Original Article
, Volume: 15( 4)Ultrasound-Assisted Extraction of Anthocyanin Pigments from Hibiscus sabdariffa (Rosella) and its Phytochemical Activity at Kingdom of Saudi Arabia
- *Correspondence:
- Almahy HA Faculty of Science and Education (Alkhurma), Taif University, Alkhurma, Kingdom of Saudi Arabia
Tel: 00966556304068; E-mail: abuamna4@hotmail.com
Received Date: August 17, 2017 Accepted Date: September 08, 2017 Published Date: September 23, 2017
Citation:Almahy HA, Abdel-Razik HH, El-Badry YA, et al. Ultrasound-Assisted Extraction of Anthocyanin Pigments from Hibiscus sabdariffa (Rosella) and its Phytochemical Activity at Kingdom of Saudi Arabia. Int J Chem Sci. 2017;15(4):196.
Abstract
Ultrasound-assisted extraction of anthocyanin pigments and the main phytochemical constituents from Hibiscus sabdariffa (Rosella) was performed using an ultrasonic bath at 30°C using a mixture of EtOH:H2O (80:20) for 20 min. The results indicated that Roselle flowers contained anthocyanins (61.55%), flavonoids (14.22%) and polyphenol (24.23%) which are the main phytochemical groups with biological activities. A reverse phase HPLC analysis gave four different Hibiscus sabdariffa anthocyanin components. Such components were separated and known as delphinidin 3-O-sambubioside and cyanidin 3-O-sambubioside are the major anthocyanins; while the absorbance of these two anthocyanins is four-fold lower than that of cyanidin 3-O-glucoside and delphinidin 3-O-glucoside. Moreover, in the present study, we identified 18 phenolic acids detected at 280 nm.
Keywords
Karkade; Malvaceae; Ultra sonication; Anthocyanin; Foodstuff; Beverages
Introduction
Hibiscus sabdariffaor (Roselle) is a member of the hibiscus family, Malvaceae. This plant has been originated from West Africa, also can be found in middle region of Kingdom of Saudi Arabia. This plant is found to have many unique properties in which different parts of the plant are used for various purposes. In countries like India, Tropical Africa, Philippines and Indonesia, Roselle has been utilized for a long time in producing refreshing beverages, jellies, jams, sauces and preserves [1,2].
A beverage that is made from Roselle calyces are rich in natural antioxidants such as anthocyanins and vitamin C. Anthocyanin pigments can be found in almost all parts of the plant including Roselle. The flowers of Hibiscus sabdariffa are rich in anthocyanins [3]. With the various use of anthocyanins, many researchers are attracted to study on anthocyanins of Roselle because of their potential commercial value and it has high possibility to be marketed widely. Recently the research for food components such as anthocyanins and other phenolic compounds has been grown due to their possible linkages to health benefits including reduction in heart disease and cancer, based on their antioxidant activity [4].
Owing to efficiency, environmentally-friendship as well as inexpensive ultrasound-assisted extraction (UAE) techniques compared to others method which requires low instruments, simple yet efficient. This technique is easy and efficient and it is less time consuming among other methods of extraction. It offers also a mechanical effect allowing greater penetration of solvent into the sample matrix, increasing the contact surface area between the solid and liquid phase and as a result, the solute quickly diffuses from the solid phase to the solvent [5].
In this circumstance, time needed for extraction is shorter. Instead, the use of UAE may prevent the possible chemical degradation of targeted compounds due to decreased chemical involvement and reduction in extraction time [6]. Other than that, it was claimed by Melecchi et al. [7] that ultrasound-assisted extraction was considered as an efficient method for extraction bioactive compounds from Hibiscus flowers.
Experimental Method
Plant materials
The fresh plant Hibiscus sabdariffa (Karkade) was collected from gabbier (Al-khurma Province), the plant collected was further shelled and dried organs were ground to a fine powder using Thomas-Willey Milling Machine.
Ultrasound-assisted extraction process
A sample of the calyces of H. Sabdariffa powder (500 mg) was placed in a 50 mL quartz tube topped by a vapor condenser. The volume was made to 50 mL with the extraction solvent ratio of ethanol: water is 80:20. Thereafter, the ultrasound-assisted extraction was taking place in an ultrasonic bath at 30°C for 20 min. After extraction, the flask was immediately cooled to room temperature by using chilled water. The extract was filtered through filter paper and concentrated to dryness. Before proceeding to the next process, the samples were allowed to cool at room temperature.
