Original Article
, Volume: 13( 1)The Adsorption Performance Research for Several Commercial Materials: The CaSO4 Scaling Removal
- *Correspondence:
- Chengtun Qu, Department of Chemical Engineering, College of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an 710065, P. R. China, Tel: +86 (29) 8838 2114, E-mail: xianquct@163.com
Received: March 30, 2018; Accepted: March 31, 2018; Published: April 15, 2018
Citation: Wang Y, Chengtun Qu, Wang F, et al. The Adsorption Performance Research for Several Commercial Materials: The CaSO4 Scaling Removal. Chem Technol Ind J. 2018;13(1):123.
Abstract
The scaling of oilfield sewage will cause serious damage to production equipment, pipelines and strata. According to heterogeneous nucleation theory, the adsorption material added into the sewage will induce the scaling to grow on its surface, to achieve the purpose of scaling removal. In this paper, the adsorption property of four kinds of filling materials, such as cellucotton, sponge, gauze and retinervus luffae fructus, were detailed studied. It was found that the adsorption effect of cellucotton was the best and its adsorption rate was up to 80%. Moreover, a series of influence factors of cellucotton for the scaling adsorption were also analyzed. The results showed that the adsorption capacity of cellucotton was the largest at experimental temperature of 30°C. When the contact time is 3 days, the u adsorption rate and adsorption capacity of cellucotton are larger. Therefore, the commercial cellucotton can be used as an adsorption material to remove the scaling in the water
Keywords
Scaling; Adsorption; Commercial materials; Static analysis; Simulated water
Introduction
The scaling problem of oil-gas gathering and transportation system is a common problem in various oilfields; the formation of scaling has unfavorable effects on pipelines, equipment and stratum [1-3]. Scaling may lead to pipeline blockage, corrosion of equipment, lower oil and gas production, water injection pressure and energy consumption increased, which may even cause oil well shutdown and bring serious harm to the oilfield [4-6].
Scaling refers to some salt ions with low solubility in water, along with physical or chemical conditions changed, these ions in solution reached a saturated state and then combine to form insoluble compounds and deposited into the solution [7,8]. For slightly soluble salts, primary nucleation is the main nucleation mechanism and it includes homogeneous nucleation and heterogeneous nucleation. Heterogeneous nucleation results in the reduction of nucleation potential energy, which is attributed in the presence of impurity particles in the solution and then it will accelerate the formation of scaling nucleation [9,10]. When the heterogeneous nucleation occurs in the solution, the supersaturation of the solution is relatively lower and the particle size is relatively larger. Meanwhile, the single scaling has a certain crystal structure and can be adsorbed on the uneven rough surface after forming. The crystal core grows continuously and finally the hard and dense scale is formed [11- 13].
Based on the above theory, we put some solid filling material with pore structure into the water, as a carrier, thereby inducing water saturated ion on the surface of the material to form the scaling. Finally, the adsorption effects of different filling materials for the scaling removal was investigated in detail.
Experimental
Materials
Chemicals were mostly purchased from Shanghai Chemicals Inc, including calcium chloride anhydrous (CaCl2), anhydrous sodium sulfate (Na2SO4), cellucotton, sponge, gauze and retinervus luffae fructus. In our experiments, a variety of chemicals used were of analytical purity and no further purification.
Experimental process
For preparing the simulated water, the experimental procedure was as follows: Firstly, an appropriate amount of CaCl2 and Na2SO4 were dissolved in 100 mL deionized water, respectively and stirred at ambient conditions for 30 min to obtain a stable solution A and B with the same concentration. After that the two solutions mentioned above were mixed (according to 1:1 volume ratio) to form a simulated water with an initial concentration of 0.005 mol/L, 0.01 mol/L, 0.03 mol/L, 0.05 mol/L, 0.1 mol/L, respectively, transferred into a conical flask and kept at 30°C for 12 h. Then, 1 gm of cellucotton was immerged in the simulated water solution above with the different concentration of BaSO4, sealed with preservative film and transferred to the oven. After a certain amount of time, the cellucotton is taken out and weighed and the weight gained was calculated. In addition, the other experimental materials were analyzed under the same condition.
