Original Article
, Volume: 16( 1)The Effect of Nickel Ion on the Morphology and Adhesion Properties of Zinc Phosphate Coating
- *Correspondence:
- R Hosseini Rad, Faculty of Engineering, Tarbiat Modares University, Tehran, Iran, Tel: +254721857685; E-mail: rad.reza1371@rocketmail.com
Received: March 12, 2018; Accepted: March 24, 2018; Published: March 30, 2018
Citation: Zarei H, Rad RH. The Effect of Nickel Ion on the Morphology and Adhesion Properties of Zinc Phosphate Coating. Mat Sci Ind J. 2018;16(1):128
Abstract
In this work, nickel ion was added to a phosphate solution in various amounts (0, 3, 5, 7, 10, 15 g/L). After applying phosphate coating as primer, epoxy coating using electrostatic spray was applied on the substrate. The scanning electron microscopy (SEM) images of the surface of the samples show that the coatings formed in the phosphate bath containing 5 g/L of nickel are more uniform and have smaller porosity. While the coatings formed in the electrolyte without nickel ion have a coarse structure with larger porosities. The results of adhesion test also show that adhesion strength for samples formed in a bath containing 5 g/L is higher than others.
Keywords
Phosphate coating; Scanning electron microscopy; Corrosion products; Adhesion
Introduction
Metals are always subject to the corrosion, therefore protecting metals against corrosion is economically important. Aluminum and its alloys are widely used because of their low density and good mechanical properties for various applications such as aerospace, automobile, etc. [1]. Aluminum is destroyed due to corrosion reactions due to the destruction of oxide layer over time. Based on the reported researches there is a direct relation between corrosion resistance of coating and its adhesion strength. To prevent the destruction of the oxide layer, application of the coating is important. Many coatings, such as chromate coatings, are being replaced with newly-created environmentally friendly coatings because of environmental issues [2,3]. Conversion layers also make it possible to modify the substrate by creating a surface with better adhesion, free of contamination, and even a coating or layer that can contain inhibitor materials. The resulting oxide layer, although in a complex structure, provides a solid bond with the substrate and provides a combination of properties such as high abrasion resistance with low energy consumption [2]. Zinc phosphate conversion coating (ZPCC) is one of the most attractive methods of creating chemical conversion coating (CCC). Because of some properties such as low cost, the impact of friendly-environment and simplicity operation, it has been typically used as a primer coating on steel and aluminum alloys as a replacement for chromate conversion coatings [3]. According to the presuppositions it is expected that nickel ion in the phosphate bath have a positive impact on the properties of the formed coating. Results show that by phosphate surface treatment, adhesion strength increases.
Materials and Methods
Materials and preparation of the substrate
Aluminum samples in dimension of 1 × 50 × 60 mm3 were prepared, surface treatment began using sandpapers 800, 1500 and 2000, then samples were degreased in acetone and washed with distilled water and dried in air prior to phosphate process.
Phosphate coating solution preparation
According to Table 1, five types of phosphated solution containing five different concentrations of nickel ion (3, 5, 7, 10, 12 g/L) along with a phosphated solution without nickel ion have been prepared, aluminum samples after degreasing and acid washing were immersed in a solution with temperature of 90°C and pH of almost 3 for 30 minutes. Finally, samples were washed with distilled water, dried with wind and at the end to do more operations were put in the desiccator.
| Composition | H3PO4 (ml/L) | HNO3 (ml/L) | ZNO (g/L) | NaNO2 (g/L) | NaF (g/L) | Ni (NO3)2.6H2O (g/L) |
| 8 | 2 | 5.3 | 1 | 0.4 | 0-12 |
Table 1: Composition of phosphate bath.
Applying of organic coating
After surface modification by phosphate, in order to apply the epoxy powder coating on the aluminum substrate, a high-voltage DC Gun device (IRS Model-Iran) was used to spray electrostatic powder. The voltage of 100 kV was used and the distance between the nozzle and the substrate was 0.25 m. Using Gann, after the powders were charged, directly attached to a substrate, at the end samples were kept in oven at 190°C for 12 minutes to complete the bake. For better comparison and perception of effect of phosphate improvement, epoxy coating was applied on a non-modified aluminum substrate were also used without improved.
