Original Article
, Volume: 15( 1)Synthesis, Structural and Morphological Studies of CdS Nanopowder
- *Correspondence:
- Thirumala Rao G Physics Division, Department of Basic Science and Humanities, GMR Institute of Technology, Rajam, Andhra Pradesh, India, Tel: 08941251592; E-mail: thirumalaphy@gmail.com
Received: February 03, 2017 Accepted: February 27, 2017 Published: March 03, 2017
Citation:Santhosh Kumar N, Govinda D, Thirumala Rao G. Synthesis, structural and morphological studies of CdS nanopowder. Int J Chem Sci 2017;14(1):101.
Abstract
Hexagonal wurtzite structured CdS nanopowder was synthesized by using chemical precipitation method. Various spectroscopic technics were used for the characterization of the prepared sample. X-ray diffraction pattern shows the high intense crystalline peaks and the average crystallite size is found to be 19 nm. SEM micrographs reveal the non-uniformly distributed spherical shaped structures. EDS analysis confirms the stoichiometric composition and presence of target elements. FT-IR spectrum exhibited a metal sulfide band at 615 cm–1 and other functional groups.
Keywords
Nanopowder, Hexagonal wurtzite structure, Chemical precipitation, X-ray diffraction
Introduction
Semiconductor nanostructures with typical dimensions of 1-100 nm have been extensively studied because of their optical, electrical properties are dramatically changed while reducing the particle size, which can be controlled during synthesis. Promising applications are expected and already has been realized in many fields of technology such as optoelectronics [1], photocatalysis [2], solar energy conversion [3], water pollutant photo-degradation [4] and biology [5]. Chalcogenide semiconductors such as CdS is a well characterized II-VI group inorganic semiconductor with a wide direct bandgap of 2.42 eV and it has wide application potential in optoelectronics, such as nonlinear optics, visible-light emitting diodes and lasers [6]. Size, morphology and dimensionality can strongly affect the properties of nanostructured materials. Recent technological developments in nanoscience lead to the synthesis of nanostructured materials with various structures and morphologies have received much attention due to their novel applications, fascinating properties and quantum size effects [7]. Most of the researchers focused on the synthesis of low dimensional nanomaterials for the functional nano devices including electrically driven transistors, lasers, light emitting diodes and chemical sensors [8-11].
Numerous methods have been developed for the synthesis of CdS nanostructures including chemical precipitation method [12,13] mechanochemical synthesis [14], ion exchange strategy [15,16] solvothermal [17], hydrothermal [18], microwave assisted sonochemical synthesis [19], sputtering [20] and chemical vapor deposition [21]. Among the processes chemical precipitation method is well suitable for the preparation of nanomaterials having significant advantages including a low reaction temperature, size selective growth, morphological control, cost effective and large-scale production [22]. In the present investigation, the chemical precipitation method is adopted for the synthesis of CdS nanopowder. As prepared nanopowder was characterized by X-ray diffraction, scanning electron microscopy with EDS analysis and Fourier transformed infrared (FT-IR) spectroscopy.
Experimental procedure
To prepare the CdS nanopowder, the following chemical reagents were used. Cadmium acetate, sodium sulfide and ethanol were used as precursors. All the chemical reagents were analytical grade without further purification and purchased from Merck chemicals, Mumbai, India. Deionized water was used for all dilution and sample preparation. All the chemicals are above 99% in purity. All the glassware used in this experimental work was acid washed.
CdS nanopowder was synthesized by a simple chemical precipitation method. In a conventional method, 0.2 M of cadmium acetate in 50 mL of deionized water-ethanol matrix and an equal molar amount of sodium sulfide in another deionized water-ethanol matrix was mixed drop by drop with continuous stirring until a homogeneous precipitation was obtained and then stirred for 4 h. The obtained dispersions were washed several times with deionized water and ethanol to remove impurities. After washing, the solution was centrifuged at 10,000 rpm about 30 min. The settled powder was collected and dried in a hot air oven at 120°C for 2 h. As prepared CdS nanopowder was characterized by following techniques.
Shimadzu XRD 6000 diffractometer was used for the XRD analysis (λ=1.5406 Å). Morphological details were collected from SEM (ZEISS EVO 18) instruments. Oxford EDS analyzer (attached with SEM instrument) was used for the chemical composition analysis. Shimadzu IR-Affinity 1S FT-IR spectrophotometer (using KBr pallets) was used the FT-IR analysis.
