Research
, Volume: 16( 3)Trichoderma harzianum 857 is an Effective Destructor of Cellulose-Containing Raw Materials
- *Correspondence:
- Jakhongir Alimov
National University of Uzbekistan
Faculty of Biology
Microbiology and Biotechnology Department
Almazar district, University Street 4, 100174
Tashkent, Uzbekistan
Tel: +998909143366
E-Mail: jahongir.alimov@gmail.com
Received: May 11, 2020; Accepted: May 18, 2020; Published: May 30, 2020
Citation: Alimov J, Abdusamatov S, Shurigin V, et al. Trichoderma harzianum 857 is an Effective Destructor of Cellulose-Containing Raw Materials. Indian J Environ Sci. 2020;16(3):113.
Abstract
The purpose of research was to assess the possibility of using Trichoderma harzianum 857, isolated from the soil of Uzbekistan, as a source of cellulolytic enzymes for processing of cellulose-containing substrates. It was established that the fungus Trichoderma harzianum 857, due to the synthesis of extracellular cellulolytic enzymes, can be used for hydrolysis of cellulose-containing substrates (wheat straw). The conditions for pretreatment of the cellulose-containing substrate (temperature 100°C, pressure 1 atm., time 1.5 h) were selected to increase the efficiency of its enzymatic cleavage. It was shown that adding ground green part of amaranth to a composition of cellulose-containing substrates contributes to the enrichment of the final product with protein, vitamins and trace elements, which makes it possible to use it as a high-protein product.
Keywords
Cellulose; Enzyme straw; Wheat; Amaranth; Trichoderma harzianum
Introduction
Due to the ability to synthesize and secrete a complex of cellulolytic enzymes: endo-1,4-β-glucanase; exo-1,4-β-glucanase (cellobiohydrolase or 1,4-β-D-glucoside glycoside hydrolase) and cellobiose β-glucosidase, fungi of the Trichoderma genus are widely used in many biotechnological processes associated with the processing of cellulose-containing materials. These fungi are very technological, undemanding to the substrate, and resistant to environmental effects [1-4].
A characteristic feature of the fungi of the genus Trichoderma is the synthesis of extracellular cellulases. In the process of their development, these fungi accumulate a large number of proteins in the nutrient medium. Due to these properties, some strains play a leading role among industrial producers of cellulolytic enzymes, which is explained by at least two reasons: firstly, their high secretory capacity, and secondly, the diversity of the composition of the synthesized enzyme complex. The composition of cellulase complexes of various microorganisms includes 4 different types of enzymes: endoglucanases, exoglucanases, cellobiohydrolases, and cellobiases. Depending on the source (i.e. producer) of the cellulase complex, each of these types of enzymes may contain multiple molecular forms of the enzyme, differing in a specific activity, stability, the strength of adsorption on the surface of an insoluble substrate, substrate specificity, degree of inhibition by hydrolysis products, etc. [4].
Exoglucanases or cellobiohydrolases (1,4-β-glucan cellobiohydrolases), which cleave cellobiose from the end of the polysaccharide chain, usually have high activity towards crystalline cellulose. Trichoderma reesei synthesizes two molecular forms of cellobiohydrolase: the first form (CBHI), attacks the cellulose chain from its non-reducing end, while the second one, endoglucanases catalyze the cellulose cleavage reaction in the middle, producing new ends of the chains for impact cellobiohydrolase [5]. Due to studies conducted in recent years, a detailed understanding of the basic laws of enzymatic hydrolysis of cellulose has been achieved, while the practical implementation of the process of enzymatic production of glucose from cellulose-containing materials is still not well understood [6].
The objective of our study was to assess the possibility of using Trichoderma harzianum 857, isolated from the soil of Uzbekistan, as a source of cellulolytic enzymes for the processing of cellulose-containing substrates. The strain was deposited into Collection of industrially Trichoderma important microorganisms of the Institute of Microbiology, Academy of Sciences of Uzbekistan under the number 857.
Materials and Methods
Study of morphological properties of Trichoderma harzianum 857
As the object for cellulolytic enzymes the fungi Trichoderma harzianum 857, isolated from the soil of Uzbekistan was used. The strain was deposited into the collection of industrially important microorganisms of the Institute of Microbiology, Academy of Sciences of Uzbekistan under the number 857. To study morphological properties of harzianum 857 it was cultivated on a Chapek-Doks nutrient medium and wort-agar.