Qualitative and quantitative phytochemical screening
The presence of some phytoconstituents was investigated by standards phytochemical methods. Phytochemical analysis of anthocyanins, flavonoids and polyphenols was performed according to the methods described in literature [8-10]. Quantitative and qualitative chemical analysis of phenols, flavonoids and anthocyanins compounds were done by employing spectrophotometric and high performance liquid chromatographic techniques.
Determination of total anthocyanin
Total anthocyanin content (TAC) of the freeze-dried extract was determined using the method described by Laima et al. 10 mg of freeze-dried extract was mixed in 5 mL of methanol acidified with trifluoroacetic acid 0.1% (v/v). Aliquots of the extracts were taken in a 10 mL glass tube and adjust to a volume of 3 mL with methanol acidified with trifluoroacetic acid TFA) and the absorbance was measured at 530 nm using a Jenway 6705 UV/Vis spectrophotometer against the blank sample containing the mixture methanol/TFA 0.1% without the sample extract, TAC was estimated as cyanidin 3-O-glucoside at 530 nm using a molar extinction coefficient of 26, 900 L/mol/cm) and molar mass (449 g/mol) [11] and was expressed as mg cyanidin-3-glucoside (mg Cya3G)/g of freeze-dried extract (g FDE).
Determination of total phenolic content
Total phenolic content (TPC) of the freeze-dried extract was determined using Folin-Ciocalteu essay [12,13]. 0.2 mL of sample extract (1 mg of freeze-dried extract was dissolved in 1 mL of methanol) was mixed with 0.8 mL of distilled water, 0.5 ml of Folin-Ciocalteu’s reagent (1:9 with water) and 1.5 ml of sodium carbonate (17%, w/v). The tubes were incubated for 30 min in the dark at room temperature before absorbance was measured at 765 nm using a Jenway 6705 UV/Vis spectrophotometer against the blank sample contained the same mixture solution without the sample extract. A standard calibration plot was generated at 404 nm using known concentrations of gallic acid (20 μg/mL to 120 μg/mL). TPC was calculated from the calibration plot and expressed as mg gallic acid equivalents (mg GAE) of phenol/g of freeze-dried extract (g FDE). The calibration equation for gallic acid was y=0.004x+0.124, R2=0.998, where y is absorbance and x is concentration of gallic acid in μg/mL.
Determination of total flavonoids
Total flavonoids content (TFC) of the freeze-dried extract was determined using the method described by Hariri et al. [13]. 50 mg of freeze-dried extract was mixed in 5 mL of methanol 70% (v/v). After 24 h, 0.5 mL of filtrate were mixed with 50 μL of Neu reagent. The absorption was determined at 404 nm using a Jenway 6705 UV/Vis spectrophotometer against the blank sample containing the same mixture solution without the sample extract and compared to the one of standard quercetin (0.05 mg/ml) treated with the Neu reagent. A standard calibration plot was generated at 404 nm using known concentrations of quercetin (10-100 μg/mL). TFC was calculated from the calibration plot and expressed as mg quercetin equivalents (mg QE)/g of freeze-dried extract (g FDE). The calibration equation for quercetin was y=0.0156x+0.07, R2=0.987, where y is absorbance and x is concentration of quercetin in μg/ml.
Analyzation of sample using HPLC
The anthocyanins were quantified according to the Lee and Wrolstad method [14] with modifications. Roselle extract was injected into the C-18 column and was eluted in an isocratic manner using acetonitrile: formic acid HPLC-grade solutions. The acetonitrile: formic acid mixture was applied at a flow rate of 1 mL/min. The column temperature was 25°C throughout the experiment. Then, the result was detected. Primary detection was at 260 nm of wavelength.
Results and Discussion
Extraction process with ultrasound has several parameters must be adjusted to get the maximum yield of anthocyanin. The parameters like time, temperature and solvent ratio are involved. The recovery of these components is commonly preceded through a solvent extraction procedure and the concentration of solvent, time and temperature are important parameters to be optimized [15].
It is well known that all the parameters may affect the production of anthocyanin. The study of Cisse et al. [16] showed that, "it has been found that the solid-to-solvent ratio and the particle size had a strong effect on both extraction velocity and anthocyanin extraction yield". The effect of temperature on the production of anthocyanin showed that greater extraction temperature and time contributed to less brilliant red in color and also less in the amount of total anthocyanin contents, 15. In this circumstance, the mentioned three parameters are adjusted for the optimal extraction procedure; our results showed about 12.5% improvement in the % yield of extract due to the use of ultrasonic as compared to the control process.
