Research
, Volume: 21( 2)The Effect of Variation of Diffusion Length on the Current Parameters in Solar Cells for Safranine-NTA System
- *Correspondence:
- Regar OP
Department of Chemistry, Guru Nanak Dev Institute of Technology, New Delhi, India
Tel: 919868002702
E-mail: opmanavvihar@gmail.com
Received: December 14, 2022, Manuscript No. TSIJCS-22-83571; Editor assigned: December 16, 2022, PreQC No. TSIJCS-22-83571 (PQ); Reviewed: December 30, 2022, QC No. TSIJCS-22-83571; Revised: February 16, 2023, Manuscript No. TSIJCS-22-83571 (R); Published: February 24, 2023; DOI: 10.37532/0972-768X.2023.21(2).432
Citation: Regar OP, Jatolia SN, Singh R. The Effect of Variation of Diffusion Length on the Current Parameters in Solar Cells for Safranine-NTA System. Int J Chem Sci. 2023;21(2):432.
Abstract
In the present work, safranine has been used as a photosensitizer in solar cells as safranine-NTA systems. The short circuit current (isc) and open circuit Voltage (Voc) of the photo galvanic cells were measured with the help of a multimeter (keeping the circuit closed) and with a digital pH meter (keeping the other circuit open), respectively. The current and potential value in between these two extreme value were recorded with the help of a carbon pot (log 470 K) connected in the circuit of multimeter, through which an external load was applied.
Keywords
Solar energy; Circuit voltage; pH meter; Safranine; Safranine-NTA systems
Introduction
The photogalvanic effect was first of all reported by Rideal and Williams but the iron-thironine system was systematically investigated by Rabinowitch [1-3]. Later on, it was investigated by many workers time to time [4-14]. Many workers have used different photosensitizers in different photogalvanic systems and observed a reasonably electrical output compared to iron-thionine system. Some of the photosensitizers used in solar cells are proflavine, tolusafranine, methylene blue, niboflavin, flavin mononucleotide, poly (N-acylamidomethylthionine), toluidine blue, brilliant cresyl blue etc. [15-17]. In the present work, safranine has been used as a photosensitizer in solar cells for safranine-NTA systems. The effects of variation of the different parameters on electrical parameters of photogalvanic cell were studied in detail. For many years, people have sought to overcome the energy problems by using solar radiation to produce electricity. If that could be easily and achieved at low cost, then the electricity would become the prime source of power for motor vehicles, trains, heating, lighting, industry and fuels could be reserved for the more important aspects of conversion to necessary chemical and industrial materials.
Materials and Methods
A digital pH meter (keeping the other circuit open) was used to measure the open circuit voltage (Voc) whereas short circuit current (isc) (keeping the other circuit closed) was measured by micrometer. The electrical parameters in between those extreme values (Voc and isc) were determined with the help of a carbon pot (log 470 K) connected in the circuit of micrometer, through which an extreme load was applied. It was observed that i-V curve deviated from its regular rectangular shape. A point in i-V curve, called power point (pp) was determined where the product of current and potential was maximum and the fill factor was calculated using the formula.

Where vpp and ipp represent the value of potential and current at power point, respectively.
Results and Discussion
Conversion of efficiency of the cell
With the help of the current and the potential values at power point and the incident power of radiations, the conversion efficiency of the cell was determined as 0.0841% by using the formula:
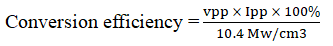
The important observations of different systems are given in Tables 1-4 which are reflecting the overall outcome of the present studies. (saf.)= 4.00 × 10-6 M, (NTA)=2.00 × 10-2 M, light intensity=10.4 mW cm-2; pH=12.6, Temp=303 K.
| Potential* (mv) | Photocurrent (µA) | Fill factor (n) |
|---|---|---|
| 665.0 | 0.0 | |
| 610.0 | 5.0 | |
| 550.0 | 10.0 | |
| 540.0 | 15.0 | |
| 390.0 | 20.0 | |
| 350.0 | 25.0 | 0.3759 |
| 65.0 | 30.0 | |
| 0.0 | 35.0 | |
| Note: *absolute values. | ||
TABLE 1. Safranine-NTA system current voltage (i-v) characteristics of the cell.
(saf.)=4.00 × 10-6, (NTA)=2.00 × 10-2 M, light intensity=10.4 mWcm-2, pH=12.6, Temp=303 K.
| Diffusion Length DL (mm) | Maximum photocurrent (Ma) | Equilibrium photocurrent (µA) | Rate of initial generation of current Ieq (µAmin-1) |
|---|---|---|---|
| 35.0 | 47.0 | 36.0 | 5.8 |
| 40.0 | 47.0 | 35.0 | 5.9 |
| 45.0 | 48.0 | 35.0 | 6.0 |
| 50.0 | 49.0 | 34.0 | 6.1 |
| 55.0 | 50.0 | 34.0 | 6.2 |
TABLE 2. Safranine-NTA system effect of diffusion length.