The treatment of cellulose-containing substrates with enzymes of Trichoderma harzianum 857
As a source of cellulolytic enzymes, a culture fluid of 4-5 daily fungi or an aqueous extract from superficially grown Trichoderma harzianum 857 were used. The ground green part of amaranth and wheat straw was used as a substrate.
For the enzymatic treatment of cellulose-containing substrates, the culture fluid or aqueous extract from the surface-grown Trichoderma harzianum 857 was standardized as 10 units of cellulolytic activity per 1 gram of the processed substrate. For deep cultivation, the modified medium described by Mandels M [7] was used, which included a cellulose-containing substrate (chopped amaranth straw) as a carbon source, instead of 2% sucrose and (NH4)2SO4 as a nitrogen source, instead of NH4)2HPO4 . In this method of cultivation, the fungal mycelium was separated from the culture fluid by centrifugation at 3000 rpm for 15 minutes, and in the case of surface cultivation, at the end of the fermentation time, either the entire nutrient medium mixture with the grown fungi or the aqueous extract of this mixture was used, obtained by pressing while respecting the rule: 10 units of cellulolytic activity per 1 g of the processed substrate.
When developing modes of solid-phase fermentation of cellulose-containing substrates, previously prepared above-ground parts of amaranth and winter wheat straw were used as raw materials.
The seed was the mycelium of a 5-day culture of the Trichoderma harzianum 857 grown on Chapek-Dox agar medium at a temperature of 28°C-32°C, as well as the mineral medium described by Mandels M [7] with a filter paper disk as the sole carbon source and cellulose agar.
A suspension of Trichoderma harzianum 857 culture medium was used as an inoculum obtained by cultivating it in a nutrient medium, the main part of which consisted of ground green part of amaranth and wheat bran. The cellulolytic activity of the inoculum was 25 μ/ml-27 μ/ml. Cultivation was carried out in conical flasks with a volume of 1-l (laboratory experiments) and on special cells measuring 30 cm × 40 cm in a sterile substrate with a substrate moisture of 10%, the height of the substrate layer did not exceed 50 mm-55 mm on a medium consisting of wheat bran (50%) and ground green part of amaranth or straw (50%) with a moist mineral medium described by Mandels M [7] (semi-industrial experiments). The initial pH value was 4.7-4.9.
Enzymatic processing materials were secondary crop waste-biomass of the aerial part of the amaranth plant and winter wheat straw, dried to a residual moisture content of 6%-7% and ground to a particle size of 1.5 mm-2.0 mm. Isolation and study of the properties of the culture were performed using standard techniques [8]. Enzymatic hydrolysis of cellulose-containing substrates was carried out in the following mode: 10 ml of tap water was added to 1 g of the substrate (ground green part of amaranth or winter wheat straw) and heated to 100°C, boiled for 5 min. After cooling to room temperature, the culture fluid of the Trichoderma harzianum 857 was added to a mixture at the rate of 10 units of enzyme per 1 g of substrate. Stirred with a stirrer at speed of 150 rpm and a temperature of 32°C-35°C. The initial pH value was 4.7-4.9. The destruction efficiency was evaluated by the accumulation of reducing substances. The method is based on the recovery of copper ions (Cu+2) from an alkaline solution of Muller to copper hemi oxide (Cu2O) with reducing substances by adding an excess amount of iodine solution and titrating its excess with sodium thiosulfate solution [9]. Another method is based on the quantitative determination of reducing sugars resulting from the hydrolysis of cellulose under the action of cellulolytic complex enzymes [10]. Per unit of cellulolytic activity (1 μ CLA) was taken the amount of enzyme that catalyzes the hydrolysis of cellulose with the formation of 1 μmol of reducing sugars (in terms of glucose) for 1 hour at a temperature of 32°C at pH of 4.7. The content of reducing sugars, formed because of the enzymatic reaction, as determined by the colorimetric method using potassium ferrocyanide (red blood salt, potassium hexacyanoferrate) and was calculated according to the calibration curve constructed for glucose. The measurement range of the monitored indicator was 0.5-25.0 units CLA. To prepare standard glucose solutions, a basic standard solution was initially prepared with a glucose concentration of 1 μmol/cm3 (180 μg/cm3). A sample of anhydrous glucose (90 mg), taken to the nearest 0.2 mg, was introduced into a volumetric flask with a capacity of 500 cm3, dissolved (30 cm3-50 cm3), made up to volume with distilled water and mixed. From the basic standard glucose solution, a series of dilutions were prepared in accordance with TABLE 1.