The results indicated that Roselle flowers contained anthocyanins, flavonoids and polyphenol which are the main phytochemical groups with biological activities. The composition of the aqueous extract of Roselle cultivated at Gabbier (Al-Khurma province) was similar to reference data, with some differences that may be due to genetic variability, type of soil and extractive solvent [17-19]. Anthocyanins were present in calyces of Roselle and have healing properties. The anthocyanins have been found to be cardioprotective, hypocholesterolemic, antioxidative and hepatoprotective [20,21]. They also have an antioxidant activity [22] and inhibit low-density lipoprotein (LDL) oxidation [23]. Flavonoids are well known for their anti-inflammatory, antioxidant activity, anti-viral, cytotoxic and also used in the treatment of diabetes, hypertension and rheumatic fever [24-26]. Roselle shows the presence of flavonoids, it can be of use to cure above mentioned disorders and as an antioxidant agent. In the present study, polyphenols were detected. Polyphenols have attracted a great attention in relation to their potential for beneficial effects on health. Over the last few years, several experimental studies have revealed biological and pharmacological properties of polyphenols compounds, especially their anti-inflammatory activity, antiviral and cytotoxic activity [27,28]. The fact that most medicinal plants is a well-documented, are enriched with polyphenol compounds that have excellent antioxidant properties [29,30]. It is clear that calyces of Roselle possess good phytoconstituents that will be helpful in future for the cure of different types of diseases.
Table 1 show the content of anthocyanins, flavonoids and phenols calyces extracts of Hibiscus sabdariffa. We noted that anthocyanins are the majority compound with 17.53 mg/g and represent 61.55% of the three compounds, followed by the phenols with 6.90 mg/g (24.23%) and finally flavonoids with 4.05 mg/g (14.22%). These results are close to those of Lin et al. [29] because they have a similarity in the relative amounts of chemical constituents (anthocyanins, phenols, flavonoids) in the different extracts. The differences are probably due to the discrepancy between geographical areas and to climatic conditions [31]. Roselle which has a higher content of anthocyanin is also sourced as a good food colorant. Roselle’s extracts have low content in phenols and flavonoids. Nevertheless, they are beneficial to the health of consumers. Indeed, they are a potential source of natural antioxidant.
| Compounds | Contents (mg/g) | Ratio |
|---|---|---|
| Anthocyanins | 17.53 | 0.6155 |
| Flavonoids | 04.05 | 0.1422 |
| Polyphenols | 06.90 | 0.2423 |
Table 1. Quantitative data of various phytochemicals in the calyces extract of Hibiscus sabdariffa.
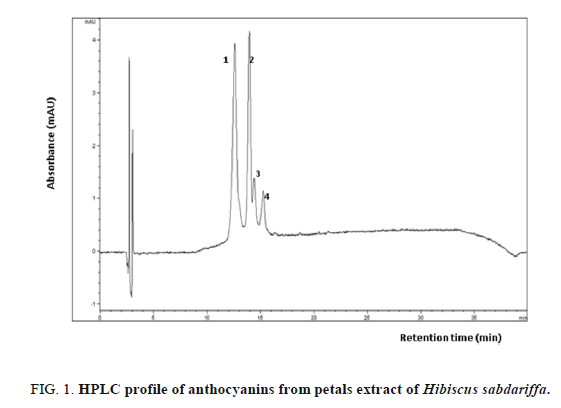
Four different Hibiscus sabdariffa anthocyanins were separated by reverse phase HPLC (Figure 1). Peak assignments are based on matching UV-vis and identical HPLC retention time with known anthocyanins from a reference library of compounds previously purified and identified by anthocyanin identified in Roselle extracts. Chromatograms showed that delphinidin 3-O-sambubioside (peak 1) and cyanidin 3-O-sambubioside (peak 2) are the major anthocyanins. Indeed, the absorbance of these two anthocyanins is four-fold lower than that of cyanidin 3-O-glucoside (peak 3) and delphinidin 3-O-glucoside (peak 4). The presence these anthocyanins in calyces of Roselle were mentioned by many authors [32]. However, the major anthocyanins vary depending on the varieties and also the cultured country. This clearly shows the influence of soil and climatic conditions on the anthocyanins biosynthesis.
