical process including its leaching, recovery and concentration from its ore. Other techniques can be applied to recover uranium from its leach liquors; namely, ion exchange resin [3-9], solvent extraction [10-17], Liquid Membrane Methods [18-21], direct precipitation [22-26], resin-in-pulp [27] and solvent-in-pulp [28]. The solvent-in-pulp (SIP) was also developed to eliminate the high cost solid/liquid separation stage, where the solvent passed through the slurry directly. Although successful pilot plant trials have been achieved in Canada, the SIP processes described her deals mainly with the recovery of uranium and limited work has been done on the recovery of other metals, (i.e., copper) using different types of contactors [29]. No plant applying this process has yet been installed in Canada. Beijing Research Institute of Uranium Ore Processing and Hengyong Uranium Mill in China considerable research works upon the SIP process using sieve-plate pulse columns and other equipment based on previous work in Canada. Their investigation was carried out upon leach slurries produced by conventional atmospheric acid leach and acid pressure leaching of a powdered lignite ore after its combustion to generate electric power [30,31]. The Chinese SIP extraction development has proven greatly feasible particularly for a rich uranium ore and focused on uranium extraction from leach slurries and successful development of technique to treat such slurries may lead to wider application. The main objective of the present investigation is studying uranium recovery from GV composite sample by SIP technique. Accordingly, proper conventional agitation leaching of GV working sample was performed by sulfuric acid. In order to obtain leach slurry, from which uranium was recovered using SIP technique. Where uranium (as it well known) i.e., exists in sulfuric acid in the form of cationic, anionic and neutral states; [UO]2+, [UO2(SO4)2]2-, [UO2(SO4)3]4- and UO2SO4. So, it was decided to use TOA (Trioctyl amine) which could extract uranium selectively in its neutral form [32,33]. namely:
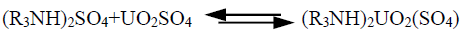
Experimental Materials and Methods
Materials
Proper leaching experiments: The relevant leaching factors were studied using 20 g portions of the powdered sample (-5:+60 mish size). The studied leaching factors involved, effect of sulfuric acid amounts (5% to 50%), effect of solid/liquid ratio (ranged from 1/1 up to 1/6), effect of time intervals (ranged from 1 h up to 6 h) at different temperatures (ranged from room temperature (~25°C) to 60°C). the obtained reaction slurry was filtrated acid thoroughly washed. Afterwards, the uranium leaching efficiencies were calculated.
Solvent in pulp (SIP) experiments: For obtaining adequate amount of leaching slurry (stock slurry), the achieved optimum leaching factors were applied using 1 kg from the studied composite El-Hammamat sediments sample (GV). Accordingly, solvent-in-pulp study was performed by carrying out the effects of the following factors, namely; solvent concentration, slurry dilution, shaking time, solid/liquid phase ratio and diluent type.
The following factors were studied in order to investigate their effects upon reducing solvent consumption, i.e., surfactant type and surfactant/ slurry ratio. Finally, uranium stripping was investigated by studying the effect of stripping agents, and effect of aqueous/organic phase ratios.
Solutions and reagents
A uranium standard solution assaying 1000 mg/l was prepared by dissolving 0.1782 g of uranyl acetate (UO2(CH3COO)2.2H2O (BDH Chemicals Ltd. Poole, England), in 100 ml distilled water. Different concentrations of Trioctylamine (TOA) Mol. Wt 353.76 of Riedel-deHaen of and assaying 98% were used for uranium extraction after its proper dilution in Sunrise kerosene.
Analytical procedures
Uranium was analyzed in the corresponding aqueous phases using ArsenazoIII reagent under different conditions [34]. For this purpose, a Lambda UV/VIS spectrophotometer (Perkin-Elmer, USA) was used. In addition, uranium was also analyzed by an oxidimetric titration method against ammonium metavanedate in the presence of diphenylamine sulfonate indicator prior to titration; proper reduction of uranium was performed using ferrous sulfate [32].
The representative El Hammamat sediment GV composite sample used in this study was obtained from Gabal Gattar by Nuclear Material Authority (NMA), Egypt having the chemical composition of 0.104% REE, 0.130% U, 2.09% SO4-2, 1.77% PO43- , 68.3% SiO2, 0.22% CaO, 10.88% Fe2O3 and 0.09% TiO2.
Results and Discussion
Relevant factors of sulfuric acid leaching of uranium from the composite sample of El Hammamat sediment GV
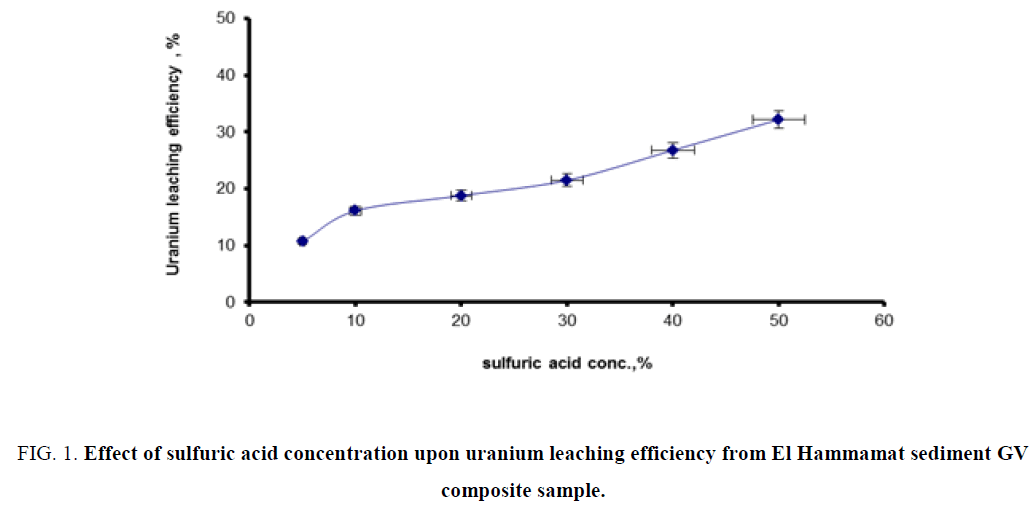
Effect of sulfuric acid concentration: In order to study the effect of acid concentration upon uranium leaching efficiency from GV composite sample, a series of leaching experiments was performed using different acid concentrations ranging from 5% up to 50%. The experiments were carried out under fixed conditions of 25°C leaching temperature for 2 h, 1/3 solid/liquid ratio. The obtained data were plotted in Figure 1. From this figure, it is clearly obvious that using the 30% acid concentration is the economically preferable (after taking the leaching factors in consideration).