| The volume of a standard solution* with molar (mass) glucose concentration, µmol/ cm3 | Volume of buffer solution, cm3 | The concentration of glucose in dilution | |
|---|---|---|---|
| Mass, µg/cm3 | Molar, mol/cm3 | ||
| 2 | 8 | 36 | 0.20 |
| 3 | 7 | 54 | 0.30 |
| 4 | 6 | 72 | 0.40 |
| 5 | 5 | 90 | 0.50 |
| 6 | 4 | 108 | 0.60 |
| 7 | 3 | 126 | 0.70 |
| 8 | 2 | 144 | 0.80 |
*standard glucose solutions were prepared on the day of construction the calibration curve with three parallel weights
Table 1: Standard dilutions of glucose.
To construct the calibration graph, 2 cm3 of standard glucose solution and 6 cm3 of potassium hexcyanoferrate solution were added to a series of tubes. The tubes were placed into a boiling water bath for 10 minutes, then taken out and cooled to room temperature. The optical density of the colored solutions was measured at a wavelength of 400 nm-440 nm and an absorbing layer thickness of 10 mm against distilled water.
Based on obtained results, a calibration curve was plotted for the dependence of the absorbance values on the glucose concentration (μmol/cm3). The molar concentration of glucose μmol/cm3 was laid off along the axis of abscissas, and the optical densities in OD units were plotted along the ordinate axis. The graph has an inverse linear relationship. The working area of the calibration curve was in the range from 0.3 μmol/cm3 to 0.6 μmol/cm3 of glucose, which corresponds to the absorption in units of optical density from 0.50 to 0.65 units OP. To build each point of the calibration curve, the arithmetic average value of the optical density of three parallel measurements was calculated. The working solution of the enzyme was prepared by dilution in distilled water so that when determining the activity, the optical densities of the test and control solutions were within the working area of the calibration curve.
Statistical analysis
The results were analyzed with ANOVA using SPSS-22 statistical software (SPSS, Inc., Chicago, IL, USA).
Results and Discussion
Morphology of Trichoderma harzianum 857
Trichoderma harzianum 857 strain formed fast-growing colonies on the Chapek-Doks medium and wort agar. Mycelium of the fungus was colorless, septate, and prostrate. On the 4th-5th day of growth, the turfs with conidiophores appeared the cushion-shaped at first white, with a time of yellow or dark green color. Butyles (9 μ-12 μ) were arranged in whorls of 3 or more. On every fialida conidia were formed, glued in the head. Conidia of fungi were round, smooth, small (3 μ-5 μ), pale green in transmitted light, and dark in daylight.
Chemical composition of the amaranth and wheat straw
The aerial part of both amaranth and grain crops is a cellulose-containing material, which is of low nutritional value and consists mainly of polysaccharides TABLE 2 [11]. The characteristic feature of the chemical composition and limited nutritional value of these materials is a high content of cellulose and fiber, and very small amount of protein and fat, poverty with minerals, and lack of vitamins. Also, the straw of grain crops in its pure form is poorly eaten by animals, and its components are poorly digested.
| Ingredients | Content,% a.d.w. | |
|---|---|---|
| Wheat straw | Amaranth phytomass | |
| Cellulose | 41.52 ± 0.40 | 29.2 ± 0.07 |
| Hemicellulose | 23.23 ± 0.13 | 18.2 ± 0.12 |
| Lignin | 21.37 ± 0.81 | 21.4 ± 0.62 |
| Crude fiber | 34.02 ± 0.84 | 24.0 ± 0.05 |
| Crude protein | 4.52 ± 0.09 | 28.0 ± 0.3 |
| Fat | 1.46 ± 0.17 | 3.5 ± 0.004 |
| Ash | 4.14 ± 0.04 | 10.01 ± 0.04 |
Table 2: The chemical composition of wheat straw and the aerial part of amaranth [11].
The nutritional components of straw are enclosed in a strong lignin-cellulose complex, poorly destroyed in the gastrointestinal tract of animals. Wheat straw fiber consists of 35%-45% cellulose, 14%-22% lignin, 20%-30% pentosans, and 3%-5% silicon salts. The higher the content in fiber straw, the lower its nutritional value. However, wheat straw is a promising cellulosic raw material, a potential food product. The development of optimal conditions for the bioconversion of this type of raw material can contribute to the production of several valuable products TABLE 2 [12].