Results and Discussion
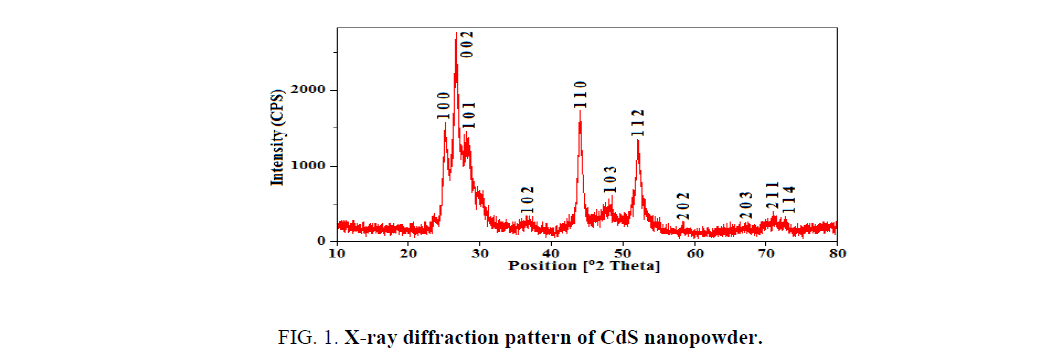
X-ray diffraction analysis was performed to obtain the information about the structure and crystallinity of the material. Figure. 1 depicts the X-ray diffraction pattern of CdS nanopowder. The sharp and high intense peaks in the XRD pattern may be arises due to the well-ordered crystal structure of the prepared sample. All the diffraction peaks observed at 2θ=25.09, 26.53, 27.93, 36.76, 43.85, 48.10, 51.92, 58.13, 66.92, 71.16 and 72.64 are indexed to the diffraction planes of (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), (2 0 2), (2 0 3), (2 1 1) and (1 1 4) respectively. The diffraction data exhibits the hexagonal wurtzite structure of CdS with the lattice cell parameters a=0.4122 and c=6718 nm which are in good agreement with the standard JCPDS data (65-3414). All the diffraction peaks shift towards higher angles (2θ) and lattice cell parameters are decreases as compared to the standard values which arises due to the reduction of crystallite size of the crystalline nanopowder. The average crystallite size is evaluated for the maximum intense peak using the most familiar Debye Scherrer’s formula,
D=0.9 λ/β cosθ
where D is the average crystallite size, λ is the incident wavelength (Cu Kα=1.5406), β is full width at half maxima (FWHM) and θ is the diffraction angle. The average crystallite size is found to be 19 nm. The induced micro strain in the CdS nanopowder is evaluated using Stokes-Wilson equation,
ε=β cosθ/4
The micro strain is evaluated as ε=1.8477 × 10–3. The volume of the hexagonal CdS unit cell (V), internal parameter (u) and Cd-S bond length (L) are also evaluated using the following relations,
V=0.866 a2 c
u=a2/3c2+0.25
L=[0.3a2+(0.5–u)2 c2]1/2
Where a, c are the lattice cell parameters. The volume (V), internal parameter (u) and bond length (L) are evaluated as 0.9885 nm3, 0.3755 and 0.2407 nm. It is clear that the lattice volume and bond length are slightly decreases compared to the standard ones which leads to the lattice contraction of the CdS.
