Review
, Volume: 14( 3)The Diels Alder Reaction-FMO and Stepwise Radical Ion Cycloaddition Pathways
- *Correspondence:
- Zargar ND , Department of Chemistry University of Kashmir, Srinagar (190006), Jammu and Kashmir, India, Tel: 91-9906518251189; E-Mail: nded.1092@rediffmail.com
Received: October 11, 2018; Accepted: October 26, 2018; Published: November 05, 2018
Citation: Zargar ND, Khan KZ. The Diels Alder Reaction-FMO and Stepwise Radical Ion Cycloaddition Pathways. Org Chem Ind J.2018;14(3):131
Abstract
Carbon (4+2) cycloaddition diels alder reactions have been a subject of research. Varied dienes and dienophiles have been employed to carry out these normal cycloadditions based on the symmetries of ground state orbitals.1,3-indandione also offers an interesting example of diels alder reaction. Imine (4+2) cycloaddition reactions have been investigated where the imine or imminium ions function as dienophiles and provide a powerful tool for the construction of six membered nitrogen heterocycles.
Stepwise radical cation diels alder reactions of aryl vinyl ethers and allied substrates have been modafinil bestsellers online carried out by electrocatalysis triggered by an oxidative single electron transfer (SET) process and proceed via unique multiple pathways.
Keywords
Cycloadditio; Diels alder reaction; 1,3-indandione; Imine; Radical cation; Dienophile; Stepwise; Nitrogen heterocycles
Introduction
A normal (4+2) cycloaddition Diels Alder reaction involves an overlap of in-phase orbitals with HOMO-LUMO combination between a conjugated diene and an alkene. Though a number of dienophiles have been used in Diels Alder reaction, of late, imines have also been employed as dienophiles in a hetero-Diels Alder reaction which proceeds via (4+2) cycloaddition pathway under thermal or acid catalyzed reaction conditions. Vinyl ketones upon (4+2) cycloaddition with imines afford dihydropyridones with excellent yields [1]. In a similar reaction, vinyl allenes have also been found to react in presence of Lewis acid resulting in high diastereoselective products [2].
In more advanced research work, stepwise radical cation Diels Alder reaction via multiple pathways has been carried out by electrocatalysis triggered by an oxidative SET process. The recent examples of radical cation Diels Alder reaction have employed styrenes but the scope is not limited only to such electron rich dienophiles. Bauld and Yoon observed that aryl vinyl ethers,enol-ether equivalents, and aryl vinyl sulfides also work as promising dienophiles for the reactions [3-9]. In this article, we have discussed the various mechanistic pathways of normal pericyclic (4+2) cycloaddition vis-à-vis stepwise radical cation Diels Alder reaction via multiple steps.
General Discussion and Mechanistic Pathways
Diels Alder reaction is a cycloaddition reaction in which two unsaturated molecules (diene and dienophile) add together and form a six-membered ring [10]. The diene acts as an electron-rich nucleophile and the dienophile as the electron poor electrophile.
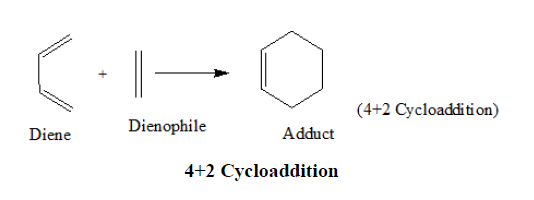
The orbital in ethylene that receives these electrons is the Lowest Unoccupied Molecular Orbital (LUMO). The electrons in the HOMO of butadiene flow smoothly into the LUMO of ethylene resulting in a concerted reaction. Radical and ionic intermediates are not involved here.