Unlike wheat, green mass and amaranth grain without pretreatment can be widely used as a food and for technical purposes, because it is superior to many traditional crops in protein, vitamins, biologically active substances, and fats content TABLE
3. As it is shown in TABLE 3, the productivity, protein yield, and lysine content of amaranth in terms of hectares are much higher as compared with many traditional crops. In the field conditions, the yield of amaranth green mass reaches 80 t/ha, and on average for Uzbekistan, it can be estimated at 40 t/ha-45 t/ha. The seed yield reaches 5 t/ha, but due to the extended ripening period and the lack of special harvesting equipment, the economic yield is 1.5 t/ha-2.0 t/ha. From 50 tons of amaranth yield, up to 2.0 tons of high-lysine protein can be obtained, whereas, in wheat and barley, this indicator is 0.05 tons TABLE 3.
| Plants | Productivity, t/ha | Protein content | Lysine content, kg/ha | |
|---|---|---|---|---|
| % | kg/hа | |||
| Winter wheat | 5.0 ± 0.012 | 11.0 ± 0.3 | 500 ± 12 | 18.0 ± 0.02 |
| Spring barley | 2.0 ± 0.011 | 11.5 ± 0.3 | 510 ± 12 | 18.0 ± 0.02 |
| Rape | 2.2 ± 0.012 | 20.0 ± 0.3 | 440 ± 11 | 26.0 ± 0.02 |
| Peas | 2.0 ± 0.011 | 24.0 ± 0.4 | 440 ± 12 | 29.0 ± 0.02 |
| Sunflower | 2.3 ± 0.012 | 18.0 ± 0.3 | 414 ± 10 | 15.0 ± 0.02 |
| Soy | 1.5 ± 0.012 | 40.0 ± 0.4 | 650 ± 12 | 33.0 ± 0.03 |
| Alfalfa (dry weight of phytomass) | 10.0 ± 0.01 | 20.0 ± 0.3 | 2000 ± 13 | 82.0 ± 0.05 |
| Amaranth (dry weight of phytomass) | 15.0 ± 0.12 | 20.0 ± 0.3 | 3000 ± 13 | 180.0 ± 0.06 |
Table 3: Comparative characteristics of the most important agricultural plants.
Talking about the chemical composition of amaranth, it is necessary to note the high plasticity of the plant, which causes large discrepancies depending on the agro technological conditions of cultivation, variety, climate, age of plants at the time of harvest [13]. It should also be noted that the chemical composition of amaranth is studied primarily from nutritional value, therefore, the protein content, its amino acid composition, the content of certain vitamins and minerals were studied in detail [14]. Other classes of plant substances in amaranth, such as cellulose, hemicellulose, and lignin were studied very poorly or were not studied at all. The available literature data convincingly show that an amaranth phytomass can be used in fodder production without additional processing.
Amaranth protein is characterized by a high content of essential amino acids. In 1 kg of amaranth dry matter, the vegetative mass contains 7.1 g-7.15 g of lysine, and in maize just 2.8 g, i.e. 2.4 times less. In a green mass of amaranth in terms of absolutely dry weight: crude protein: 15.6%-16.75% (in leaves up to 30%), fat: 2.4%-2.8%; cellulose: 16%-21.7%; Calcium: 2.1%-2.6%; phosphorus: 0.2%-0.21%; carotene: 160 mg-200 mg [15]. For comparison, the green mass of corn in the phase of milky-waxy ripeness of grain contains 7.5%-8.0% protein, which is 2 times less than in amaranth. Amaranth grain contains 15%-17.8% of protein, 5%-7% of fat, 3.2%-6.4% of fiber, 3.0%-4.1% of ash.
The protein of amaranth is among the best proteins of plant origin and surpasses soy protein in quality [15]. For the ratio of amino acids, amaranth protein is approaching the ideal protein. If we estimate the ideal protein (close to the egg) as 100 points, then casein milk protein would get 72 points, soyabean: 68, barley: 62, wheat: 58, corn: 44, and amaranth: 75 points.