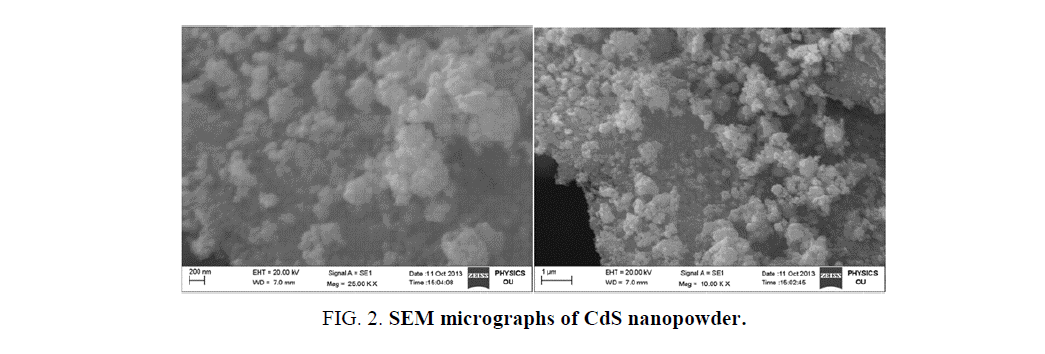
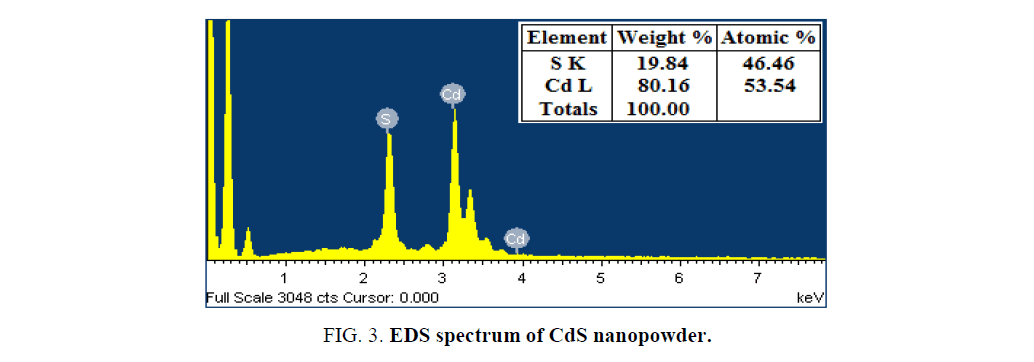
SEM with EDS is the most widely used technique for surface morphology and chemical composition analysis. SEM images of CdS nanopowder at different magnifications are shown in Figure. 2. It is clearly observed that the SEM images shows non-uniformly distributed spherical shaped particle like structures with slight agglomerations. The agglomerations may arises due to the narrow space between the particles. Further, the EDS analysis was performed in order to verify the stoichiometric chemical composition details. Figure. 3 shows the EDS spectrum of CdS nanopowder which exhibits only the characteristic peaks of Cd and S species. No other peaks observed in the spectrum indicates the purity of the samples.
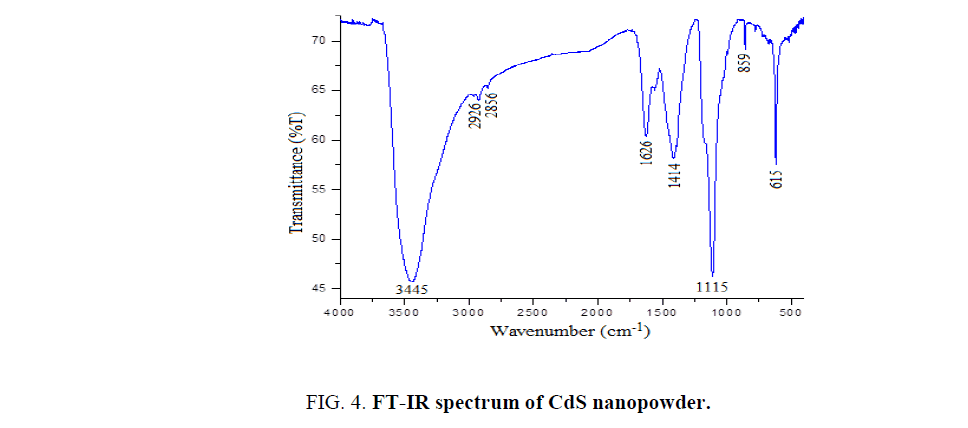
Fourier transform infrared spectroscopy (FTIR) spectra have long been used to identify organic and inorganic materials by measuring the absorption of various infrared light wavelengths. FT-IR spectrum of CdS nanopowder is shown in Figure. 4. The band centered at 615 cm-1 is attributed to the symmetric stretching vibration of Cd-S [23]. The structural changes are observed at 859 cm-1. The bands observed at 1117 cm-1 is attributed to O-H stretching vibrations of H2O molecule [24]. The band at 1414 cm-1 is assigned to the symmetric stretching vibration of C=O [25]. The vibrational band centered at 1630 cm-1 is a characteristic of symmetric bending vibration of H2O molecule [26]. The symmetric and asymmetric stretching vibrations of C–H are observed at 2855 and 2926 cm-1 respectively [27]. The broad band observed at 3445 cm-1 is assigned to the O–H stretching vibration of H2O molecule [28].
Conclusion
The CdS nanopowder was synthesized by using chemical precipitation method. X-ray diffraction pattern exhibited hexagonal wurtzite structure of CdS. The average crystallite size and Cd-S bond length are evaluated as 19 and 0.2407 nm respectively. SEM micrographs reveal the non-uniformly distributed spherical shaped particle like structures. EDS spectrum shows only target elements which reveal that the sample is in good purity and stoichiometric ratio. FT-IR spectrum shows the metal sulfide band at 615 cm-1 and other functional groups.