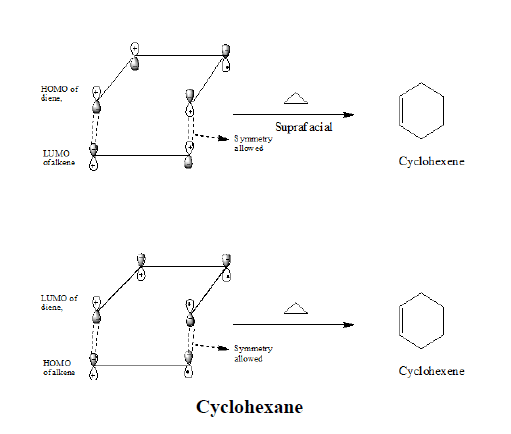
Frontier orbital analysis of a (4+2) cycloaddition reaction shows that overlap of in-phase orbitals to form the two new sigma bonds requires suprafacial orbital overlap. Both the combinations are possible that is if we use HOMO of the alkene and LUMO of the diene or LUMO of the alkene and HOMO of the diene.
The symmetries of the two ground state orbitals are such that bonding of the terminal lobes can occur with suprafacial geometry.
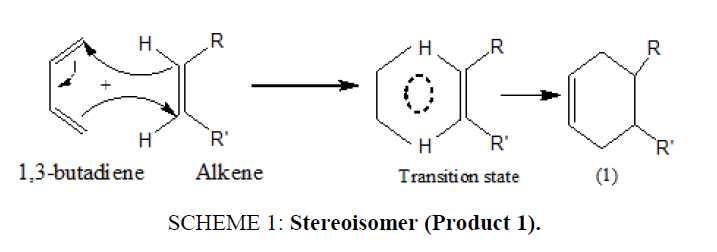
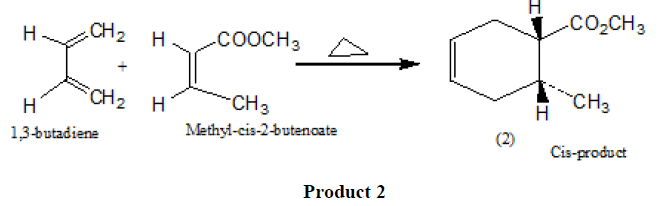
In this approach of cycloaddition, the Diels Alder reaction is stereospecific and carried out under thermal control. The stereochemistry of the starting dienophiles is maintained during the reaction and a single product stereoisomer (1) results (SCHEME 1).
SCHEME 1: Stereoisomer (Product 1).
There are simultaneous breaking and making of bonds through a six-centered transition state with no intermediate.
Similarly, if we carry out the cycloaddition with a cis dienophile such as methyl-cis-2-butenoate, only the cis-substituted cyclohexene product (2) is formed.
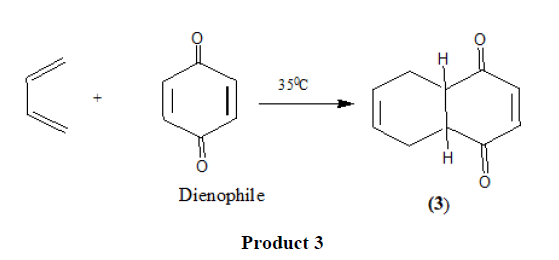
Thousands of examples of Diels Alder reaction are known that proceed by a pericyclic process.
Electron withdrawing substituents in the dienophiles such as >C=O,-CHO,-COOR,-CN [11],-NO2 [12], etc. promote the reaction.
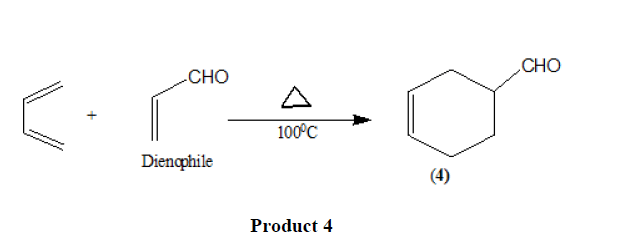
The reaction is also accelerated by the presence of electron releasing groups in the dienes.
Triple bond compounds, allenes, and benzyne may also function as dienophiles [13].
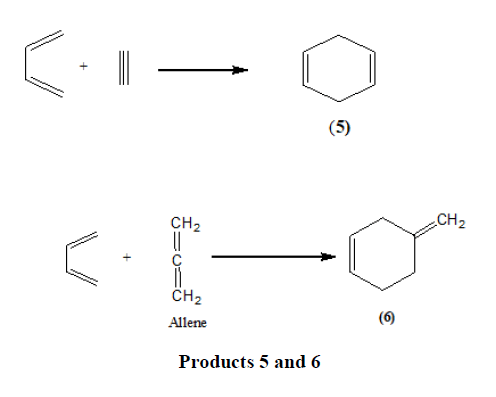
It is also possible for a diene to have one double bond in an aromatic system and the other outside it. Viz.