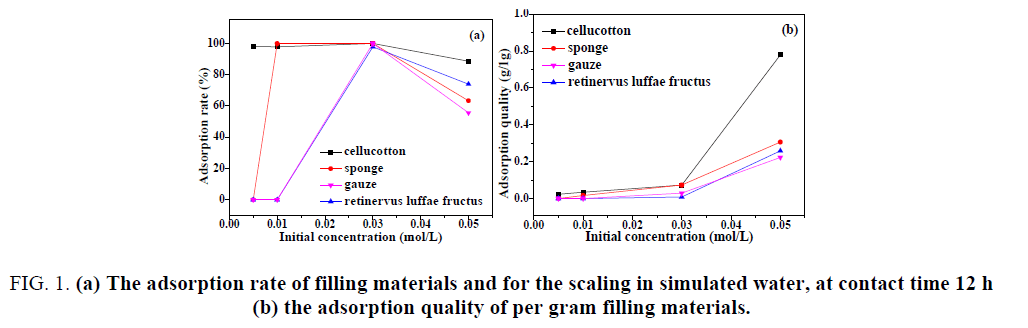
It can be seen from FIG. 1(a) that with the concentration of simulated water increased, the adsorption rate of four kinds of filling materials for the scaling first reached the maximum and then gradually decreased. When the initial concentration of the scaling in simulated water was 0.005 mol/L and 0.01 mol/L, the adsorption rate of retinervus luffae fructus and gauze was 0.
FIG. 1: (a) The adsorption rate of filling materials and for the scaling in simulated water, at contact time 12 h (b) the adsorption quality of per gram filling materials.
However, when the initial concentration of the scaling in simulated water was 0.03 mol/L, the adsorption rate of filling materials reached the maximum, the adsorption rate of cellucotton, sponge and gauze was up to 100%, as well as retinervus luffae fructus was 97.8%. When the initial concentration of the scaling in simulated water was 0.05 mol/L, the adsorption rate of cellucotton was the biggest, followed by retinervus luffae fructus, gauze was minimal.
When the experimental temperature was 40°C and contact time was 12 h, as shown in FIG. 1(b), the quality of scaling adsorbed on per gram of filling material increased along with the initial concentration of simulated water increased. When the initial concentration of the scaling in simulated water was less than 0.03 mol/L, the adsorption quality of per gram adsorption material for the scaling was relatively small. This may be attributed to the small amount of scaling itself, when the initial concentration of the scaling in simulated water was lower as well as the adsorption material did not reach saturated adsorption. When the initial concentration of the scaling in simulated water was 0.05 mol/L, the adsorption capacity of cellucotton was much higher than that of other materials. The adsorption quality of per gram cellucotton and sponge was 0.77894 g and 0.30606 g, respectively. However, the adsorption quality of per gram retinervus luffae fructus and gauze was about 0.2 g.
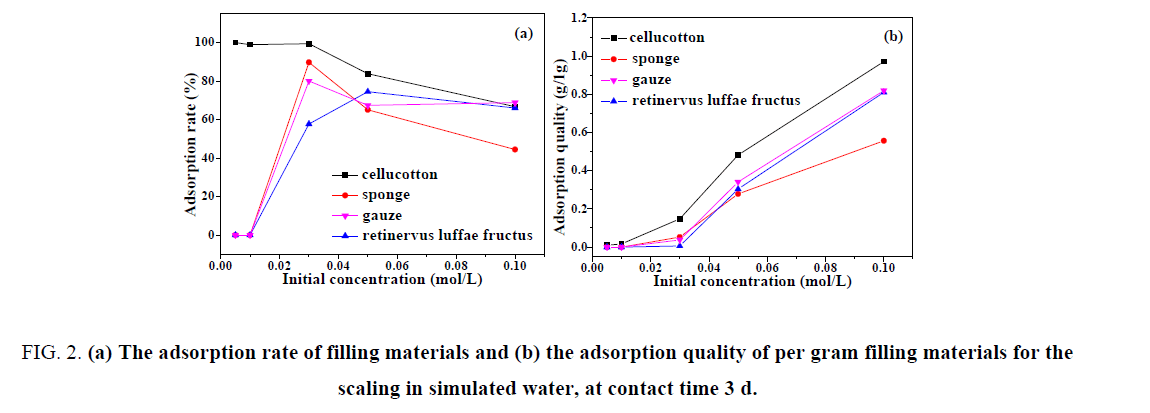
When the experimental temperature was 40°C and the contact time was 72 h, it can be seen form FIG. 2(a) that with the increase of the initial concentration in simulated water, the adsorption rate of the filling material for the scaling gradually decreased. When the initial concentration of the scaling in simulated water was 0.005 mol/L, the adsorption rate of cellucotton was 100% and that of the other materials was 0. This may be because the initial concentration of the scaling in simulated water was small, resulting in no formation of scaling in water. When the initial concentration of the scaling in simulated water was 0.01 mol/L, the adsorption rate of cellucotton was up to 98.9% and that of the other materials was also 0. When the initial concentration of the scaling in simulated water was 0.03 mol/L, the