Detection is shown at 521 nm. Peaks were identified by comparison with reference standards when available or by HNMR data (retention time). 1. delphinidin 3-O-sambubioside (12.681 min); 2. cyanidin 3-O-sambubioside (13.389 min); 3. cyanidin 3-O-glucoside (14.389 min); 4. delphinidin 3-O-glucoside (15.238 min).
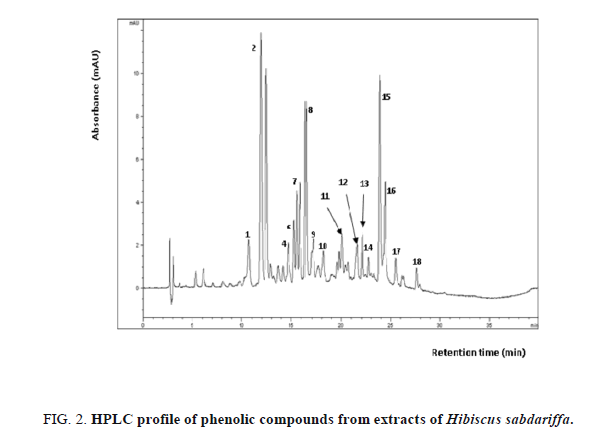
We identified 18 compounds in Hibiscus sabdariffa extract: phenolic acids i.e., chlorogenic acid (4) and protocatechuic acid (6); flavonoids i.e., gossypetrin (1); sabdaretin (2); gossypetin (3); luteolin (5); gossytrin (7); hibiscetin (8); rutin (9); hibiscetrin (10); myricetin (11); eugenol (12); nicotiflorine (13); quercitrin (14); quercetin (15); kaempferol (16); astragalin (17); cyranoside (18). The phenolic acids represent 11% of total phenolic compounds while flavonoids constitute 89%. This suggests that flavonoids are predominating. The major phenolic compounds in Roselle extract are sabdaretin (2), gossypetin (3), hibiscetin (8) and quercetin (15). The presence of sabdaretin, gossypetin, hibiscetin, eugenol, quercetin and protocatechic acid confirms the earlier reports. In addition, they reported the presence of astragalin (kaempferol 3-O-glucoside), nicotiflorine (kaempferol 3-O-rutinoside), luteolin, gossytrin and chlorogenic acid (Figure 2).
Detection is shown at 280 nm. Peaks were identified by comparison with reference standards when available or by HNMR data (retention time). 1. gossypetrin (10.671 min); 2. sabdaretin (11.919 min); 3. gossypetin (12.466 min); 4. chlorogenic acid (14.690 min); 5. luteolin (15.270 min); 6. protocatechuic acid (15.548 min); 7. gossytrin (15.863 min); 8. hibiscetin (16.418 min); 9. rutin (17.120 min); 10. hibiscetrin (18.129 min); 11. myricetin (20.045 min); 12. eugenol (21.570 min); 13. nicotiflorine (22.082 min) 14. quercitrin (22.795 min); 15. quercetin (23.866 min) ; 16. kaempferol (24.399 min); 17. astragalin (25.465 min); 18. cyranoside (25.596 min).
Conclusion
Hibiscus sabdariffa is an excellent source of dietary phytochemicals such as anthocyanins, flavonoids and phenolic acids. The use of Roselle calyces as natural antioxidants, natural colorants and an ingredient of functional foods seems to be promising.
References
- Clydesdale MF, Main HJ, Francis JF. Roselle (Hibiscus sabdariffa L.) anthocyanins as colorants for beverages and gelatin desserts. J Food Prot. 1979;42:204-7.
- Clydesdale MF, Main HJ, Francis JF, et al. Effect of anthocyanin preparations as colorants on hygroscopicity of dry-pack foods. J Food Prot. 1979;42:225-7.
- Cissé M, Dornier M, Sakho M, et al. Le bissap (Hibiscus sabdariffa L.): Composition et principales utilisations. Fruits. 2009;64(3):179-93.
- Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360-7.
- Wang W anderson BT, Phillips N, et al. Statistical analysis. Earth Interactions. 2006;10:1-27.
- Rostagno MH, Hurd HS, McKean JO. Preslaughter holding environment in pork plants is highly contaminated with Salmonella enterica. App and Env Microbiol. 200;369:4489-94.