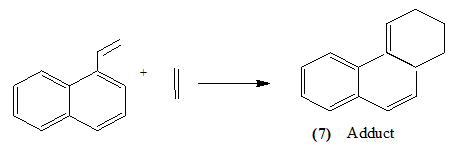
Styrene has also been shown to react in this manner [14].
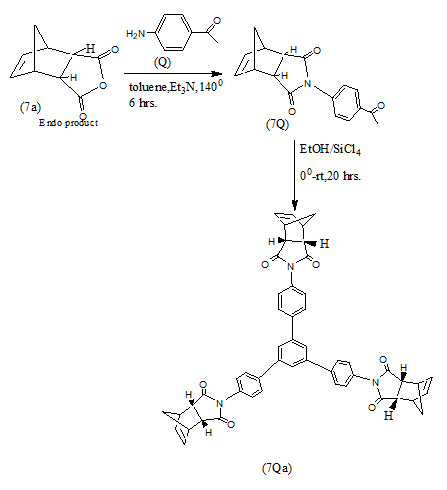
Endo products (7a and7b) result in high yields from Diels Alder Reaction when cyclopentadiene and anthracene react with maleic anhydride under varying conditions [15-18].
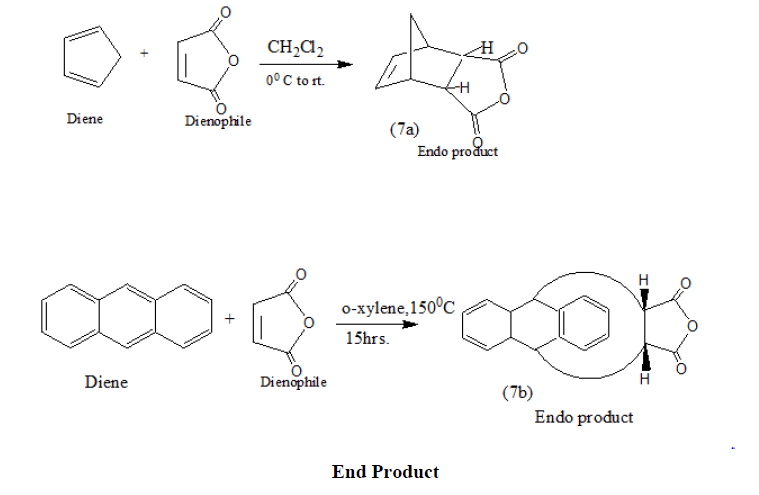
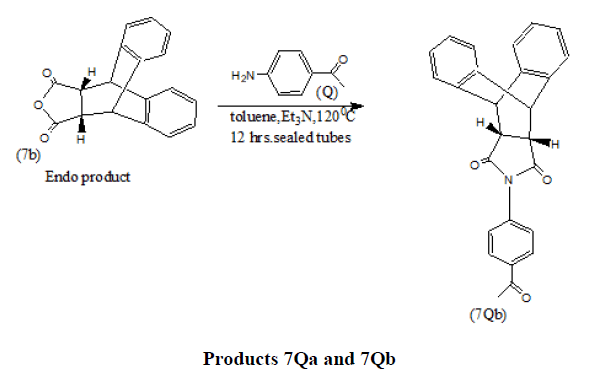
In a recent work N-heterocycles of the type (7Qa and 7Qb) have been synthesized from Diels Alder adducts (7a and 7b) which in turn have been obtained via (4+2) cycloaddition Diels Alder reactions using appropriate dienes and dienophiles [19].