Figure 1: Effect of sulfuric acid concentration upon uranium leaching efficiency from El Hammamat sediment GV composite sample.
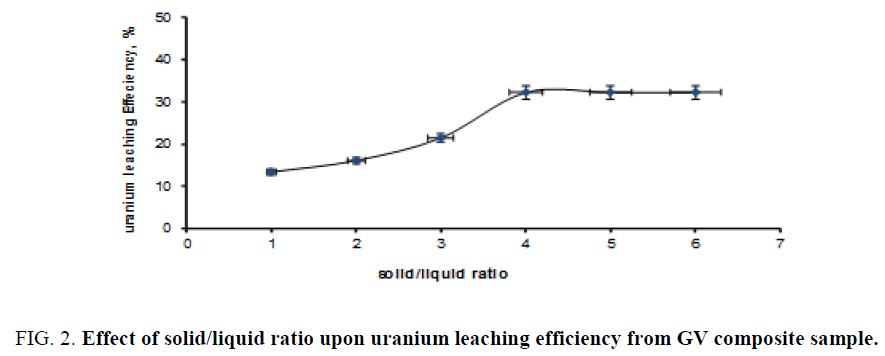
Effect of solid/liquid ratio: In order to study the effect of solid/liquid ratio upon the uranium leaching efficiency from GV composite sample, a series of leaching experiments was performed using different solid/liquid ratios ranging from 1/1 up to 1/6. The experiments were carried out under fixed conditions of 25°C leaching temperature for 2 h, 30% sulfuric acid concentration. The obtained data are plotted in Figure 2. From this figure, it is clearly obvious that uranium leaching efficiency steadily increased by decreasing the solid/liquid ratios until the fourth experiment (of 1/4), after which decreasing solid/liquid ratios gave no any improvement in uranium leaching efficiency.
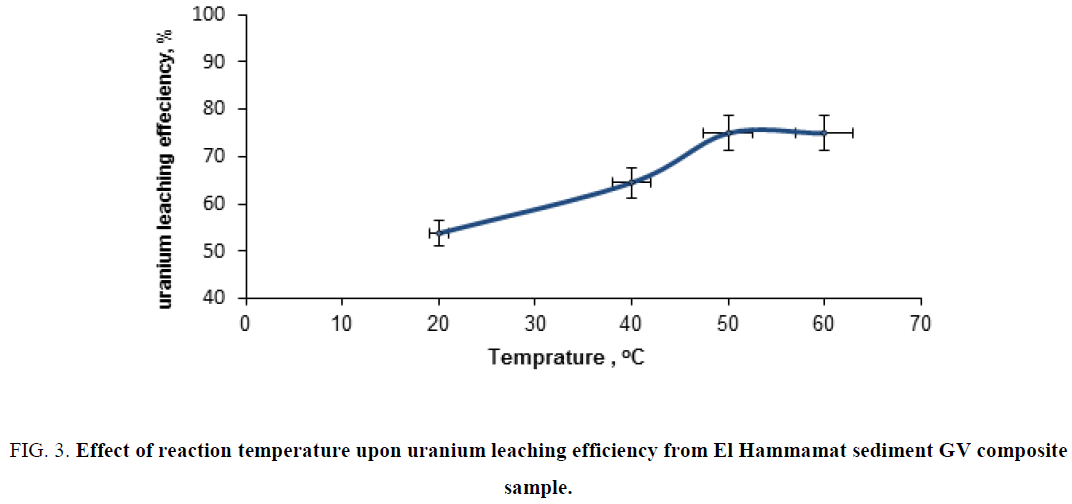
Effect of temperature upon uranium leaching efficiency from GV composite sample: To investigate the effect of temperature upon uranium leaching efficiency of uranium from El Hammamat sediment GV composite sample, a series of leaching experiments were performed using different temperatures ranging from 25°C to 60°C. The experiments were carried out under fixed conditions of 1/4 S/L ratio, reaction time of 6 h and acid concentration of 30%. The obtained data are plotted in Figure 3. From this figure, it is clearly obvious the experiment of 50°C gave the higher uranium leaching efficiency (75%).
Figure 3: Effect of reaction temperature upon uranium leaching efficiency from El Hammamat sediment GV composite sample.