adsorption rate of cellucotton, sponge and gauze reached above 80% and that of retinervus luffae fructus was 57.7%. When the initial concentration of the scaling in simulated water was 0.05 mol/L, the adsorption rate of sponge was 83.8% and that of retinervus luffae fructus reached the highest value of 74.5%. When the initial concentration of the scaling in simulated water was 0.1 mol/L, the adsorption rate of sponge for scaling decreased to less than 50% and that of other materials remained at about 70%.
FIG. 2: (a) The adsorption rate of filling materials and (b) the adsorption quality of per gram filling materials for the scaling in simulated water, at contact time 3 d.
As displayed in FIG. 2(b), the quality of scaling adsorbed on per gram of filling material increases along with the initial concentration of simulated water increased. The adsorption capacity of cellucotton for scaling uniformly increased with the increase of initial concentration of the scaling in simulated water. When the initial concentration of the scaling in the simulated water was 0.1 mol/L, the adsorption quality of per gram cellucotton to scaling reached to 0.972 g higher than that of other filling materials. When the initial concentration of the scaling in the simulated water was less than 0.03 mol/L, the adsorption quality of per gram sponge, gauze and retinervus luffae fructus for the scaling slowly increased. When the initial concentration of the scaling in simulated water was more than 0.03 mol/L, the adsorption quality of per gram these materials quickly increased. When the initial concentration of the scaling in simulated water was more than 0.1 mol/L, the adsorption quality of per gram gauze and retinervus luffae fructus was about 0.81 g.
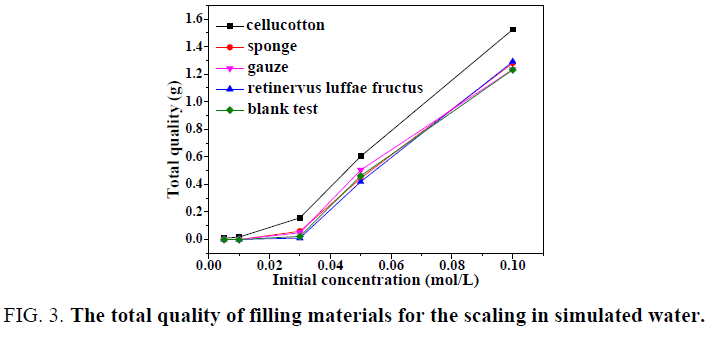
It can be seen from FIG. 3. that in the different initial concentration of the scaling, the total adsorption quality of cellucotton for scaling was relatively larger. The total adsorption quality of sponge, gauze, retinervus luffae fructus and no filling materials for scaling was almost the same. From the above discussion, the cellucotton can promote the formation of scaling in simulated water.
On the one hand, take a certain amount of filling material into a conical flask, added 100 mL distilled water and then kept at 40°C for 72 h. On the other hand, a certain amount of solution was used for titration from conical flask above and the concentration of Ca2+ in water was calculated. As can be seen from TABLE 1, the calcium release rate of four filling materials was relatively small and negligible.
The adsorption effect of cellucotton for scaling
To sum up, the adsorption capacity of cellucotton for the scaling was the biggest and the calcium release rate was not high, so the cellucotton, as a filling material, was used to study the influence of other experimental conditions on adsorption effect.