Scanning electron microscope
Scanning electron microscope (Philips XL-30) was used to investigate the surface morphology of the samples prepared by the phosphate bath and the cross-section micrographs of the duplex coatings.
Adhesion strength
To evaluate the epoxy coating adhesion strength and the effect of phosphate pretreatment, adhesion test based on pull-off technique according to ASTM D4541-02 was performed for three times and the mean of measurements was reported. To perform this test, pull stub with a diameter of 30 mm was prepared and stuck to the coating specimens using a suitable adhesive (3M, Scotch-weld, Epoxy Adhesive DP-460, Off-white), then tension machine (HOUNSFIELD, H50KS) was acted for picking up and peeling stub as far as the coating is peeled. Tension force at which the coating is peeled off is reported as the pull-off adhesion strength.
Results and Discussion
Figure 1 illustrates the micrographs corresponding to the surface morphology of phosphate coatings on the aluminum surface. Surface morphology and microstructure of the phosphate coatings markedly affected the adhesion properties of double-layer coating (phosphate coating and epoxy coating), through the amount and size of porosity.
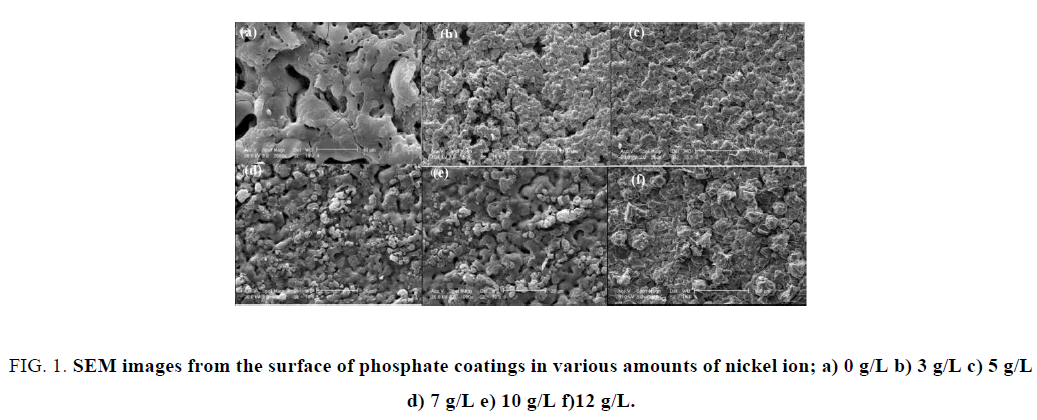
Figure 1: SEM images from the surface of phosphate coatings in various amounts of nickel ion; a) 0 g/L b) 3 g/L c) 5 g/L d) 7 g/L e) 10 g/L f)12 g/L.
According to the Figure 1, it is clear that by adding nickel ion to the phosphate bath, the amount of porosity of resulted coating decrease. Figure 1 also shows that the sample formed in nickel-free ion bath has coarse structure while for other samples that has formed in bath containing nickel ion the structure and morphology is totally different. It is clear that the densest structure is for sample formed in phosphate bath containing 5 g/L (Figure 1C), while for quantities more than 5 g/L fine structure of coating shifts toward coarse structure. These results show that 5 g/L of nickel ion can be stated as optimum amount in phosphate bath. The reason that by adding nickel ion to the phosphate bath, the morphology and surface texture of the coating change, can be noted that porosity is filled with nickel ion [4]. The SEM images show that in 5 g/L of nickel ion the large holes on the surface of sample will be filled with ions and then the finer structure will be resulted, while by adding more than 5 g/L nickel ion to the phosphate bath, in addition to the filling of present porosities on the surface of the coating, the coarse structure also will be resulted that this is very clear for the 12 g/L of nickel ion (Figure 1F).