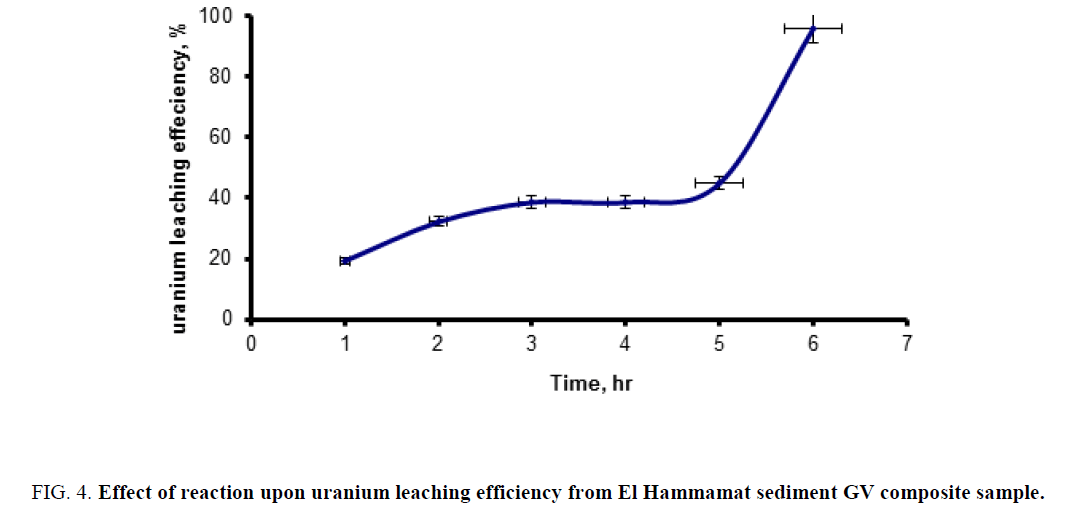
Effect reaction time: In order to study the effect of reaction time upon uranium leaching efficiency from El Hammamat sediment GV composite sample, a series of leaching experiments were performed at different time intervals ranging from 1 h to 6 h. The experiments were carried out under fixed conditions of 1/4 S/L, temperature of 25°C and acid concentration is 30%. The obtained data were plotted in Figure 4. From this figure, it is clearly obvious that the leaching efficiency increased as the reaction time increased from 1 h to 5h (from about 20% to about 40% respectively). At the 6 h reaction time experiment the uranium leaching efficiencies increased significantly to about 96%. Accordingly, one can recommend the use of 6 h as the optimum leaching time.
Figure 4: Effect of reaction upon uranium leaching efficiency from El Hammamat sediment GV composite sample.
Choice of the optimum leaching conditions: Primarily, it is important to mention herein that, careful selection of the optimum values of the obtained result to would depend upon the economic considerations. However, from the forgoing results obtained after studying the relevant factors affecting uranium leaching from El Hammamat sediment GV composite sample; it would seem economic to select the following conditions for leaching uranium from El Hammamat sediment GV composite sample: 30% sulfuric acid concentration, 6 h leaching time, 1/6 solid/liquid ratio and reaction temperature of 25°C. Performing a leaching experiment under the selected optimum condition gave about 96% uranium leaching efficiency.
Relevant factors of uranium extraction from El Hammamat sediment GV composite sample using solvent-in-pulp method
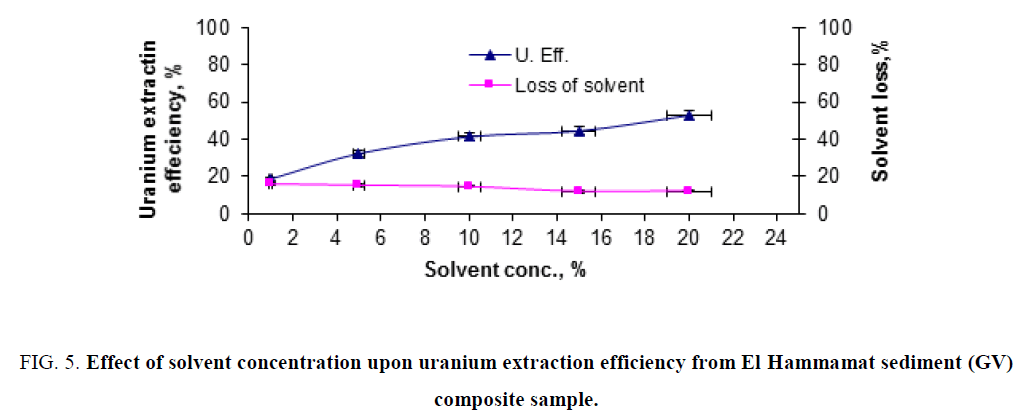
Effect of solvent concentration: To study the effect of (TOA) solvent concentration upon uranium recovery from El Hammamat sediment GV composite sample leaching slurry, a series of extraction experiments were performed by contacting fixed portions (20 ml) with the solvent of different concentrations (0.02 M to 0.54 M) in benzene. The other conditions were fixed, namely; dilution factor of 250 g/l, shaking time of 15 min and solvent/liquid phase ratio of 1/1. The obtained results were plotted in Figure 1. After two phases separate, uranium was determined in the aqueous phase and thereupon the extraction efficiency was calculated. From the obtained results, it was clearly obvious that uranium extraction efficiency increases with increasing the solvent concentration. On the other hand, one could observe that the loss of solvent ratio decreased by increasing its concentration.
Figure 5: Effect of solvent concentration upon uranium extraction efficiency from El Hammamat sediment (GV) composite sample.
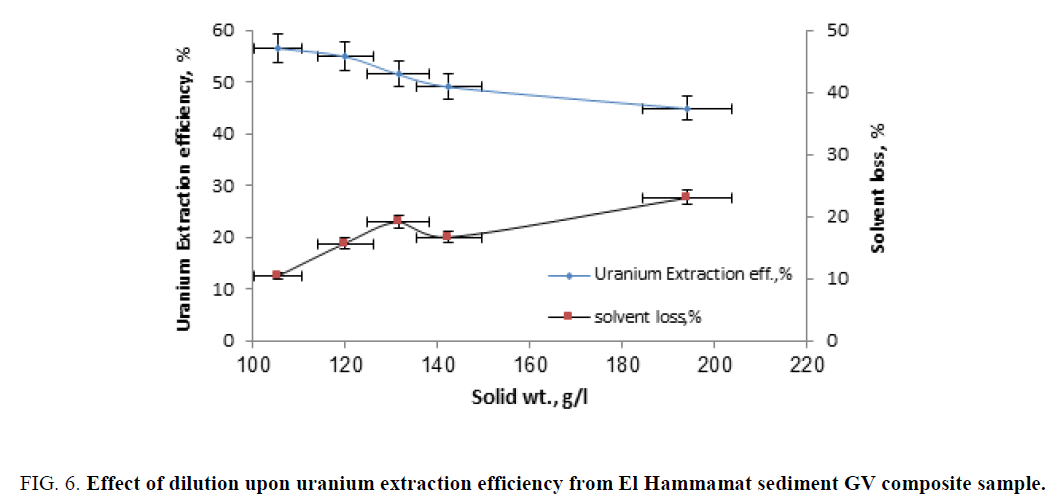
Effect of dilution: For studying the effect of dilution factor of the leaching slurry upon uranium extraction efficiency, a series of extraction experiments was performed by contacting fixed diluted slurry portions (20 ml) with different solid contents ranging from 105 g/l to 194 g/l (5:20 ml of the original slurry) by adding water to original leaching slurry. In all experiment, uranium concentration has been justified (by adding varying volumes from a stander uranium solution) in order to reach the original uranium concentration in the original leaching slurry. The other conditions were fixed at; solvent concentration of 10%, shaking time of 15 min and solvent/liquid phase ratio of 1/1. The obtained results were plotted in Figure 6.