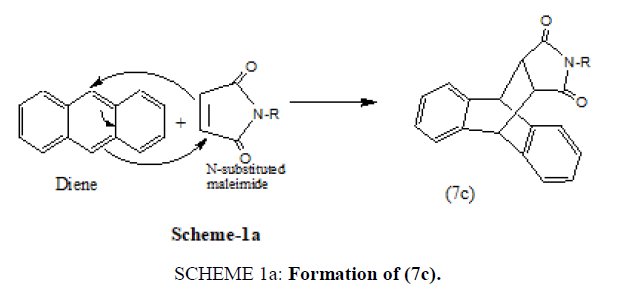
Anthracene also reacts with N-substituted maleimides to yield the Diels Alder Adduct (7c) [20].
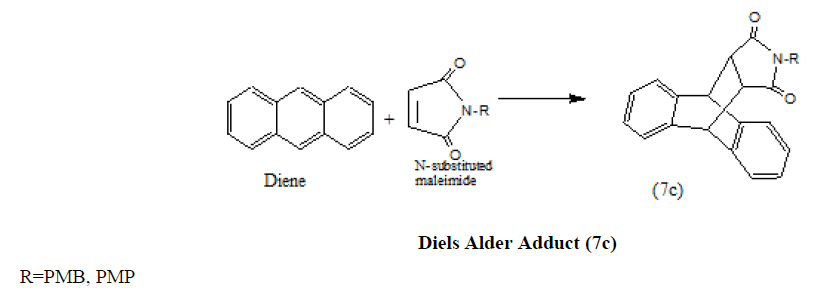
Mechanistically the formation of (7c) can be depicted as below (SCHEME 1a).
SCHEME 1a: Formation of (7c).
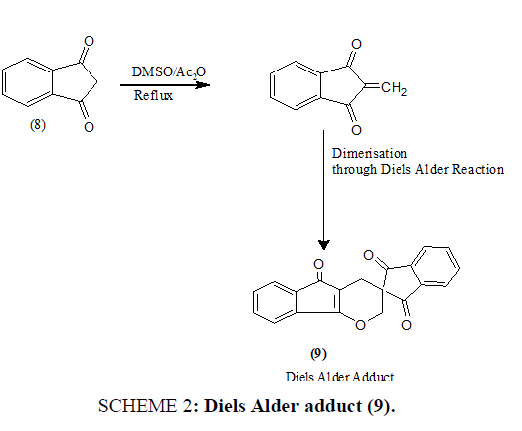
1,3-indandione (8), an important member of the class of 1,3-diketo compounds has been found to yield a series of compounds of biological and chemical interest with different substrates and reagents. When interacted with DMSO/Ac2O reagent at ~1500°C, it affords a Diels Alder adduct (9) SCHEME 2.
SCHEME 2: Diels Alder adduct (9).
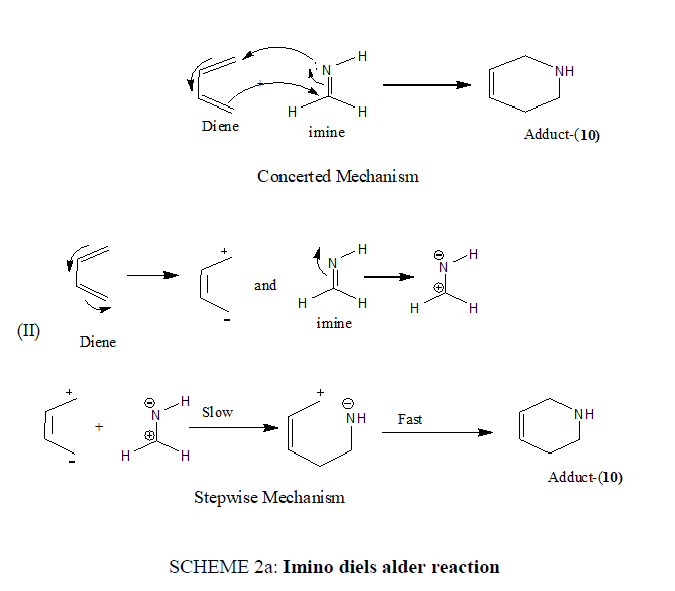
In an interesting hetero-Diels Alder reaction imines have been employed as dienophiles which involve LUMO of the imine. The reaction may be thermal or acid-catalyzed and proceed via a [4+2] cycloaddition mechanism.