The enzymatic hydrolysis of cellulose
The main attention was focused on the study of cellulose-containing raw materials, particularly wheat straw. At the same time, amaranth was used to enrich the final product with protein, amino acids, lipids, and trace elements (TABLES 2 and 3). It is known that the hydrolysis of cellulose to glucose can be carried out in two ways: chemical and enzymatic, the most promising of which is the latter. Moreover, the enzymatic hydrolysis of cellulose is an environmentally friendly technology based on the processes and mechanisms of the conversion of substances by cellulolytic enzymes [16].
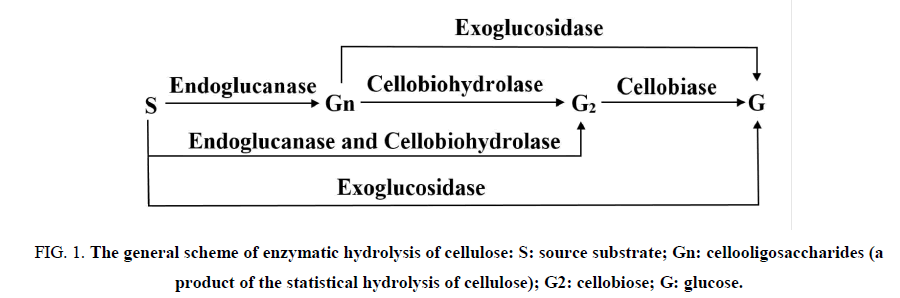
The enzymatic hydrolysis of cellulose and its derivatives involves a complex of cellulases consisting of different types of carbohydrases: endo-1,4-β-glucanase, exo-1,4-β-glucanase (1,4-β-glucan cellobiohydrolase or 1,4-β-D-glycoside glycol hydrolase) and cellobiase (β-glucosidase or β-D-glucosidoglucidrolase) [17]. The general scheme of enzymatic hydrolysis of cellulose is shown in FIG. 1.
Figure 1. The general scheme of enzymatic hydrolysis of cellulose: S: source substrate; Gn: cellooligosaccharides (a product of the statistical hydrolysis of cellulose); G2: cellobiose; G: glucose.
Depending on the physical state of the original substrate, G can be either partially destructed insoluble cellulose with a relatively low degree of polymerization compared to the initial insoluble substrate, or a set of substituted cellodextrins (upon hydrolysis of soluble cellulose derivatives, for example, CM-cellulose).
The effectiveness of the enzymatic hydrolysis of cellulose-containing substrates was greatly influenced by the preliminary testing of the latter [18]. Native wheat straw consists of rigidly bonded fibers oriented in space, without longitudinal and transverse tears. Such strength of the straw is due to twisted elementary fibers protecting the straw from any impact. During heat treatment, the spatial orientation of the fibers was disturbed depending on the temperature, pressure, and time of incubation. Thus longitudinal and transverse breaks of the cellulose fibers appeared. For example, heat treatment at a temperature of 121°C, a pressure of 1 atm., for 1.5 hours caused fiber loosening, destruction of the lignin layer and longitudinal ruptures occurred, which lead to a change in the chemical composition of straw TABLE 4.
| Sample | Mass fraction of lignin,% | Mass fraction of fiber,% |
|---|---|---|
| Original wheat straw | 21.37 ± 0.81 | 34.02 ± 0.84 |
| Wheat straw after heat treatment at t-100°С; p-1.0 atm. τ-1.5 hours | 17.9 ± 0.13 | 33.52 ± 0.78 |
| Wheat straw after heat treatment at t-120°С; p-2.0 atm. τ-15 min | 14.3 ± 0.17 | 31.14 ± 0.81 |
Table 4: Changes in the chemical composition of wheat straw during heat treatment.
Thus, when processing wheat straw at a temperature of 100°C,p=1 atm. and the incubation time is 1.5 hours, the amount of lignin and fiber in the composition of the straw decreased, which made it more accessible to the action of microorganisms and justified the expediency of using the subsequent enzymatic hydrolysis of wheat straw.