Figure 6: Effect of dilution upon uranium extraction efficiency from El Hammamat sediment GV composite sample.
From the obtained results, it was clearly evident that uranium extraction efficiency decreased (from about 56% to 45%) by the increase the solid content from 104 g/l to 194 g/l respectively, and the solvent loss increased by increasing the solid content (from about 10% to 23% of solvent weight). The decrease of uranium extraction efficiency with the increase of the solid content could be explained as that the increased of solid particles inhibit the free motion of the solvent molecules. We can clarify that, when the slurry is thick that reduce the solvent ability for uranium extraction. On the other hand, the solvent loss is happened by absorbing the solvent molecules on the surface of the thick gangue minerals particles.
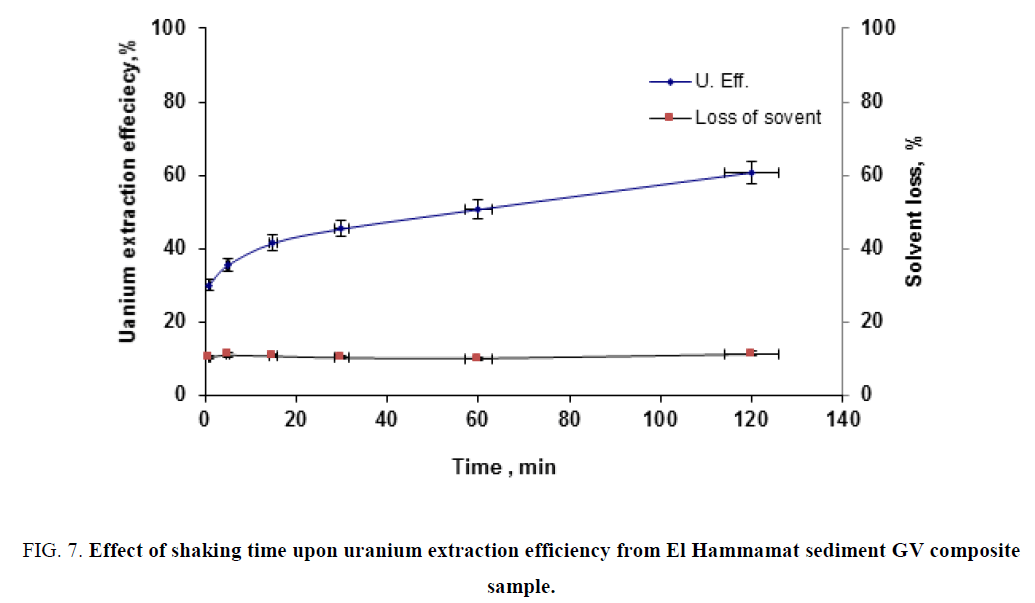
Effect of shaking time: To study the effect of shaking time upon uranium extraction efficiency, a series of extraction experiments were performed by contacting fixed slurry portions (20ml) with the solvent under time interval ranged from 1 min to 120 min. while fixing the dilution factor at 104 g/l, and solvent/liquid phase ratio at 1/1. The obtained data were plotted in Figure 7. From this figure, one could observe that uranium extraction efficiency increases with increasing extraction time while solvent loss was nearly unaffected.
Figure 7: Effect of shaking time upon uranium extraction efficiency from El Hammamat sediment GV composite sample.
Factors affecting solvent loss (consumption): Successful solvent-in-pulp extraction of uranium from the obtained leach slurry will be dictated by the efficiency of the solvent in extraction as well as by the lost amount by slurry raffinate. Accordingly, surfactant type and surfactant amount are studied as the main factors affecting the degree of solvent loss on the gangue mineral surfaces.
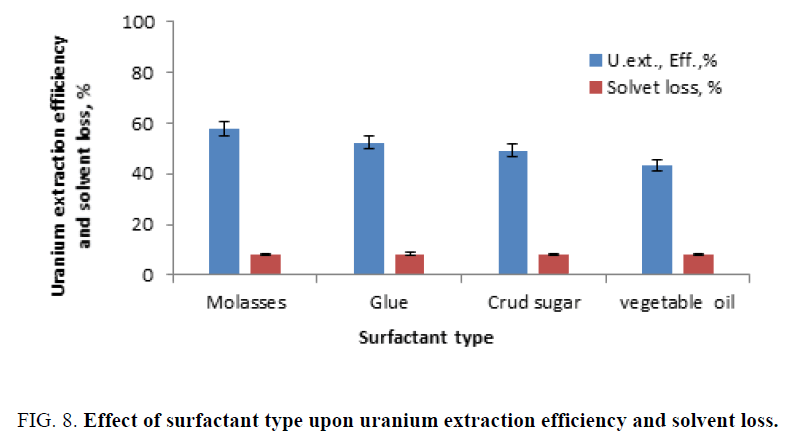
Surfactant type: In order to study the effect of surfactants upon uranium extraction efficiency, the following surfactants were tested, manly; molasses, glue, crud sugar and vegetable oil. The experiments were carries at by mixing 0.5 g of the tested surfactant with the fixed slurries portions (20 ml) for 0.5 h prior contacting with the solvent. The solvent loss was then calculated by the difference in the weight before and after the contact. The experiments were carried out under fixed solvent concentration of 10%, solid content (dilution factor) of 105 g/l, 120 min. as the contact time, and Vorganic/Vslurry ratio of 1/1. The obtained results were plotted in Figure 8. From this figure it is clearly obvious that the uranium extraction efficiency (about 60%) was by using molasses as an effective surfactant.
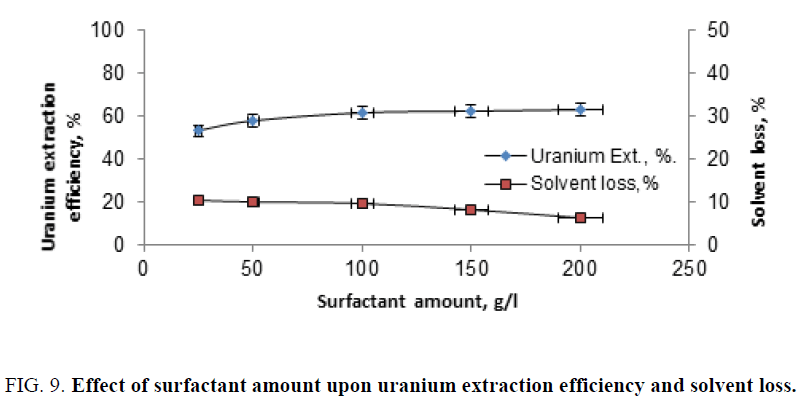
Effect of surfactant amount: In order to study the effect of the surfactant amount upon uranium extraction efficiency, a series of extraction experiments were performed by contacting fixed slurry portion (20 ml) with the solvent. After mixing with deferent amounts of molasses. The mixed amounts of the latter, ranged from 25 g/l to 200 g/l. The experiments performed under fixed condition of solvent concentration of 10%, contact time of 120min, dilution factor (solid amount) of 105 g/l and using molasses as the surfactant. The obtained data were plotted in Figure 9. From this figure one on detected that increasing the surfactant (molasses) amount lead to a pronounced loss in the solvent. On the other hand, increasing the former (molasses) amount showed uranium related increase in uranium extraction efficiency.
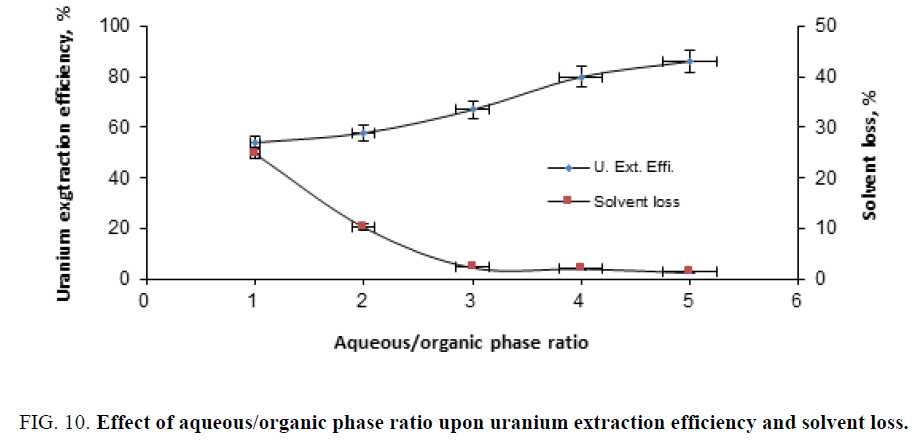
Effect of aqueous/organic phase ratio: To study the effect of aqueous (diluted slurry)/organic phase ratio upon uranium extraction efficiency, a series of extraction experiments were performed by contacting diluted slurry with TOA solution in benzene using different ratios ranged from 0.5/1 up to 4/1. The experiments were carried surfactant under fixed solvent concentration (of 10%) for 120 min contact time, dilution factor (solid content) of 105 g/l and 100 g/l of the surfactant (molasses). The obtained results were graphically represented in Figure 10. From this figure, it is clearly evident that the uranium extraction efficiency increases with the increases of the phase ratios. The latter phenomenon could be explained as due to the deceases in the slurry viscosity (dilution) a matter which enhances uranium extraction efficiency (about 85% for the last experiment). On the other hand, solvent loss (consumption) decreased by increasing aqueous/organic phase ratios.
Figure 10: Effect of aqueous/organic phase ratio upon uranium extraction efficiency and solvent loss.
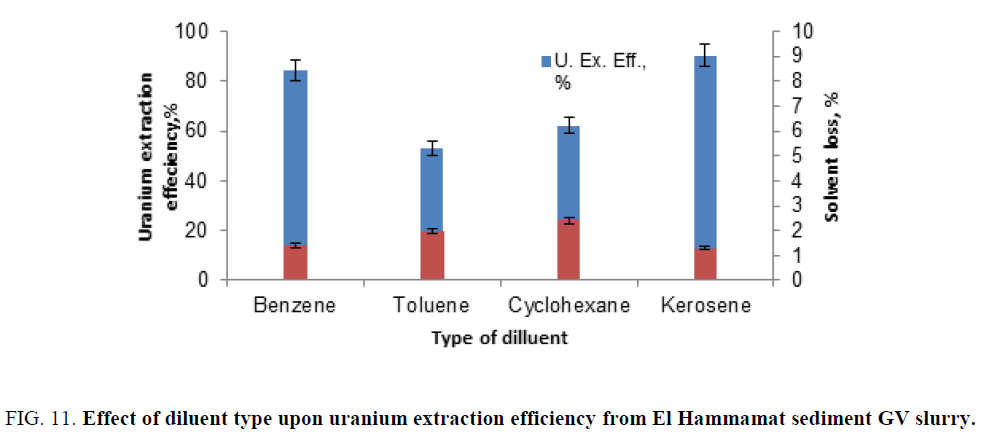
Effect of diluent type: To study the effect of diluent type upon uranium extraction efficiency, many diluents were tested namely; benzene, toluene, cyclohexane and kerosene. The experiments were performed under fixed condition of the solvent concentration of (10%), contact time (120 min), dilution factor (solid content) 105 g/l, surfactant (molasses) 100 g/l and the diluted slurry/organic phase ratio (4/1). The obtained data were plotted in Figure 11. From this figure, it is clearly evident that kerosene gave maximum uranium extraction efficiency (about 90%) and minimum solvent loss (about 1.25%).
Figure 11: Effect of diluent type upon uranium extraction efficiency from El Hammamat sediment GV slurry.
Choice of the optimum conditions of uranium extraction from El Hammamat sediment GV composite sample: From the previous study of relevant factors of uranium extraction from the study sample, it is clearly obvious that it would be possible to attain comparable extraction efficiencies by different combinations of the studied relevant factors. Careful selection of the optimum conditions would depend primarily on economic considerations. In the light of the studied relevant factors, it would seem economically to select the following extraction conditions. Solvent concentration of 10%, extraction time of 120 min, solid content (slurry dilution) of 105 g/l, the surfactant molasses of 100 g\l, aqueous (diluted slurry volume)/organic phase ratio of 4/1 and kerosene as the diluents substance.
Uranium stripping
Uranium stripping from the loaded working solvent, obtained after applying the above mentioned optimum conditions (assay about 1.006 gU/l) was studied by studying the effects of the following factors, namely, stripping agent, stripping agent concentration, shaking time and organic/aqueous phase ratios.
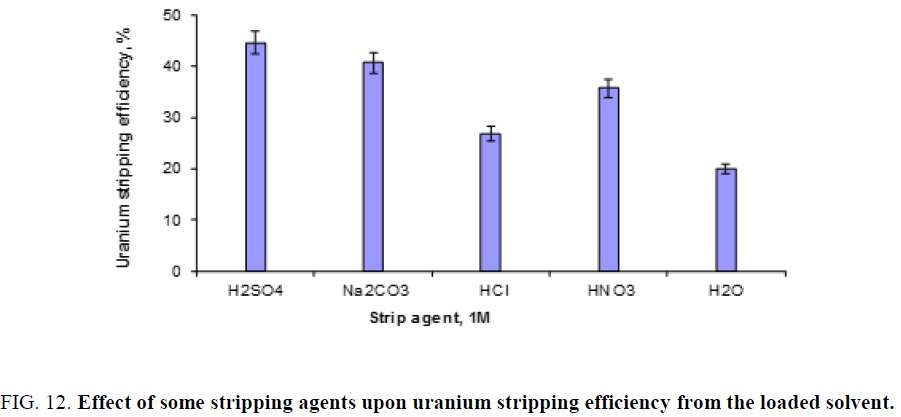
Effect of stripping agent: The following stripping agents, namely; H2SO4, Na2CO3, HCl, HNO3 and H2O, 1 M of each shaked with the loaded solvent of 2/1 aqueous/organic phase ratio for 30 min. shaking time. The obtained results are plotted in Figure 12. From this figure it is clearly obvious that sulfuric acid could be selected as a suitable stripping agent.
Figure 12: Effect of some stripping agents upon uranium stripping efficiency from the loaded solvent.
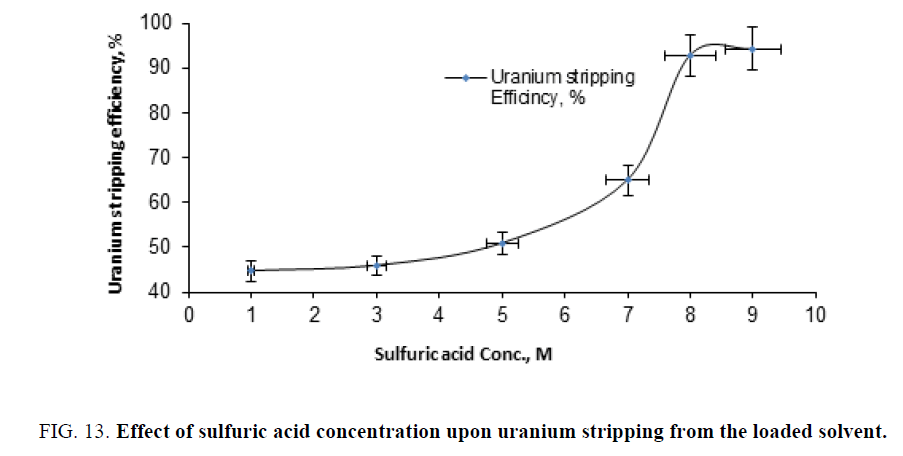
Effect of stripping agent concentration: For investigating the effect of stripping agent concentration, a series of stripping experiments were performed by shaking different concentrations of sulfuric acid ranged from 1 M to 9 M. with the loaded solvent of 2/1 aqous/organic phase ratio for 30 min. shaking time. The results were plotted in Figure 13. From this figure, it is clearly obvious that the 9 M sulfuric acid solution (stripped about 90% of the loaded uranium) could be selected as a suitable concentration.