The imino Diels Alder reaction may occur either by a concerted mechanism or stepwise process (SCHEME 2a).
SCHEME 2a: Imino diels alder reaction
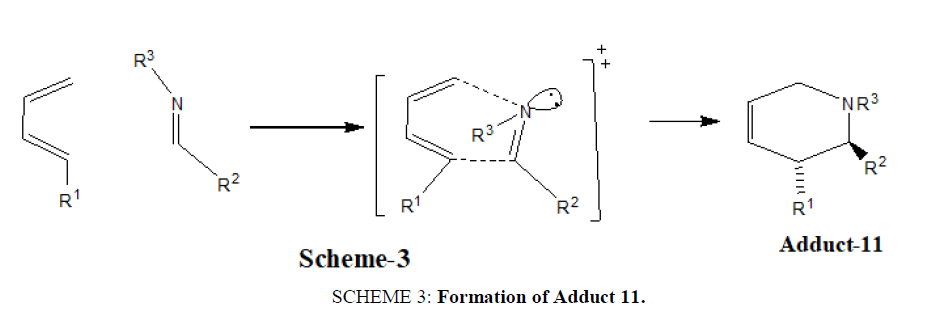
For the concerted process, the lowest energy transition state places the lone pair in an exo-position. Thus (E) imines in which the lone pair and the larger imine carbon substituents are cis, tend to give exo-products [21] (SCHEME 3).
SCHEME 3: Formation of Adduct 11.
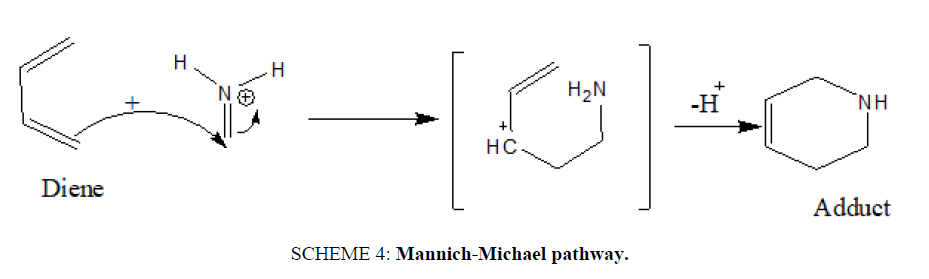
If the imine nitrogen is protonated or co-ordinated to a strong Lewis acid, the mechanism shifts to a stepwise, Mannich-Michael pathway [22] (SCHEME 4).
SCHEME 4: Mannich-Michael pathway.
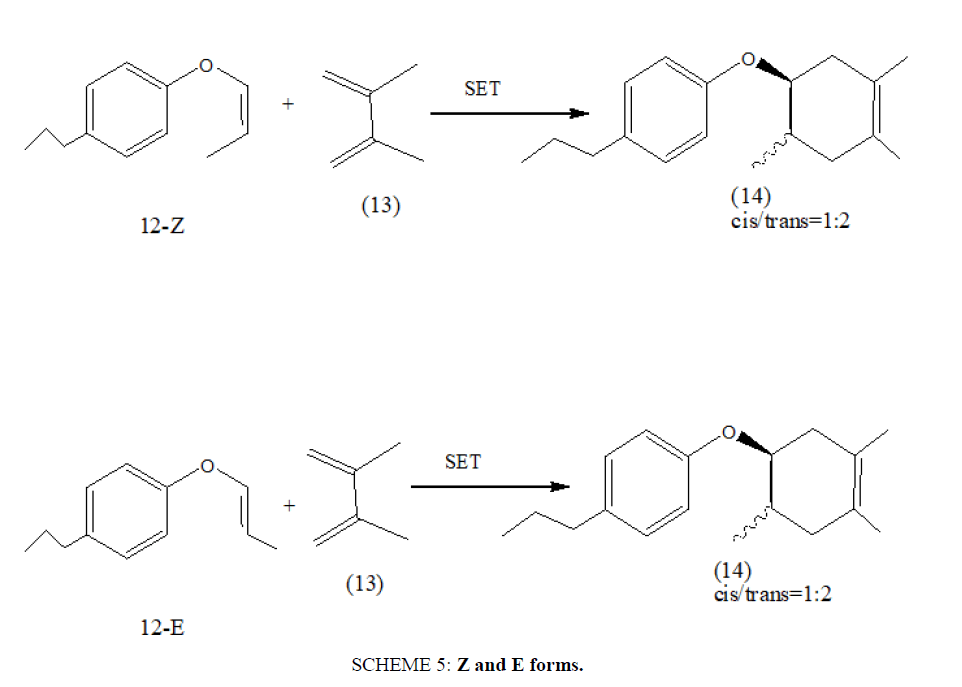
In a more recent work, radical cation Diels Alder reaction has been employed between aryl vinyl ether (12) and 2,3-dimethyl- 1,3-butadiene (13) under Single Electron Transfer (SET) process to afford the Diels Alder adduct (14) with excellent yield. The adduct (14) has been found approximately cis/trans=1:2 mixture so, the reaction must proceed in a stepwise manner. Both Z and E forms afforded the same synthetic products [22] (SCHEME 5).