- Melecchi S, Martinez MM, Abad FC, et al. Caramão, chemical composition of Hibiscus tiliaceus L. flowers: A study of extraction methods. J Sep Sci. 2002;25:86.
- Harborne JB. Phytochemical methods: A Guide to Modern techniques of plants Analysis. Chapman and Hall London, UK. 1998;pp:40-82.
- Phillipson JD, Phillipson JD. Phytochemistry and medicinal plants. Phytochemistry. 2001;56(3):237-43.
- Cesonien? L, Daubaras R, Viškelis P, et al. Determination of the total phenolic and anthocyanin contents and antimicrobial activity of Viburnum opulus fruit juice. Plant foods for human nutrition. 2012;67(3):256-61.
- Chaovanalikit A, Wrolstad RE. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J Food Sci. 2004;69(1).
- Siriwoharn T, Wrolstad RE, Finn CE. Influence of cultivar, maturity and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics and antioxidant properties. J Agri Food Chem. 2004;52:8021-30.
- Hariri EB, Sallé G andary C. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma. 1991;162(1):20-6.
- Lee J, Wrolstad RE. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J Food Sci. 2004;69(7):564-73.
- Spigno G, Tramelli L, De Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng. 2007;81:200-8.
- Cisse M, Ly I, Nianogo AJ, et al. Grazing behavior and milk yield of Senegalese Sahel goat. Small Rumin Res. 2002;43(1):85-95.
- Olaleye MT. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J Med Plants Res. 2007;1(1):9-13.
- Mungole A, Chaturvedi A. Hibiscus sabdariffa L a rich source of secondary metabolites J Pharma Sci Rev and Res. 6(1), 83-87 (2011).
- Ongoka PR, Matini L, Moutou JM, et al. Annales de l'Université Marien NOUGABI. 2006;7(3):138-46.
- Jonadet M, Bastide J, Boyer B, et al. In vitro enzyme inhibitory and in vivo cardioprotective activities of hibiscus (Hibiscus sabdariffa L.). J Pharmacol Belgium. 1990;45(2):120-4.
- Wang CJ, Wang JM, Lin WL. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411-6.
- Zhang M, Hettiarachchy NS, Horax R, et al. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. J Med Plants Res. 2011;5(30):6672-80.
- Rio DD, Rodriguez-Mateos A, Spencer JPE, et al. Dietary (poly) phenolics in human health: Structures, bioavailability and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18(14): 1818-92.
- Usoh IF, Akpan EJ, Etim EO, et al. Antioxidant actions of dried flower extracts of Hibiscus sabdariffa L. on sodium arsenite-induced oxidative stress in rats. Pakistan J Nut. 2005;4(3):135-41.
- Akanbi WB, Olaniyan AB, Togun AO, et al. The effect of organic and inorganic fertilizer on growth, calyx yield and quality of Roselle (Hibiscus sabdariffa L.). Am-Eurasian J Sustainable Agri. 2009;3(4):652-7.
- Azevedo J, Fernandes I, Faria A, et al. Antioxidant properties of anthocyanidins, anthocyanidin-3-glucosides and respective portisins. Food Chem. 2010;119:518-23.
- Zhang M, Hettiarachchy NS, Horax R, et al. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J Med Plants Res. 2011;5(30):6672-80.
- Vilasinee H, Anocha U, Noppawan PM, et al. Antioxidant Effects of aqueous extracts from dried calyx of Hibiscus sabdariffa L. (Roselle) in vitro using rat low-density lipoprotein (LDL). Biol and Pharmaceutical Bulletin. 2005;28(3): 481-4.
- Lin TL, Lin HH, Chen CC, et al. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nut Res. 2007;27:140-5.
- Salazar-Gonzalez C, Vergara-Balderas FT, Ortega-Regules AE, et al. Antioxidant properties and color of Hibiscus sabdariffa extracts. Ciencia Investigacion Agraria. 2012;39(1):79-90.
- Segura-Carretero A, Puertas-Mejia MA, Cortacero-Ramírez S, et al. Selective extraction, separation and identification of anthocyanins from Hibiscus sabdariffa L. using solid phase extraction-capillary electrophoresis-mass spectrometry (time-of-flight/ion trap). Electrophoresis. 2008;29(13):2852-61.
- Mahadevan N, Shivali K, Kamboj P. Hibiscus sabdariffa Linn-An overview. Natural Product Radiance. 2009;8(1):77-83.