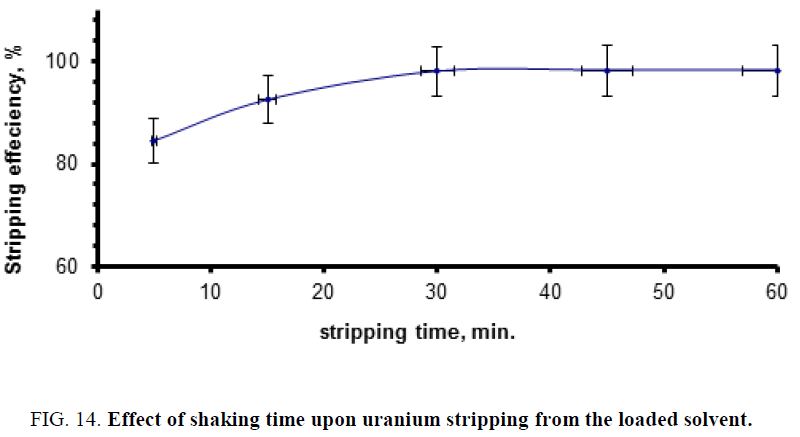
Effect of shaking time upon uranium stripping efficiency: For studying the effect of contact (shaking) time upon uranium stripping from the uranium-loaded solvent of El Hammamat sediment GV composite sample diluted slurry, a series of experiments was performed using time intervals varying from 5 min to 60 min. The performed experiments were carried out using fixed sulfuric acid concentration of 9 M and 2/1 A/O ratio. The obtained results are plotted in Figure 14. From this figure, one can observe that 30 min shaking time would be quite sufficient for stripping about 98% of uranium from the loaded solvent of El Hammamat sediment GV composite sample.
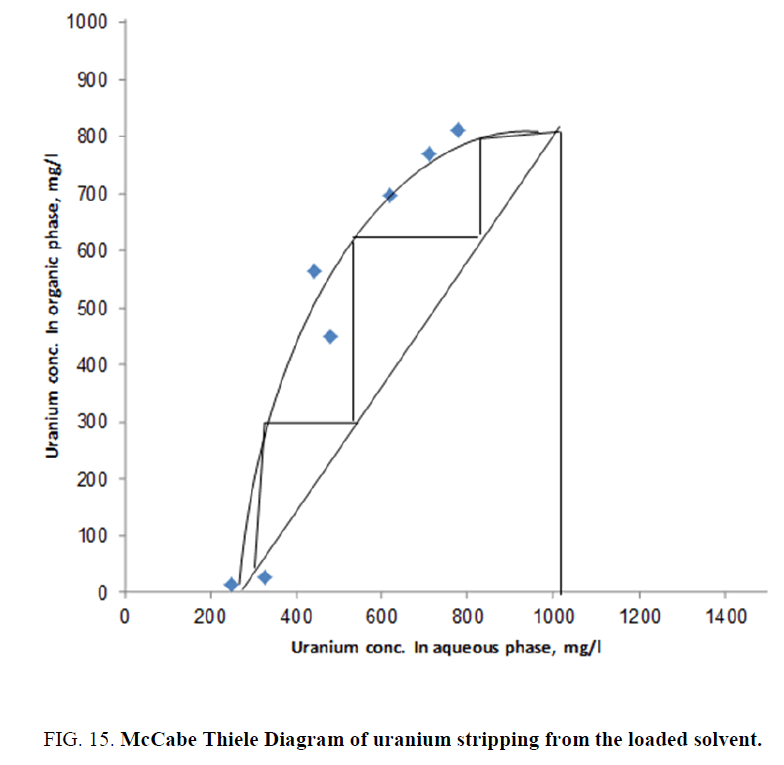
Effect of O/A phase ratio upon uranium stripping efficiency and construction of mccabe thiele stripping diagram: In order to study the effect of aqueous/organic phase ratio upon uranium stripping efficiency from the loaded solvent (of El Hammamat sediment GV composite diluted slurry sample), a series of stripping experiments were performed using O/A ratios ranging from 4/1 down to 1/4. In these experiments, 9 M sulfuric acid concentration was used and the contact time was fixed at 30 min. The obtained data are summarized in Table 1. This table was used to construct the equilibrium isotherm in the McCabe Thiele diagram [34] (Figure 15).
| O/A | U Conc., mg/l | Sao | Stripping efficiency, % | |
|---|---|---|---|---|
| Ratio | Org. | Aq. | ||
| 4/1 | 811 | 780 | 1.03 | 19.3 |
| 3/1 | 769.3 | 710 | 1.08 | 23.5 |
| 2/1 | 696 | 620 | 1.12 | 30.8 |
| 1/1 | 563.3 | 442.2 | 0.73 | 44.2 |
| 1/2 | 450 | 478 | 0.1 | 95 |
| 1/3 | 27.7 | 326.1 | 0.08 | 97.2 |
| 1/4 | 13.2 | 248.2 | 0.05 | 98.6 |
Table 1: Effect of O/A phase ratio upon uranium stripping efficiency from the uranium loaded solvent of El Hammamat sediment GV diluted slurry sample.
The latter was used to calculate the theoretical stages required for almost complete uranium stripping from the uranium-loaded solvent of El Hammamat sediment GV composite diluted slurry sample. From the constructed diagram, it is clearly obvious that an almost three theoretical stages are required for the removal of almost all uranium from the loaded solvent sample prepared from El Hamm mat sediment GV composite sample.
Conclusion
By applying the solvent-in-pulp method, about 90% uranium extraction efficiency was attained (and a minimum solvent loss of about 1.5%) by the application of the extraction optimum conditions of El Hammamat sediment GV diluted slurry sample. In addition, about 98% of the loaded uranium could be stripped using 9 M sulfuric acid as an effective stripping agent.
References
- Sayyah TA, Attawiya MY. Contribution of the mineralogy of uranium occurrence of Gabel Gattar area Eastern Desert, Egypt. Arab J Nucl Sci Appl. 1990;23:171-84.
- Mhamoud KF. Mineralogical and geochemical characteristics of some uranium occurrences in Gattar area as a basis for preparation of high grade uranium concentrate. Ph.D. thesis. Faculty of Science, Ain Shams University; 2000.
- Roz MA. Geology and uranium mineralization of G. Gattar area north eastern desert, Egypt. M.Sc. thesis. Faculty of Science, El Azhar University; 1994.
- Gaudin AM. Principles of mineral dressing. New York: McGraw-Hill; 1939. p. 89.
- Muller LD, Burton CJ. The heavy liquid density gradient and its application in ore dressing mineralogy. Proceedings of 8th Commonwealth Mining and Metallurgy Congress, Melbourne, Australia; 1965.
- Gasps P, Gonzalaz E, de Bacarra JO, et al. Experimental installation for uranium ores alleradas, Aetas 1 Congreso Mcditerraneo de Ingenieria Ouimica; 1978.
- Ortiz I, Alonso A, Urtiaga A. An integrated process for the removal of Cd and U from wet phosphoric acid. Ind Eng Chem Res. 1999;38:2450.
- Kabay N, Demiricioglu M, Yayh S. Recovery of uranium from phosphoric acid solution using chelating ion exchange rersins. Ind Eng Chem Res. 1988;37:1983-90.
- Weintzler TH, Neil M. Uranium recovery by ion exchange/solvent extraction – an economic sensitivity analysis. Proceedings of Annual Conference of Metallurgists. The Canadian Institute of Mining and Metallurgy, Montreal, Canada; 1978.
- Gonzalez-Luque S, Streat M. Uranium sorption from phosphoric acid solution using selective ion exchange resins. Part I. Isotherms of extraction and adsorption. Hydrometallurgy. 1983;11:207.
- Gonzalez-Luque S, Streat M. Uranium sorption from phosphoric acid solutions using selective ion exchange resin, Part II, Kinetic studies. Hydrometallurgy. 1983;11:227.
- Woody RJ, George DR. Acid leaching of uranium ores. In: Clegg JW, Foley DO, editors. Uranium Ore Processing. Boston: Addison Wesley; 1958. p. 115-52.
- Brown KB, Coleman CF, Crouse DJ. Solvent extraction processing of uranium and thorium ores. Proceedings of International Conference on the Peaceful Uses of Atomic Energy; 1958 Sep 1-15, Geneva, Switzerland.
- Taha MH. Chemical and technological studies on the extraction of uranium from egyptian commercial phosphoric acid produced from hemihydrate process. M.Sc. thesis. Faculty of Science, Helwan University; 2005.
- Taha MN. Solvent extraction Studies on phosphoric acid produced by wet-process. M.Sc. thesis. Faculty of Science, Helwan University; 2006.
- Singh S, KDhami PS, Tripathi SC, et al. Studies on the recovery of uranium from phosphoric acid medium using synergistic mixture of (2-Ethyl hexyl) Phosphonic acid, mono (2-ethyl hexyl) ester (PC88A) and Tri-n-butyl phosphate (TBP). Hydrometallurgy. 2009;95:170.
- Singh H, Mishra S, Vijayalakshmi R. Uranium extraction from partially neutralised and diluted phosphoric acid using a synergistic organophosphorous solvent mixture. Hydrometallurgy. 2004;70(1):197-203.
- Nazari K, Jabbari RA, Ghannadi MM. Studies on extraction of uranium from phosphoric acid using PN-1200 extractant. Hydrometallurgy. 2004;71:371-7.
- Amin MI. Removal of some metallic and non-metallic impurities from wet process phosphoric acid, using different separation techniques. Ph.D. thesis. Faculty of Sciences, Mansoura University; 2010.
- Bock J, Valnty P. Uranium extraction from wet-process phosphoric Acid - A liquid membrane approach. Am Chem Soc. Annals Meeting; 1980.
- Hayworth HC. The advantages of liquid membrane technology for the extraction of uranium from wet process phosphoric acid. Am Chem Soc. Annals Meeting; 1980.
- El-Hazek NT, El- Sayed MS. Direct uranium extraction from dihydrate and hemi-dihydrate wet process phosphoric acids by liquid emulsion membrane. Radioanal Nucl Chem. 2003;257:347.
- Saidi M, Khalaf H. Using microemulsion for recovery of uranium from phosphoric acid of Annaba (Algeria). Hydrometallurgy. 2004;74:85.
- Fournier P. Milling high-grade vanadium ore at Cluff Lake, Saskatchewan. Proceedings of Meeting of Canadian Uranium Producers, Metallurgical Committee; Bancroft, Canada. 1982.
- Anderson JS, Ritchie MI, Solution mining of uranium. Mining Congress. 1967;54(1):20-6.
- Roshdy. Removal of uranium from crude phosphoric acid by precipitation technique. Arab J Nucl Sci Appl. 2013;46(5): 38-47.
- Brown AJ, Hayden BC. A Comparison of liquid and resin ion exchange processes for purification and concentration of uraniferous solutions. Can Min Metall. 1979;72(805): 141-9.
- Ritcey GM, et al. Solvent-In-Pulp extraction of gold from cyanide leach slurries. Proceedings of International Solvent Extraction Conference; Moscow, Russia. 1988.
- Wang J, Zhang R. Proceedings of Symposium on Hydrometallurgy; 1981 June; Manchester, England. London: Society of Chemical Industry; 1982.
- Qin WF, You YS, Ru ZY, et al. Beijing Research Institute of Uranium Ore Processing, Report No. 2371, Restricted.
- Ritcey GM, Ashbrook AW. Solvent Extraction: Principles and Applications to Process Metallurgy. Amsterdam: Elsevier; 1984.
- Mackenzie JMW. Uranium solvent extraction using tertiary amines. Uranium Ore Yellow Cake Seminar, 1997 February; Melbourne, Australia.
- Marczenko Z. Spectrophotometric determination of elements. New York: John Wiley & Sons Inc; 1976.
- McCabe WL, Thiele EW. Graphical Design of Fractionating Columns. Ind Eng Chem.1925;17(6):605-11.