SCHEME 5: Z and E forms.
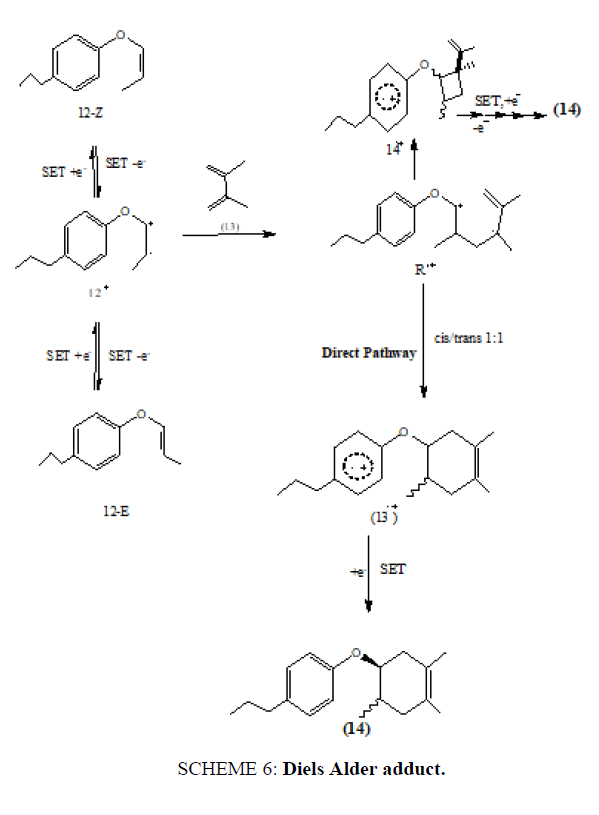
The plausible mechanism proposed for the reaction can be rationalized on the basis that when aryl vinyl ether (12,E or Z) was subjected to oxidative SET conditions, it afforded a radical cation (12+) which then gets trapped by 2,3-dimethyl-1,3- butadiene (13) to get the acrylic radical cation intermediate (R.+) . This acyclic radical cation gets converted into the aromatic radical cation with a six-membered ring (13+) or a four-membered ring ( R.+ ) via the rapid intramolecular SET process. This proposed mechanism suggests that the six- membered ring closure of the acyclic radical cation intermediate ( ) is a stepwise process leading to the Diels Alder adduct (14) (SCHEME 6) (TABLE 1).
SCHEME 6: Diels Alder adduct.
| Name of Reaction | Mechanism | Type of addition | Conditions |
|---|---|---|---|
| Normal Diels Alder Reaction | Concerted mechanism | 4+2 cycloaddition | Proceeds under thermal conditions |
| Imine Diels Alder Reactions | The concerted and Stepwise mechanism | 4+2 cycloaddition | Thermal and acid- catalyzed |
| Radical cation Diels Alder Reaction | Multiple mechanisms | 4+2 cycloaddition | Single electron transfer (SET) process |
Table 1: Diels alder reaction under different reaction conditions
Synthetic Applications
Normal Diels Alder reaction, being stereospecific in nature, has found broad synthetic applications It has been used to synthesize many natural products and to determine the configuration of the reactants by studying the adduct. Imine Diels Alder reaction has been found as a powerful tool for the synthesis of a number of natural products, often in a region, diastereo and enantioselective manner. Some important examples of Diels Alder reaction are given for illustration.