References
- Korgel BA, Monbouquette HG. Synthesis of Size-Monodisperse CdS Nanocrystals Using Phosphatidylcholine Vesicles as True Reaction Compartments, J Phys Chem. 1996;100:346.
- Grazel CK, Gratzel M. Hydrogen evolution from the photolysis of alcoholic benzophenone solutions via redox catalysis, J Am Chem Soc. 1979;101:7741.
- Ramsden J, Gratzel M. Photoluminescence of small cadmium sulphide particles, J Chem Soc. Faraday Trans. 1984;80:919.
- Mills A, Williams G. Photosensitised oxidation of water by CdS-based suspensions, J. Chem. Soc. Faraday Trans. 1989;185:503.
- He J, Scholes GD, Qu YL. Preparation, structural and its enhanced green upconversion luminescence in rare-earth doped CdMnS nanopowders, J Appl Phys. 2008;104:023110.
- Pan A, Yang H, Yu R, et al. Fabrication and photoluminescence of high-quality ternary CdSSe nanowires and nanoribbons, Nanotechnology. 2006;17:1083.
- Cui Y, Lieber CM, Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks, Science 2001;291:851.
- Mahdi MA, Hassan JJ, Ahmed NM, et al. Growth and characterization of CdS single-crystalline micro-rod photodetector, Superlattices Microstruct. 2013;54:137.
- Chen J, Xue K, An J, et al. Wang, Photoelectric effect and transport properties of a single CdS nanoribbon, Ultramicroscopy 2005;105:275.
- Raut BT, Godse PR, Pawar SG, et al. Development of nanostructured CdS sensor for H2S recognition: structural and physical characterization, J. Mater. Sci.: Mater. Electron. 2012;23:956.
- Yordanov GG, Gicheva GD, Dushkin CD, Optical memory based on photo-activated fluorescence of core/shell CdSe/CdS quantum dots embedded in poly (butylmethacrylate), Mater Chem Phys. 2009;113:507.
- Rajesh Kumar M, Murugadoss G, Synthesis and study of optical and thermal properties of Mn doped CdS nanoparticles using polyvinylpyrrolidone, J Lumin. 2014;146:325.
- Chauhan R, Kumar A, Ram P, Visible-light photocatalytic degradation of methylene blue with Fe doped CdS Nanoparticles, Appl Surf Sci. 2013;270:655.
- Reyes P, Velumani S. Structural and optical characterization of mechanochemically synthesized copper doped CdS nanopowders, Mater Sci Eng. 2012;177:1452.
- Xiang Q, Cheng B, Yu J, Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance, Appl Catal B: Environ. 2013;138-39:299.
- Yu J, Zhang J, Jaroniec M, Preparation and enhanced visible-light photocatalytic H2-production activity of CdS quantum dots-sensitized Zn1−xCdxS solid solution, Green Chem. 2010;12:1611.
- Yu J, Yu Y, Cheng B. Enhanced visible-light photocatalytic H2-production performance of multi-armed CdS nanorods, RSC Adv. 2, 2012;11829.
- Zhai T, Fang X, Li L, et al. One-dimensional CdS nanostructures: synthesis, properties, and applications, Nanoscale 2. 2009;168.
- Yang H, Huang C, Li X, et al. Luminescent and photocatalytic properties of cadmium sulfide nanoparticles synthesized via microwave irradiation, Mater Chem Phys. 2005;90:155.
- Rodr?guez RC, Perez PB, Delgado DP, et al. S diffusion at the CdTerCdS interface grown by rf magnetron sputtering, Mater Lett. 1998;37:281.
- Uda H, Yonezawa H, Ohtsubo Y, et al. Thin CdS films prepared by metal organic chemical vapor deposition, Sol. Energy Mater Sol Cells. 2003;75:219.
- Hu X, Li G, Yu JC. Design, Fabrication, and Modification of Nanostructured Semiconductor Materials for Environmental and Energy Applications, Langmuir. 2010;26:3031.
- Ziabari AA, Ghodsi FE, Influence of Cu doping and post-heat treatment on the microstructure, optical properties and photoluminescence features of sol–gel derived nanostructured CdS thin films, J Lumin. 2013;141:121.
- Kumar K, Chitkara M, Singh SI, et al. Photocatalytic, optical and magnetic properties of Fe-doped ZnO nanoparticles prepared by chemical route, J Alloys Comp. 2014;588:681.
- Babu B, Thirumala Rao G, Pushpa Manjari V, et al. