Original Article
, Volume: 16( 1)Synthesis, Structural Characterization and Solid-State NMR Studies of New Defect Pyrochlore-Type Antimoniates (H3O+)0.36lnxsb2o6+Y (with Ln=Gd3+, Eu3+ and 0<X, Y<1) Prepared by Exchange Method
- *Correspondence:
- El Haimouti ALaboratory of Chemistry and Mathematical Modelling, Department of Physics, Faculty Polydisciplinaire, Khouribga, University of Hassan, Morocco, Tel: +212-667 130655; E-mail: elhaimouti@yahoo.fr
Received Date: December 20, 2017 Accepted Date: January 12, 2017 Published Date: January 16, 2018
Citation: El Haimouti A, Dubois M, Zambon D, et al. Synthesis, Structural Characterization and Solid-State NMR Studies of New Defect Pyrochlore-Type Antimoniates (H3O+)0.36lnxsb2o6+Y (with Ln=Gd3+, Eu3+ and 0<X, Y<1) Prepared by Exchange Method. Int J Chem Sci. 2018;16(1):238
Abstract
A new cubic nonstoichiometric pyrochlore type antimoniates (H3O+)0.36LnxSb2O6+y (with Ln = Gd3+, Eu3+ and 0
Keywords
Pyrochlore; Exchange method; X-Ray diffraction; Infrared spectra; Magic angle spinning; Nuclear magnetic resonance
Introduction
The study of the solid protonic conductors constitutes a field of investigation in the range of the physiochemistry of materials. Many researchers showed the potential of the oxides pyrochlores such as NH4TaWO6 [1], MNbWO6 (M=H [2] or NH4 [3]), Na+(NaTaWO6 [4], K+(K1+xW1-xO6 [5]), Rb+(Rb3/2Cr1/2Te3/2O6 [6] and Tl+(Tl1+xTa1+xW1-xO6 [7]) for ionic conduction. Duan et al showed that the exchange properties of the hydroxylated phases KTa2 (O, OH)6 exhibit interesting catalytic properties [8]. Moreover, the hygroscopicity of some of these compounds can be considered an asset. Since the conductivity of pyrochlores with formula (M2O)x WO3, zH2O (M=H+, Li+, Na+, Ag+, NH4+) is strongly dependent of the degree of hygroscopy of the medium, these compounds can then be used as sensors of moisture [9]. In fact, intense research during these last years is devoted to the development of new ion exchanger materials.
In addition to many works concerned the exchange properties of lamellar perovskite type containing niobate [10-12], some antimoniates were also investigated [13-15]. One of approach of the problem, already tested successfully for various ions, consists to take into account the structural aspect: the structure is characterized by an open framework, favouring the ionic mobility. Accordingly, synchexs protonic conductor performed, for example, by substitution of a monovalent ion, generally alkaline ion.
Some protonic conductors can be directly prepared; as exemplified by HUP (HUO2PO4, 4H2O) which results from the reaction of the phosphoric acid on uranyl nitrate [16]. An indirect preparation is also possible: it consists in substituting by acid way a monovalent element in structural materials likely to allow a good ionic mobility. This approach leads to the synthesis of the phases HMO3, xH2O or M=Sb, Nb and Ta [17], pyrochlores HNbWO6 xH2O [18] and NH4NbWO6 [19].
Recently, we reported the conditions to obtain new defect pyrochlores in the systems (A=Na, K, Rb, Cs or Tl; Ln=Y, Gd and Eu)-Sb-O [20,21]. Because their high content of defects, ionic exchange properties could be expected for these pyrochlores. It appeared interesting to investigate the exchange in aqueous medium of the sodium ion by a monovalent ion such as hydronium H3O+ and ammonium NH4+ ions in the Na0,36LnxSb2O6+y phase. This process could be facilitated for three main factors:
• Position of the Na+ cations in large-sized cages, suggesting a high mobility of the charge carriers.
• Presence of the defect in the sites occupied by sodium.
• Insolubility of the materials.
The ideal structure of pyrochlore A2B2X6X' (or AA'B2X6X') has been previously described [22-25]. The crystal structure exhibits a three-dimensional framework builds with edge-sharing regular BX6 octahedra and sharing channels along the <110> directions. The cages, centred on the positions 8b (3/8, 3/8, 3/8) and occupied by the X' ion, are created by the intersection of six channels. Because of their large size, they can theoretically accommodate ions with ionic radius less than or equal to 1.80 Å, approximately and surrounded by 18 anions X.
The synthesis and the structural characterization of new pyrochlores with composition (H3O+)0.36LnxSb2O6+y (with Ln=Gd3+, Eu3+ and 0<x, y<1) obtained by exchange method have been investigated in this this work. Infrared spectroscopy and 23Na and 1H MAS-NMR measurements were performed in order to confirm the ionic exchange.
Experimental
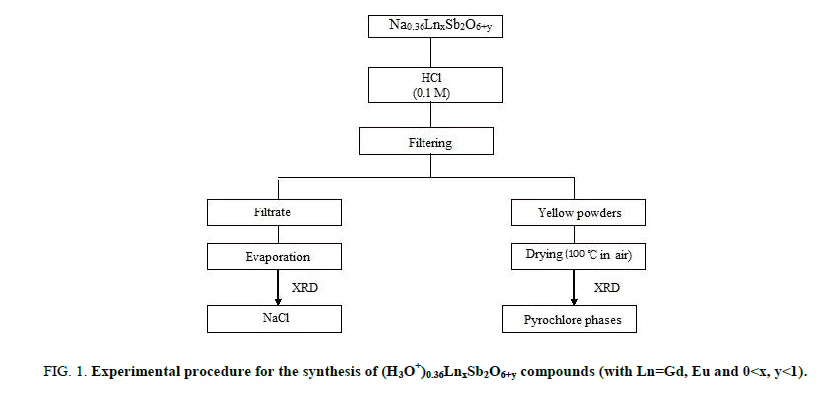
The (H3O+)0,36Gd0,80Sb2O6,38 and (H3O+)0,36Eu0,82Sb2O6,41 compounds were prepared by exchange method. Figure 1 displays the experimental process used for the synthesis of the samples. The starting materials for the preparation of Na0,36LnxSb2O6+y was NaNO3, Sb2O3 and Ln2O3, 6H2O. The preparation for this compound was carried out by using the same method as previously described [20].
Figure 1: Experimental procedure for the synthesis of (H3O+)0.36LnxSb2O6+y compounds (with Ln=Gd, Eu and 0<x, y<1).
The Na0,36LnxSb2O6+y agitated by magnetic stirring for 48 hours and then treated by a hydrochloric solution (0.1 M). The obtained powders are separated from the solution by filtration and were oven-dried at 100°C for 12 hours. The X-ray analysis reveals that pure pyrochlore phases were obtained and the characterization of the filtrate obtained after exchange shows the formation of sodium chloride what is in favour of the exchange.
The structural characterization of the resulting powders by the Rietveld method [26] was performed from X-ray powder patterns recorded on the 2θ range 10° to 120° with a step scan of 0.028° using a SIEMENS D-501 diffractometer (λ of CuKα=1.5405 Å). The data processing was performed using the FULLPROF program [27].
Fourier-transform infrared spectroscopy performed with a spectrophotometer with Fourier transform NICOLET 55XC. The sample spectra were recorded by transmission in a dry air atmosphere between 400 cm-1 and 4000 cm-1. X spectra accumulation was performed through a pellet made of 2 mg of the sample material deluted in KBr (200 mg).
The 1H and 23Na NMR spectra were recorded at room temperature with a Bruker MSL300 spectrometer operating at 300.1 and 79.4 MHz respectively for 1H and 23H experiments. MAS spectra were performed at 8 KHz with Bruker 4 mm MAS probes using a simple sequence ( acquisition). The processing and acquisition parameters were recycling at time 0.5 and 6 s, 5 and 6 s single π/2 pulse duration and 64 and 2400 scans for 1H and 23Na NMR measurements, respectively. The 1H chemical shifts are given in ppm and referred to tetramethylsilane (TMS) by using adamantane as an external standard (1.74 ppm from TMS). The chemical shifts for 23Na NMR experiments are quoted relative to NaCl (solid) as external standard referenced to 0 ppm. The reproducibility of the measured chemical shift values was in order to 0.1 ppm.
acquisition). The processing and acquisition parameters were recycling at time 0.5 and 6 s, 5 and 6 s single π/2 pulse duration and 64 and 2400 scans for 1H and 23Na NMR measurements, respectively. The 1H chemical shifts are given in ppm and referred to tetramethylsilane (TMS) by using adamantane as an external standard (1.74 ppm from TMS). The chemical shifts for 23Na NMR experiments are quoted relative to NaCl (solid) as external standard referenced to 0 ppm. The reproducibility of the measured chemical shift values was in order to 0.1 ppm.
Results and Discussion
Crystallographic study
The obtained (H3O+)0,36Gd0,80Sb2O6,38 and (H3O+)0,36Eu0,82Sb2O6,41 compounds exhibit a yellow colour. The X-ray powder patterns of these compounds were indexed in the cubic system with the space group F d3m (n°127). The element of framework (Sb2O6) were positioned on the sites 16 (c) (0, 0, 0) and 48 (f) (x, 1/2, 1/2) respectively for antimony and oxygen atoms as for pyrochlores Na0,36LnxSb2O6+y [20]. The scattering factors were used supposing that the elements are in an ionic state, those of H3O+ ions are identical to atomic oxygen having the same number of electrons.
XRD powder study does not allow to precisely locating the hydronium ions taking into account scattering factor of oxygen atom in comparison with the metal elements. By analogy with the pyrochlore Na0,36LnxSb2O6+y [20], both H3O+ and Ln cations are located on 16 (d) sites (1/2,1/2,1/2). The reliability factors obtained during the refinement are unsatisfactory (RB>16); nevertheless, this permits to exclude the hypothesis of the occupation of the 16 (d) sites by the hydronium ions.
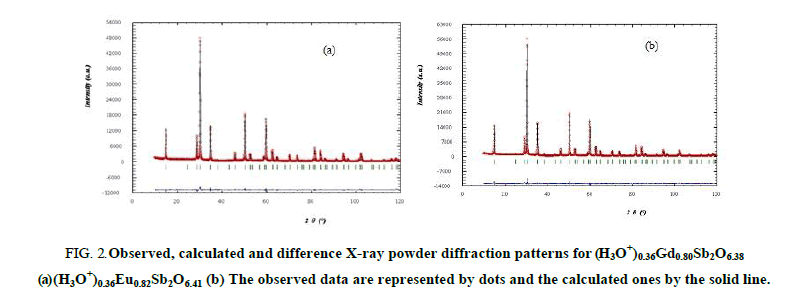
In order to obtain better reliability factors, one second assumption was done consisting of a statistical occupation of the 8 (b) sites (3/8,3/8,3/8) by the hydronium and oxygen (O') ions; isotropic thermal displacement parameters for these ions were selected by analogy with pyrochlore K0,42Eu0,74Sb2O6,32 [20]. After refinement, the reliability factors RB converge towards satisfactory values (Table 1) confirming the statistical and alternative occupation of the site 8 (b) by either H3O+ or O’2 ions and a partial occupation of the 16d sites by rare earth ions with site occupancy factors less than 0.5. When H3O+ (resp. O’2-) occupies the 8 (b) sites, the 16 (d) position is vacant (resp. occupied). The atomic coordinates the isotropic thermal displacement parameters, the occupancy factors, the reliability factors and the unit cell parameters are gathered in Table 1. The mean interatomic distances and bond angles are listed in Table 2. The observed cations-oxygen bond distances are in good agreement with sum of the ionic radii [28] and compatible with a statistical repartition of H3O+ and O’2- ions over the 8 (b) sites. The observed, calculated and difference patterns for (H3O+)0,36LnxSb2O6+y are shown in Figure 2.
Figure 2: Observed, calculated and difference X-ray powder diffraction patterns for (H3O+)0.36Gd0.80Sb2O6.38 (a) (H3O+)0.36Eu0.82Sb2O6.41 (b) The observed data are represented by dots and the calculated ones by the solid line.
| Atoms | Wyckoff | x/a | y/b position | z/c | B (Å)2 | τ |
|---|---|---|---|---|---|---|
| Gd | 16 (d) | 1/2 | 1/2 | 1/2 | 3.13 (4) | 0.39 |
| Sb | 16 (c) | 0 | 0 | 0 | 1.50 (3) | 1 |
| O | 48 (f) | 0.3294 (2) | 0.125 | 0.125 | 4.83 (9) | 1 |
| H3O+ | 8 (b) | 0.375 | 0.375 | 0.375 | 1.48 (5) | 0.36 |
| O’ | 8 (b) | 0.375 | 0.375 | 0.375 | 1.48 (5) | 0.36 |
| - | Rp = 10.90 | Rwp = 10.90 | χ2 = 4.26 | RB=4.31 | RF=4.28 | a = 10.2829 (7) Å |
| (H3O+)0.36Eu0.82Sb2O6O’0.41 | ||||||
| Atoms | Wyckoff | x/a | y/b | z/c | B (Å)2 | τ position |
| Eu | 16 (d) | 1/2 | 1/2 | 1/2 | 3.13 (4) | 1/2 |
| Sb | 16 (c) | 0 | 0 | 0 | 1.35 (9) | 1 |
| O | 48 (f) | 0.3335 (2) | 0.125 | 0.125 | 5.24 (9) | 1 |
| H3O+ | 8 (b) | 0.375 | 0.375 | 0.375 | 1.97 (8) | 0.36 |
| O’ | 8 (b) | 0.375 | 0.375 | 0.375 | 1.97 (8) | 0.41 |
| - | Rp=10.90 | Rwp=11.4 | χ2=4.66 | RB=4.04 | RF=4.15 | a=0.2826 (8) Å |
Table 1. Atomic coordinates, isotropic thermal parameters B (Å)2, relative occupancies τ, reliability factors and unit cell parameters in (H3O+)0.36LnxSb2O6O’y (H3O+)0.36Gd0.80Sb2O6O’0.38.
| (H3O+)0.36Gd0.80Sb2O6O’0.38 | (H3O+)0.36Eu0.82Sb2O6O’0.41 | |
|---|---|---|
| (H3O+)…O’ | 3.038 (2) ( × 2) | 2.996 (1) ( × 6) |
| Ln…O | 2.525 (3) ( × 6) | 2.225 (7) ( × 2) |
| Ln…O' | 2.226 (3) ( × 6) | 2.496 (2) ( × 6) |
| Sb…O | 1.992 (3) ( × 6) | 2.009 (1) ( × 6) |
| O’-Ln-O’ | 180.0 | 180.0 |
| O-Ln-O | 63.4 (1), 116.6 (1) | 63.8 (4), 116.1 (6) |
| O-Ln-O' | 79.2 (4) | 78.5 (5) |
| O-Sb-O | 83.5 (3), 96.4 (7) | 83.0 (6), 97.9 (1) |
| Sb-O-Sb | 131.6 (1) | 129.4 (2) |
Table 2. Interatomic distances (Å) and bond angles (°) for (H3O+)0.36LnxSb2O6O’y.
In these phases, antimony exhibit a low distorted octahedral environment in agreement with the values of the oxygen (O) positional parameter x (ideal theoretical value calculated for a regular octahedron: x=0.3125). The Sb-O distances are of the same order of magnitude in comparison with those raised in many oxide pyrochlores [20,21,29] and close to the theoretical value (DShannon=0.62+1.40=2.02 Å) [28].
Spectroscopy data infrared
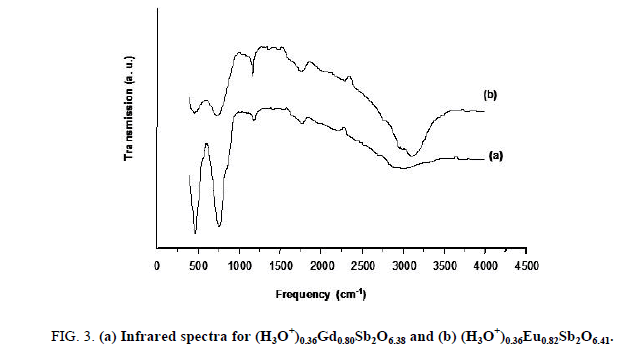
The ions exchange reactions observed in the case the Na0,36LnxSb2O6+y phase in acid medium and the reversibility of this phenomenon, could not constitute a formal proof of the existence of (H3O+) ions in the compound with expected formula (H3O+)0,36LnxSb2O6+y. In order to identify the accommodation of H3O+ ions in our compounds, the pyrochlore (H3O+)0,36LnxSb2O6+y was studied by infrared spectroscopy. In spite of the data of the vibrational frequencies of H3O+ ion is obtained with systems chemically different from the samples studied in this work. They have given information about the presence H3O+ ion. We were inspired to benefit our spectra from work of Michel et al. for the compounds pyrochlores H2 (H2O) M2O6 with M=Nb, Y and HSbWO6, H2O. The FT-IR transmission spectrum (Figure 3) exhibits:
• Two bands located in the 450-750 cm-1 range, which characterize the antisymmetric vibration of valence of the connection antimony-oxygen Sb-O.
• The broad band in the 2900-3200 cm-1 range, it related the symmetrical and antisymmetric vibration modes of H3O+ ion.
• A diffuse band centered around 1750 cm-1 could be attributed to the antisymmetric deformation of H3O+ ion.
• A weak band centered around 1100 cm-1 which can be assigned to the symmetrical deformation of H3O+ ion.
These FT-IR data must be considered carefully because of the possible presence of water absorbed on the surface sample. A study by NMR spectra is then necessary to confirm the results obtained by FT-IR spectroscopy.
Study of the Na0,36Gd0,80Sb2O6,38, (H3O+)0,36Gd0,80Sb2O6,38 and (H3O+)0,36Eu0,82Sb2O6,41 by 23Na and 1H solid state NMR
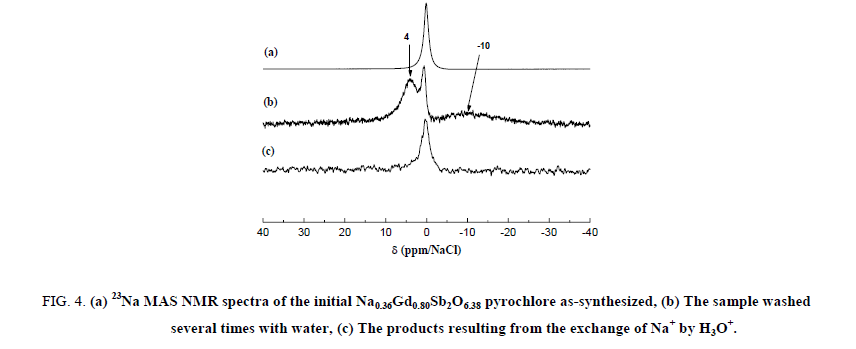
23Na MAS NMR spectra of the Na0,36Gd0,80Sb2O6,38 starting material and the H3O+ exchanged samples are shown in Figure 4. The spectrum of the as-synthesized Na0,36Gd0,80Sb2O6,38 compound exhibits a strong narrow band centred at 0.0 ppm. When this sample was washed several times with water, this narrow line decreased drastically in intensity, it is then related to impurities deposed on the powder surface during the synthesis. As a matter of fact, NaNO3 was added in excess during the preparation of the samples. A consequent washing of these ones results in the decrease of its concentration.
Figure 4: (a) 23Na MAS NMR spectra of the initial Na0.36Gd0.80Sb2O6.38 pyrochlore as-synthesized, (b) The sample washed several times with water, (c) The products resulting from the exchange of Na+ by H3O+.
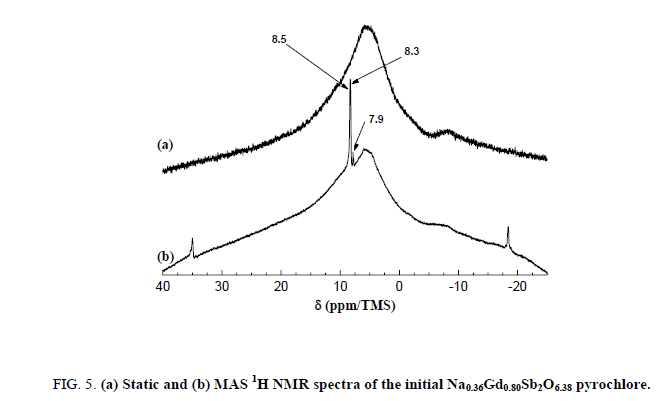
After the washing, two broad bands are then observable at -10.0 and +4.0 ppm. The first one is consistent with hydrated sodium cations. Both the two broad lines could be attributed to the sodium ions located in the center of the hexagonal cavities of the pyrochlore framework. After exchange of Na+ by H3O+, these bands disappeared contrary to the narrow line of the impurity indicating the quasi-total exchange. The signal/noise ratio indicates a low content of residual 23Na nuclei. A strong broad band between -25 and 40 ppm is present both in the static and 1H MAS NMR spectrum of the Na0,36Gd0,80Sb2O6,38 sample (Figure 5).
Figure 5: (a) Static and (b) MAS 1H NMR spectra of the initial Na0.36Gd0.80Sb2O6.38 pyrochlore.
The absence of spinning bands for this line indicates that this contribution is not sensitive to the rotation of the sample. This peak is then certainly related to a disordered system with a distribution of chemical shifts which are not averaged by the MAS rotation. On the other hand, three fine lines, at 8.5 ppm, 8.3 ppm and 7.9 ppm (and their spinning side bands, which are well defined), are narrowed in the MAS conditions and are then related to two 1H nuclei in ordered sites with one chemical shift tensor.
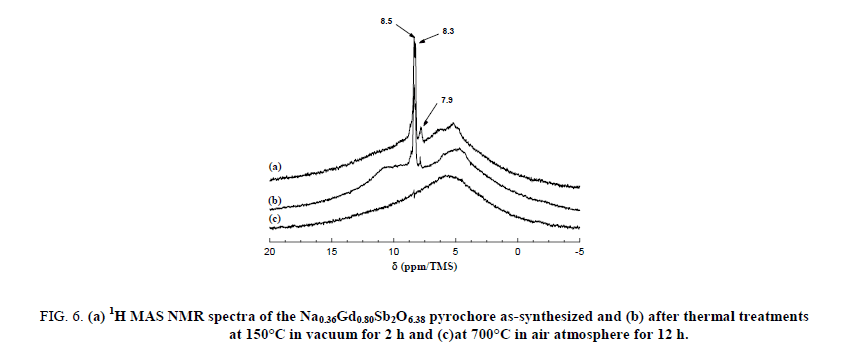
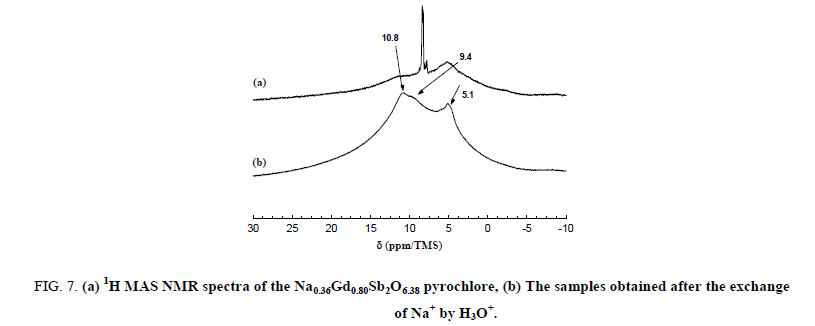
The narrow lines are attributed to the water molecules located along the hexagonal tunnels of the host structure. An annealing treatment at 150°C for 2 hours in dynamic primary vacuum results in a low decrease of the relative intensity of the bands at 8.3 and 7.9 ppm in comparison with the one at 8.5 ppm (Figure 6). On the contrary, after a thermal treatment at 700°C for 12 h in air atmosphere, all the narrow bands disappeared indicating that the water molecules are totally removed from the host inorganic matrix in these conditions. Figure 7 shows the 300 MHz 1H MAS NMR spectra of pyrochlore sample Na0,36Gd0,80Sb2O6,38 before and after ions exchange of Na+ by H3O+. The spectrum of the pyrochlore after exchanged by H3O+ is complex with at least 3 contributions at 10.8, 9.4 and 5.1 ppm.
Figure 6: (a) 1H MAS NMR spectra of the Na0.36Gd0.80Sb2O6.38 pyrochore as-synthesized and (b) after thermal treatments at 150°C in vacuum for 2 h and (c)at 700°C in air atmosphere for 12 h.
Figure 7: (a) 1H MAS NMR spectra of the Na0.36Gd0.80Sb2O6.38 pyrochlore, (b) The samples obtained after the exchange of Na+ by H3O+.
This suggests the existence of different H species in this latter sample; such a phenomenon was reported by Abraham et al. in the case of pyrochlore containing bismuth after exchange of potassium by proton. The presence of the band at 10.8 ppm indicates that the hydronium ions H3O+ are introduced into pyrochlore during the ions exchange processes; as a matter of fact, chemical shift value of these cations is expected at about 10 ppm. By comparison with NMR data concerning inorganic hydrated oxides, we can propose that the signal at 5.4 ppm is caused by protons of absorbed H2O molecule.
13Na and 1H MAS NMR were also performed to characterize Na0,36Eu0,82Sb2O6,41 pyrochlore; this study reveals similar behaviours of these compound in comparison with (H3O+)0,36Gd0,80Sb2O6,38 pyrochlore during the exchange of sodium ions by H3O+. This 13Na and 1H MAS NMR data show clearly the exchange of the sodium ions by H3O+ because of the disappearance of the bands of sodium ions and the presence of lines typical of the hydronium ions and H2O after the exchange process.
Conclusion
Rietveld refinements of their X-ray powder patterns clearly schow the formation of defect pyrochlore phases with a partiel occupancy of the 16d sites by Gd3+ and Eu3+ ions and the 8 (b) site by H3O+ and O’2- ions. The ionic exchange properties in aqueous solution of pyrochlores containing sodium, rare earth and antimony ions were investigated for the first time. Such a process allows to form new pyrochlores where as a direct reaction of mixed appropriate oxides is unaffective, thus could be synthesized. The structural refinements are in good agreement with the 23Na and 1H solid state NMR and infrared spectroscopy characterization.
References
- Butler MA, Biefeld RM. Ionic motion in the defect pyrochlore NH4TaWO6. Sol Stat Comm. 1979;29(1):5-7.
- Binesh N, Bhat V, Bhat SV. Mechanism of protonic conduction in defect pyrochlore HNbWO6· xH2O using MAS NMR. Sol Stat Ion. 1996;86:665-8.
- Brunner DG, Tomandl G. A new proton-conducting ceramic: Part II. Preparation of proton-conducting NH sub 4 NbWO sub 6 and NH sub 4 TaWO sub 6. Adv Ceram Mat. 1987;2(4).
- Goodenough JB, Hong HP, Kafalas JA. Fast Na+-ion transport in skeleton structures. Mat Res Bull. 1976;11(2):203-20.
- Grins J, Nygren M, Wallin T. Studies on composition, structure and ionic conductivity of the pyrochlore type system K1+ × Ta1+ × W1− × O6· nH2O, 0<x<1. Mat Res Bulletin. 1980;15(1):53-61.
- Isasi J, Lopez ML, Veiga ML, et al. Ionic conductivity of a new defect pyrochlore. Sol Stat Ion. 1996;89(3-4):321-6.
- Michel C, Guyomarc'h A, Deschanvres A, et al. Nouveaux conducteurs ioniques caracterises par une structure a tunnels entrecroises. Mat Res Bull. 1978;13(3):197-203.
- Duan N, Tian ZR, Willis WS, et al. Hydrothermal synthesis and structure of a potassium tantalum defect pyrochlore. Inorg Chem. 1998;37(18):4697-701.
- Li YJ, Tsai PP. Lacunar pyrochlore-type tungsten oxides as humidity-sensing materials. Sol Stat Ion. 1996;86:1001-4.
- Sato M, Abo J, Jin T. Structure examination of NaLaNb2O7 synthesized by soft chemistry. Sol Stat Ion. 1992;57(3-4):285-93.
- Toda K, Honma T, Ye ZG, et al. Synthesis and structure determination of new layered perovskite compound, KLaTa2O7. J Alloys and Comp. 1997;249(1-2):256-9.
- Gopalakrishnan J, Bhat V, Raveau B. AILaNb2O7: A new series of layered perovskites exhibiting ion exchange and intercalation behavior. Mat Res Bull. 1987;22(3):413-7.
- Baetsle LH, Huys D. Structure and ion-exchange characteristics of polyantimonic acid. J Inorg and Nuclear Chem. 1968;30(2):639-49.
- Weigel F, Hoffmann G. The phosphates and arsenates of hexavalent actinides. Part I. Uranium. J Less Common Met. 1976;44:99-123.
- Chowdhry U, Barkley JR, English AD, et al. New inorganic proton conductors. Mat Res Bull. 1982;17(7):917-33.
- Perottoni CA, Haines J, Da Jornada JA. Crystal structure and phase transition in the defect pyrochlore NH4NbWO6. J Sol Stat Chem. 1998;141(2):537-45.
- El Haimouti A, Zambon D, M. EL-Ghozzi, D. Synthesis, structural and physico-chemical characterization of new defect pyrochlore-type antimonates K 0.42 Ln y′ Sb 2 O 6+ z′ and Na 0.36 Ln y Sb 2 O 6+ z (0<y,y′,z,z′<1; Ln= Y, Eu and Gd) prepared by soft chemistry route. J Allows Comp. 2004;363:130-4.
- El Haimouti A, Zambon D, EL-Ghozzi M, et al. Synthesis and structural characterization of new defect pyrochlore type A × Ln y Sb2O6+ z antimoniates (0<x, y, z<1; A=Rb, Cs, Tl; Ln=Eu, Gd and Y) prepared by coprecipitation. Mat Res Bull. 2003;38:1423-36.
- Fourquet JL, Jacoboni C, De Pape R. Les pyrochlores AIB2X6: Mise en evidence de l'occupation par le cation AI de nouvelles positions cristallographiques dans le groupe d'espace fd3m. Mat Res Bull. 1973 Apr 1;8(4):393-403.
- Darriet B, Rat M, Galy J, et al. Sur quelques nouveaux pyrochlores des systemes MTO3 WO3 et MTO3 TeO3 (M K, Rb, Cs, Tl; T= Nb, Ta). Mat Res Bulletin. 1971;6(12):1305-15.
- Rietveld H. A profile refinement method for nuclear and magnetic structures. J App Crystall. 1969 ;2(2):65-71.
- Carvajal JR. Full prof: A program for the Rietveld Refinement and Pattern Matching Analysis?, Abstracts of the satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France. 1991;pp:127.
- Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica section A: Crystal Physics, Diffraction, Theoretical and General Crystallography. 1976;32(5):751-67.
- Piffard Y, Tournoux M. Structure du pyrochlore, Tl0. 51SbIII0. 71SbV2O6. 32. Acta Crystallographica Section B: Struct Crystall and Crystal Chem. 1979;35(6):1450-2.
- Piffard Y, Dion M, Tournoux M. Structure cristalline du pyrochlore, K0, 51SbIII0, 67SbV2O6, 26. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 1978;34(2):366-8.
- Michel C, Groult D, Raveau B. Ion exchange properties of pyrochlores AB2O6-I Preparation and structural study of new pyrochlores AMWO6. H2O (A=Li, Na, M=Nb, Ta, Sb). J Inorg Nuc Chem. 1975;37(1):247-50.
- Ciraolo MF, Hanson JC, Grey CP. Solid-state rubidium exchange of zeolite NH 4 Y. Microporous and Mesoporous Materials. 2001;49(1):111-24.
- Feuerstein M, Hunger M, Engelhardt G, et al. Characterization of sodium cations in dehydrated zeolite NaX by 23Na NMR spectroscopy. Solid State Nuclear Magnetic Resonance. 1996;7(2):95-103.
- Zheng L, Fishbein KW, Griffin RG, et al. Two-dimensional solid-state proton NMR and proton exchange. Journal of the American Chemical Society. 1993;115(14):6254-61.