1. In the synthesis of camphene
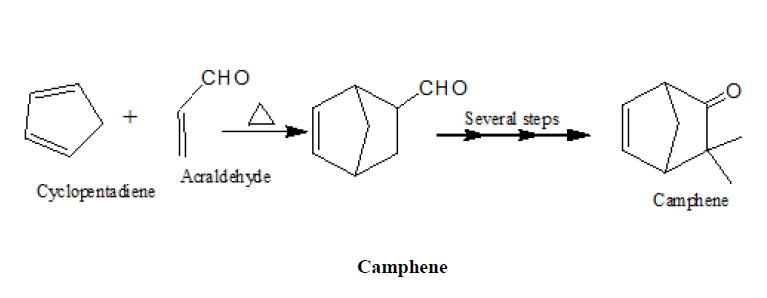
2. Synthesis of α-terpineol
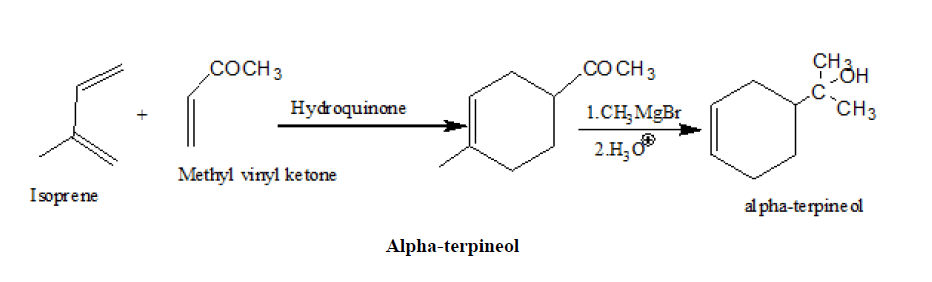
3. Johnson, s synthesis of Oestrone
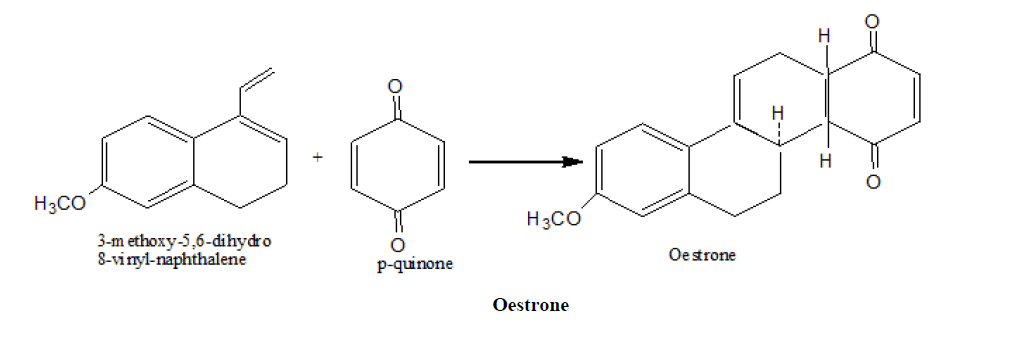
4. Imine Diels Alder reaction has been used for the synthesis of a number of alkaloid natural products
Danishefskys diene is used for the construction of a six-membered ring en-route to Phyllanthine [22].
Conclusion
In conclusion, our findings and literature survey reveals that Diels Alder reaction does not follow only the concerted mechanism but stepwise mechanistic pathways are also effective. Radical ion Diels Alder reactions are highly stereoselective including stereospecific examples and follow oxidative SET triggered cycloadditions. Besides usual dienophiles, imines have also been employed as dienophiles in hetero-Diels Alder reactions.