Different experimental temperature
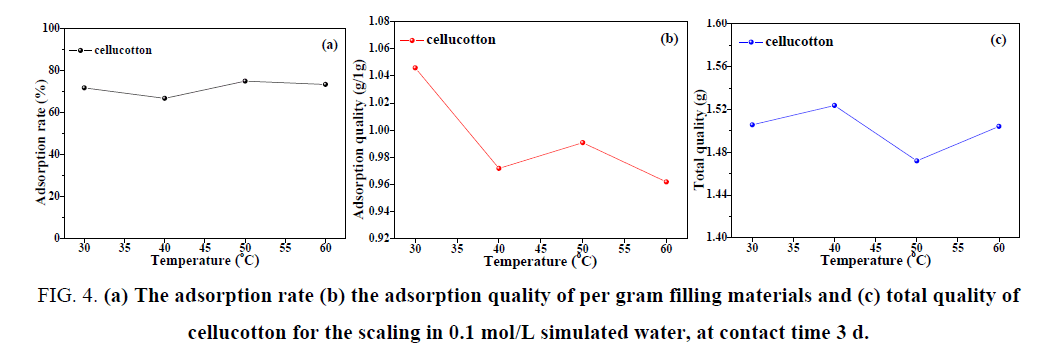
It can be seen from FIG. 4(a,b) that when the initial concentration of the scaling in simulated water was 0.1 mol/L and the contact time was 72 h, there was no obvious difference in the adsorption rate of cellucotton for the scaling at different experimental temperatures and was about 70%. It was worth noting that the adsorption quality of cellucotton for the scaling was the highest at experimental temperature of 30°C. As shown in FIG. 4(c), the total adsorption quality of cellucotton for the scaling fluctuate at different temperature, but it reached the highest place in the experimental temperature of 40°C.
FIG. 4: (a) The adsorption rate (b) the adsorption quality of per gram filling materials and (c) total quality of cellucotton for the scaling in 0.1 mol/L simulated water, at contact time 3 d.
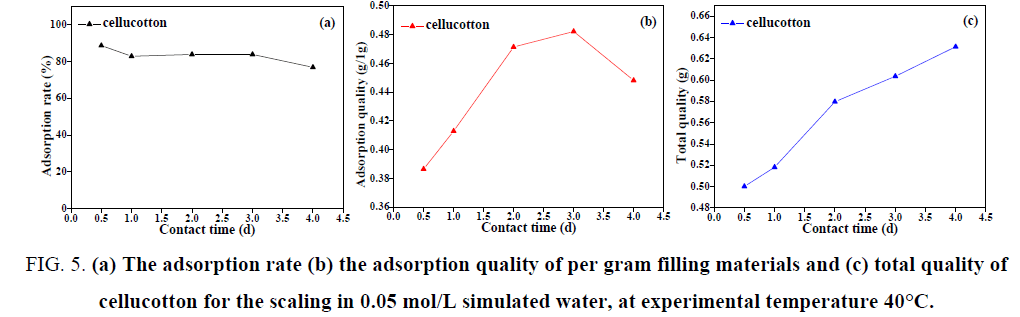
Different contact time
As illustrated in FIG. 5(a,b), it can be seen that when the initial concentration of the scaling in simulated water was 0.05 mol/L, the experimental temperature was 40°C and the contact time was 12 h, the adsorption rate of cellucotton for the scaling was the highest. However, the adsorption quality of per gram cellucotton for the scaling was lower.
FIG. 5: (a) The adsorption rate (b) the adsorption quality of per gram filling materials and (c) total quality of cellucotton for the scaling in 0.05 mol/L simulated water, at experimental temperature 40°C.