To enrich partially hydrolyzed wheat straw with microbial proteins, we used the method of deep heterophase fermentation. It is known that the fungi of the genus Trichoderma are a source of cellulolytic enzymes and their biomass contains a big number of proteins. Trichoderma harzianum 857, chosen as a producer for wheat straw bioconversion, grew well during deep heterophase fermentation and synthesized a complex of cellulolytic enzymes on the medium containing ground wheat straw, after its preliminary heat treatment at 100°C, p-1 atm. within 1.5 hours. A sporicomicellar suspension of Trichoderma harzianum 857 was used as a seed material. Temperature, pH, and time of incubation were used as the main controlled factors regulating the growth and development of Fermentation regimes the producer. with different values of the above parameters were investigated. The effectiveness of the regime was determined by the accumulation of reducing sugars in the ferment lysate, as well as by the accumulation of protein.
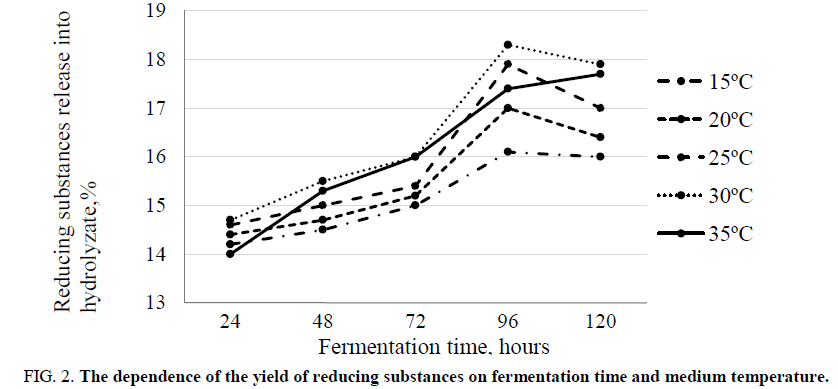
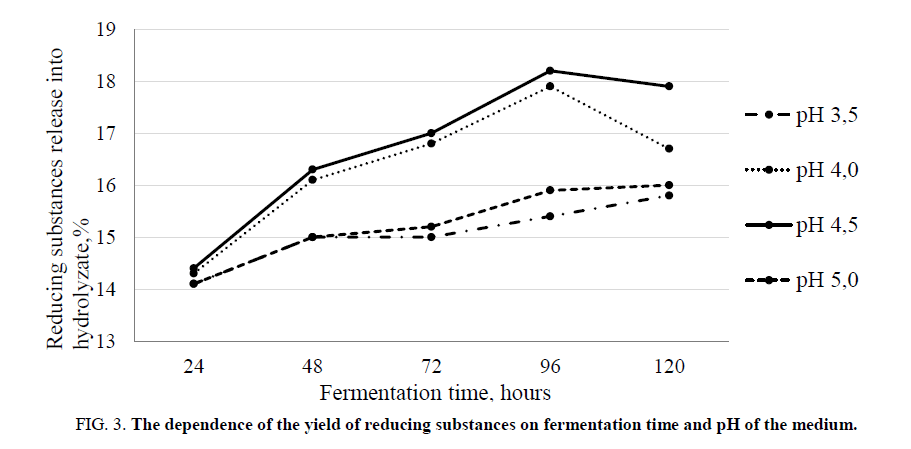
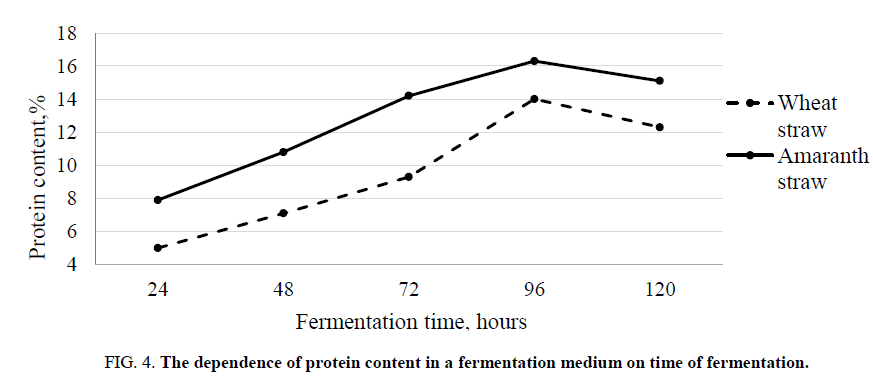
It was experimentally established that the yield of reducing substances depends on the cultivation temperature, the pH of the fermentation medium, and the time of fermentation. At a temperature of 30°C-35°C and a pH of 4.5-4.7, the maximum yield of reducing substances was observed after 96 hours of fermentation FIG. 2 and 3. By this time, the protein content in the fermentation medium reached its maximum FIG 4.