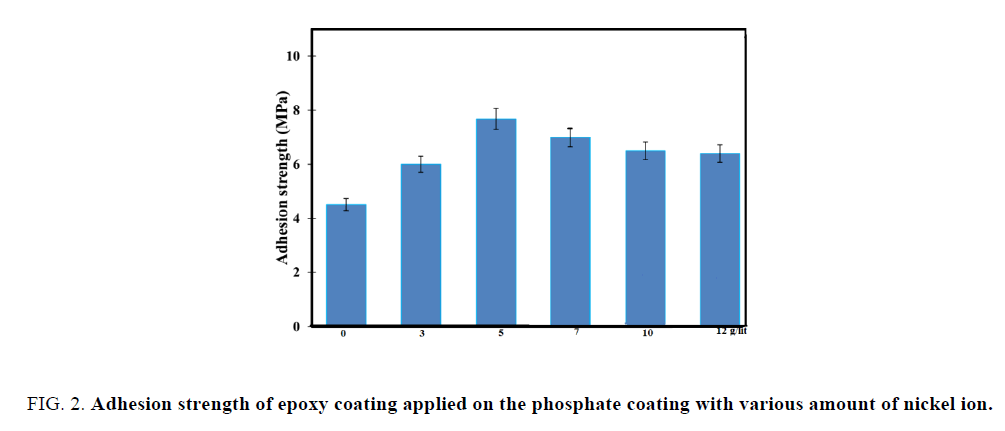
Figure 2 shows the result of adhesion test. Based on the figure it is clear that adhesion strength for epoxy coating applied on the phosphate coating containing 5 g/L nickel ion is more than others. In fact adhesion resistance is considered as a key parameter in achieving the desired performance in adhesion strength [5]. It can be stated that surface structure for 5 g/L is finer and also its porosities has distributed uniformly. In the result of mentioned structure, epoxy coating will fill all fine porosities [6]. As a result, a mechanical interlock between the coating and the substrate will be obtained and this prevents penetration of the corrosive solution into the interface of epoxy coating/substrate [7].
Figure 2: Adhesion strength of epoxy coating applied on the phosphate coating with various amount of nickel ion.
Conclusion
It was shown that applying phosphate conversion coating on the surface of aluminum substrate for preparing the surface for epoxy coating increases the properties of duplex coatings (phosphated+epoxy). This can be attributed to the sealing of porosities and defects exist in phosphate coating using epoxy coating which effectively prevents the reaching electrolyte to the epoxy coating/substrate interface. Also it is seen that adding various amounts of nickel ion to the phosphating bath changes the morphology of obtained phosphated coating, which leads to a change in the adhesion properties of epoxy coating.
References
- George FO, Skeldon P, Thompson GE. Formation of zirconium-based conversion coatings on aluminium and Al–Cu alloys. Corros Sci. 2012;65:231-7.
- Golru SS, Attar MM, Ramezanzadeh B. Effects of surface treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the epoxy coating. Appl Surf Sci. 2015;345:360-8.
- Sankara Narayanan TS. Surface pretreatment by phosphate conversion coatings: A review. Rev Adv Mater Sci. 2005;9:130-77.
- Nair UB, Subbaiyan M. Characterization of zinc phosphate coatings obtained from modified baths. J Mater Sci. 1995;30(8):2108-14.
- Rezaee N, Attar MM, Ramezanzadeh B. Studying corrosion performance, microstructure and adhesion properties of a room temperature zinc phosphate conversion coating containing Mn2+ on mild steel. Surf Coat Technol. 2013;236:361-7.
- Ramezanzadeh B, Attar MM. Evaluation of the effects of surface treatments on the cathodic delamination and anticorrosion performance of an epoxy-nanocomposite on steel substrate. J Coat Technol Res. 2013;10(1):47-55.
- Harun MK, Marsh J, Lyon SB. The effect of surface modification on the cathodic disbondment rate of epoxy and alkyd coatings. Prog Org Coat. 2005;54(4):317-21.