It can be seen from FIG. 5(c) that the total adsorption quality of scaling in simulated water was small at 12 h and most of the scaling generated in simulated water was adsorbed by cellucotton, so the adsorption rate of cellucotton was higher. The total adsorption quality of scaling in simulated water was large at the contact time of 48 h, the adsorption quality of per gram of cellucotton for the scaling was higher and the adsorption rate was up to 85%. When the contact time was 72 h, the scaling generated in simulated water continued to increase and the adsorption rate of cellucotton was kept at about 85%. The scaling generated in simulated water was still increased, but the adsorption quality of 1 g cellucotton decreased. This phenomenon was caused by the formation of scaling in process of forming, dissolving and reforming and the process of scaling on the surface of cellucotton was adsorption, shedding, resorption [14].
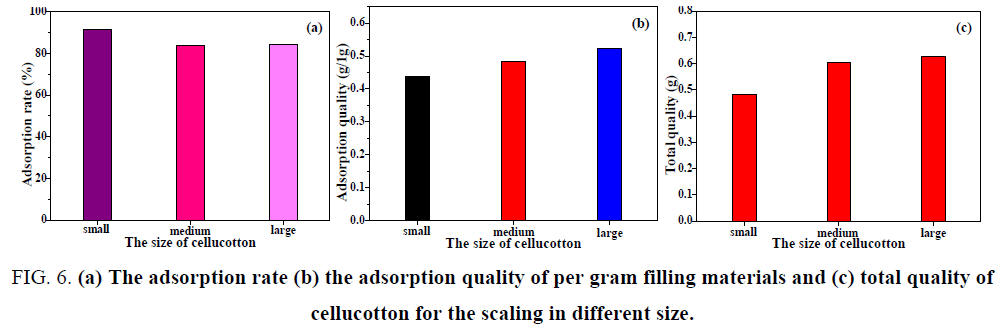
Different size
It can be seen from FIG. 6 . that when the initial concentration of the scaling in simulated water was 0.05 mol/L, the experimental temperature was 40°C and the contact time was 72 h, the adsorption rate of small cellucotton for the scaling was the highest and the total adsorption quality of scaling was low. With the increase of material size, the adsorption rate of cellucotton was unchanged, but the scaling was large. As a result, the scaling in simulated water was largest when the size of cellucotton was large, indicating that the large size of cellucotton was beneficial to the formation of scaling. This can be attributed to the small number of cellucotton and was beneficial to the formation and growth of scaling.
FIG. 6: (a) The adsorption rate (b) the adsorption quality of per gram filling materials and (c) total quality of cellucotton for the scaling in different size.
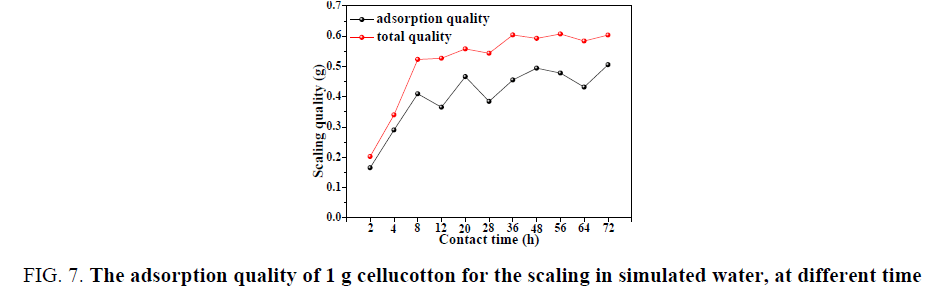
The adsorption rate and quality of cellucotton for scaling
It can be seen from FIG. 7. that when the initial concentration of the scaling in simulated water was 0.05 mol/L and the experimental temperature was 40°C, the scaling for CaSO4 was quickly increased and the total adsorption quality of the scaling was largest. The total adsorption quality of scaling was up to largest when the experimental time was 36 h and the scaling quality in 100 mL simulated water was 0.604 g. It was worth noting that after experiment of 8 h, the curve for scaling quality was ups and downs. This was because the supersaturated solution existed between the formation of anion and cation ion pairs. When the solution of saturation was higher, these ions were increased and gathered to form particles and, in the formation, dissolving and reformation dynamic balance [15,16].