Figure 2. The dependence of the yield of reducing substances on fermentation time and medium temperature.
Figure 3. The dependence of the yield of reducing substances on fermentation time and pH of the medium.
Figure 4. The dependence of protein content in a fermentation medium on time of fermentation.
Conclusion
It was established that Trichoderma harzianum 857, due to the synthesis of extracellular cellulolytic enzymes, can be used for the hydrolysis of cellulose-containing substrates (wheat straw). The conditions for pretreatment of the cellulose-containing substrate (100°C, at 1 atm. for 1.5 hours) were selected to increase the efficiency of enzymatic cleavage. It was shown that adding amaranth green mass to the composition of cellulose-containing substrates contributes to the enrichment of the final product with protein, vitamins, and trace elements, which makes it possible to use as a high-protein product.
Acknowledgements
The author of this study is thankful to Research Institute for Fundamental Sciences, University of Tabriz, Iran for supporting of this work.
References
- Saleh AM, Nevin MF, Ebtsam NH, et al. Biochemical characterization of an extracellular polygalacturonase from Trichoderma harzanium. J Biotechnol. 2006;127(1):54-64.
- Patel N, Choy V, Malauf P, et al. Growth of Trichoderma reesei RUT C-30 in stirred tank and reciprocating plate bioreactors. Process Biochem. 2009;10(1):1164-71.
- Singh HB, Singh BN, Singh SP, et al. Solid-state cultivation of Trichoderma harzianum NBRI-1055 for modulating natural antioxidans in soybean seed matrix. Bioresour Technol. 2010;101(16):6444-453.
- Dedkov V D, Gneusheva MA, Pavlonskaya NE. Bioconversion of straw of cereals by fungi of the genus Trichoderma into feed products for animal husbandry. Vestnik Oryol GAU. 2012;4(37):102-05.
- Miettinen-Oinonen A, Paloheimo M, Lantto R, et al. Enhanced production of cellobiohydrolases in Trichoderma reesei and evaluation of the new preparations in bio finishing of cotton. J Biotechnol. 2005;116(3):305-17.
- Amezcua-Allieri MA, Durán TS, Aburto J. Study of chemical and enzymatic hydrolysis of cellulosic material to obtain fermentable sugars. J Chem. 2017;2017:1-9.
- Mandels M, Parrish FW, Reese ET. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol Res. 1962;83(2):400-08.
- Gradova NB, Babusenko YS, Gornova IB. Laboratory manual on general microbiology. 2nd Edition. М. DeLi print. (In Russian), 2004.
- GOST. Sugar methods for the determination of reducing substances Intro. Minsk: IPK Standards publisher. (In Russian), 2002.
- GOST. Enzymatic preparations. Methods for determining the enzymatic activity of cellulase. Standart inform. (In Russian), 2000.
- Lovegrove A, Edwards CH, Noni DI, et al. Role of polysaccharides in food, digestion, and health. Crit Rev Food Sci Nutr. 2017;57(2):237-53.
- Robak K, Balcerek M. Review of second-generation bioethanol production from residual biomass. Food Technol Biotechnol. 2018;56(2):174-18.
- El Gendy ANG, Tavarini S, Conte G, et al. Yield and qualitative characterisation of seeds of Amaranthus hypochondriacus L. and Amaranthus cruentus L. grown in central Italy. Ital J Agron. 2017;13(1):63-73.
- Sushiln N, Suneeta P. Amaranth-a functional food. Con Dai Vet Sci. 2018;1(3).
- Andini, R, Yoshida Sh, Ohsawa R. et al. Variation in protein content and amino acids in the leaves of grain, vegetable and weedy types of amaranths. Agronomy. 2013:3(1);391-403.
- Yang B, Dai Z, Ding SY, et al. Enzymatic hydrolysis of cellulosic biomass. Biofuels. 2011;2(4):421-49.
- Ladisch MR, Lin KW, Voloch M, et al. Process considerations in the enzymatic hydrolysis of biomass. Enzyme and Microbial Technology.1983;5(2):82-102.
- Wyman CE, Decker SR, Brady JW, et al. Hydrolysis of cellulose and hemicellulose in polysaccharides: structural diversity and functional versatility. Biorefining of Biomass to Biofuels: Opportunities and Perception. Springer, 2008