Acknowledgment
I am highly thankful to my supervisor Prof. KZ Khan Department of Chemistry, the University of Kashmir for encouragement and fruitful suggestions in writing this article. I also acknowledge to Allama Iqbal Library University of Kashmir Srinagar India for providing all the literature of this article.
References
- Bennett DM, Okamoto I, Danheiser RL. Hetero [4 + 2] Cycloadditions of (Trialkylsilyl) vinylketenes. Synthesis of a,ß-Unsaturated d-Valerolactones and Lactams. Org Lett. 1999;1:641.
- Regás D, Afonso MM, Rodríguez ML, et al. Synthesis of octahydroquinolines through the Lewis acid catalyzed the reaction of vinyl allenes and imines. J Org Chem. 2003;68:7845-52.
- Lin S, Ischay MA, Fry CG, et al. Radical cation Diels-Alder cycloadditions by visible light photocatalysis. J Am Chem Soc. 2011;133:19350-3.
- Reynolds DW, Harirchian B, Chiou HS, et al. The cation radical vinylcyclobutane rearrangement. J Phy Org Chem. 1989;2:57-88.
- Pabon RA, Bellville DJ, Bauld NL. Selective cyclobutane adduct formation in competition with Diels-Alder addition in cation radical cycloadditions. J Am Chem Soc. 1984;106:2730-1.
- Harirchian B, Bauld NL. Cation radical Diels-Alder cycloadditions in organic synthesis. A formal total synthesis of (-)-beta.-selinene. J Am Chem Soc. 1989;111:1826-8.
- Aplin JT, Bauld NL. Aryl vinyl sulfides as probes for electrophilic versuselectrontransfer mechanisms. J Chem Soc. PerkinTrans-2. 1997;5:853-5.
- Harirchian B, Bauld NL. Tetrahedron Lett. 199;28:927-30.
- Lin S, Lies SD, Gravatt CS, et al. Radical cation cycloadditions using cleavable redox auxiliaries. Org Lett. 2017;19:368-71.
- Brieger G, Bennett JN. The intramolecular Diels-Alder reaction. Chem Rev. 1980;80:63-97.
- Butskus. Cyclisation reactions based on acrylonitrile. Russ Chem Rev. 1962;31:283-4.
- Novikov SS, Shvekhgeimer GA, Dudinskaya AA. Diene syntheses with nitro compounds. Russ Cheml Rev. 1960;29:79-94.
- Bastide and Henri-Rousseau, Willey, New York. 1978;67:447-552.
- Ciganek E. Negatively substituted acetylenes. II. Cycloaddition reactions with styrenes. J Org Chem. 1969;34:1923- 30.
- Kiriazis A, af Gennäs GB, Talman V, et al. Stereoselective synthesis of (3-aminodecahydro-1, 4- methanonaphthalen-2-yl) methanols targeted to the C1 domain of protein kinase C. Tetrahedron. 2011;67:8665-70.
- Kotha S, Todeti S, Aswar VR. Design and synthesis of C3-symmetric molecules bearing propellane moieties via cyclotrimerization and a ring-closing metathesis sequence. Beilstein J Org Chem. 2018;14:2537-44.
- Marsh BJ, Adams H, Barker MD, et al. Enantioselective catalytic desymmetrization of maleimides by temporary removal of an internal mirror plane and stereoablative over-reduction: synthesis of (R)-pyrrolam A. Org lett. 2014;6:3780-3.
- Obermayer D, Znidar D, Glotz G, et al. Design and performance validation of a conductively heated sealed-vessel reactor for organic synthesis. J Org Chem. 2016;81:11788-801.
- Whiting A, Windsor CM. What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reaction? Tetrahedron. 1998;54:6035-50.
- Waldmann,H. Asymmetric hetero diels-alder reactions. Synthesis. 1994;6:535-51.
- Shimizu R, Okada Y, Chiba K. Stepwise radical cation Diels-Alder reaction via multiple pathways. Beilstein J Org Chem. 2018;14:704-8.
- Han G, LaPorte MG, Folmer JJ, et al. Total synthesis of the Securinega alkaloids (+)-14, 15-dihydronorsecurinine, (-)-norsecurinine, and phyllanthine. J Org Chem. 2000;65:6293-306.