FIG. 7: The adsorption quality of 1 g cellucotton for the scaling in simulated water, at different time
It can be seen from the adsorption curve that the adsorption quality of cellucotton for the scaling was quickly increased and when the contact time was 8 h, the adsorption quality of cellucotton in 100 mL simulated water was 0.4096 g. When the contact time was 48 h, the adsorption quality of 1 g cellucotton was up to 0.4942 g. It was worth noting that after experiment of 8 h, the curve was ups and downs. This was due to the presence of adsorption, peeling and resorption process on the surface of cellucotton.
Results, Discussion and Conclusion
In this paper, we compared the adsorption effect of four materials for BaSO4 scaling and analyzed the behavior of cellucotton in different experimental conditions. Through the above experimental analysis, we can get a series conclusion, 1) the adsorption effect of cellucotton for the scaling in four materials was best and the release rate of Ca2+ was lower. 2) The adsorption quality of 1 g cellucotton for the scaling was largest in 30°C and the total adsorption quality of scaling in simulated water was largest in 40°C. 3) The adsorption rate and the adsorption quality of 1 g cellucotton for the scaling in the contact time of 72 h was large and the total adsorption quality of scaling with contact time increased was increasing. 4) With the increase of cutting size, the adsorption quality of cellucotton per gram increased and the total adsorption quality of scaling was also increased.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.21376189) and Special Scientific Research Project Foundation of the Science and Technology Department of Shaanxi Province (Grant No.14JS087).
References
- Evaluation of synthesized antiscalants for cooling water system application. Desalination.2011;268(1):38-45.
- Qinghe G, Yican W, Yumei J. Study on scaling formation characteristics and produced liquid properties in oil-wells of ASP flooding. Adv Mater Res. 2012;524:1270-8.
- Sijun L, Li L.Thermoreversible gelation and scaling behavior of Ca2+-induced ?-carrageenan hydrogels. Food Hydrocolloid. 2016;61:793-800.
- Abdel-Gaber AM, Abd-El-Nabey BA, Khamis E, et al. Investigation of fig leaf extract as a novel environmentally friendly antiscalent for CaCO3, calcareous deposits. Desalination. 2008;230:314-28.
- Atkinson G, Mecik M. The chemistry of scale prediction. J Petrol Sci Eng. 1997;17:113-21.
- Synthesis of polyaspartic acid-melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide. Desalination. 2012;286:285-9.
- Ou HH, Hsieh LHC. A synergistic effect of sodium gluconate and 2-phosphonobutane-1,2,4-tricarboxylic acid on the inhibition of CaCO3 scaling formation. Powder Technol. 2016;302:160-7.
- Palanisamy K, Subramanian VK. CaCO3 scale deposition on copper metal surface: Effect of morphology, size and area of contact under the influence of edta. Powder Technol. 2016;294:221-5.
- Jia J, Xiao D, Enzhou L, et al. Highly efficient and stable Au/Bi2MoO6/Bi2WO6 heterostructure with enhanced photocatalytic activity for no gas removal under visible light irradiation. J Phys D Appl Phys. 2017;50(14):145103.
- Gopi SP, Vijaya P, Subramanian VK. Morphological and crystallization process of CaCO3, in the presence of Aqua soft 330 (AS 330). Powder Technol. 2012;225:58-64.
- Yanbing L, Qiang L, Feiou L, et al. Process, mechanism and impacts of scale formation in alkaline flooding by a variable porosity and permeability model. Acta Mech Sin. 2016;32(3):406-21.
- Ezuber HM. Prediction of strontium sulfate scale formation in oilfield environment. J A Intern. 2007;4(6):100958-68.
- Minmin Z, Qianhong S, Xiaoli Y, et al. Effect of reverse solute diffusion on scaling in foreard osmosis: A new control strategy by tailoring draw solution chemistry. Desalination. 2016;401:230-7.
- Mingliang L, Chunsheng P, Jingwu D, et al. The prediction of inorganic scaling tendency in formation water of longhupao reservior. Oilfield Chem. 2000;17:211.
- Liu D, Hui F, Lédion J, et al. Study of the scaling formation mechanism in recycling water. Environ Technol.2011;32(9):1017.
- Stamatakis E, Haugan A, Chatzichristos C, et al. Study of calcium carbonate precipitation in the near-well region using Ca2+ as tracer. Spe Prod Oper. 2006;21(1):33-9.