(saf.)=4.00 × 10-6, (NTA)=2.00 × 10-2 M, light intensity=10.4 mWcm-2, pH=12.6, Temp=303 K.
| Light intensity (mW cm-2) | |||||
|---|---|---|---|---|---|
| 3.1 | 5.2 | 10.4 | 15.6 | 26.0 | |
| Photo potential (mv) | 389.0 | 402.0 | 415.0 | 429.0 | 444.0 |
| Photocurrent (µA) | 36.0 | 35.0 | 35.0 | 36.0 | 36.0 |
| Log V | 2.5899 | 2.6042 | 2.6180 | 2.6324 | 2.6473 |
TABLE 3. Safranine-NTA system effect of light intensity.
(saf.)=4.00 × 10-6, (NTA)=2.00 × 10-2 M, light intensity=10.4 mWcm-2, pH=12.6, Temp=303 K.
| Time (min) | Power (µW) |
|---|---|
| 0.0 | 10.50 |
| 1.0 | 10.48 |
| 2.0 | 10.25 |
| 3.0 | 10.01 |
| 4.0 | 9.57 |
| 5.0 | 8.49 |
| 6.0 | 6.20 |
| 7.0 | 5.41 |
| 8.0 | 5.24 |
| 9.0 | 5.02 |
| 10.0 | 4.16 |
| 11.0 | 3.97 |
| 12.0 | 3.61 |
TABLE 4. Safranine-NTA system performance of the cell.
Conclusion
It was observed that in the systems i-V curves deviated from their expected regular rectangular shapes. The power point in i-V curves was determined and their fill factors were also calculated. The effect of variation of diffusion length (distance between the two electrodes) on the current parameter of the cell (imax, icq and initial rate of generation of photocurrent) was studied using H cells of different dimensions. It was observed that with an increase in diffusion length, imax and rate (μAmin-1) both showed an increase but the icq showed a negligibly small decreasing behaviour with the increase in diffusion length. So virtually, it may be considered as unaffected by the change in diffusion length. The results show the effect of variation of diffusion length on the current parameters of the cell. The performance of the photo galvanic cell was observed by applying an external load (necessary to have current at power point) after terminating the illumination as soon as the potential reaches a constant value. The performance was determined in terms of t1/2, i.e. the time required in fall of the output (power) to its half at power point in dark. It was observed that the cell can be used in dark for 26 minutes.
References
- Rideal EK, Williams EG. XLIII-The action of light on the ferrous ferric iodine iodide equilibrium. J Chem Soc Trans. 1925;127:258-269.
[Crossref]
- Robinowitch E. Medalists of the royal society. J Chem Phys.1940;92(2398):551-551.
[Crossref]
- Suda Y, Shimoura Y, Sakata T, et al. Photogalvanic effect in the thionine-iron system at semiconductor electrodes. J Phys Chem. 1978;82(3):268-271.
[Crossref]
- Stevenson KL, Erbelding WF. A photogalvanic cell utilizing the photodissociation of iodine in solution. Sol Energy. 1981;27(2):139-141.
[Crossref]
- Fox MA. A new carbanionic photogalvanic cell. J Phys Chem. 1979;83(13):1800-1801.
- Albery WJ, Bartlett PN, Davies JP, et al. New thiazine dyes for photogalvanic cells. Faraday Discuss Chem Soc. 1980;70:341-357.
- de Berry DW, Viehbeck A. Photoelectrochromic behavior of Prussian blue modified TiO2 electrodes. J Electrochem Soc. 1983;130(1)249-251.
[Crossref]
- Itoh K, Nakao M, Honda K. Influence of donor density of semiconductor substrate on quantum yield of dye sensitized photocurrent in the rhodamine B/SnO2 system. J Electroanal Chem Interfacial Electrochem. 1984;178(2):329-332.
[Crossref]
- Jain PK, Jajoo OP, Ameta RC. Studies in photochemical conversion of solar energy-VI: Use of brilliant cresyl blue-iminodiacetic acid system in photogalvanic cell. Z Fur Phys Chem. 1986;267(1):1230-1232.
[Crossref]
- Eisenberg M, Silverman H. Photo electrochemical cells. Electrochim Acta. 1961;5(1-2):1-12.
- Kaneko M, Yamada A. Photopotential and photocurrent induced by a tolusafranine ethylenediaminetetraacetic acid system. J Phys Chem. 1977;81(12):1213-1215.
[Crossref]
- Murthy AS, Dak HC, Reddy KS. Photogalvanic effect in riboflavin ethylenediaminetetraacetic acid system. Int J Energy Res. 1980;4(4):339-343.
- Yamase T. Production of hydrogen by a photogalvanic cell with a flavin mononucleotide-EDTA system. Photochem Photobiol. 1981;34(1):111-114.
[Crossref]
- Genwa KR, Kumar A. Dye sensitized photogalvanic solar cells: Studies in a methyl green-NaLS system in view of energy conversion. Energy Sources A: Recovery Util Environ Eff. 2012;34(14):1261-1270.
- Decker F, Cattarin S. In encyclopedia of electrochemical power sources. 1st edition, Elsevier, Amsterdam, Netherlands, 2009;1-9.
- Koli P, Dayma Y, Pareek RK, et al. Use of Congo red dye formaldehyde as a new sensitizer reductant couple for enhanced simultaneous solar energy conversion and storage by photogalvanic cells at the low and artificial sun intensity. Sci Rep. 2020;10(1):19264.
[Crossref] [Google Scholar] [PubMed]
- Chandra M. Use of solar cell for solar energy conversion in electrical. Eur J Mol Clin Med. 2021;8(3):680-686.
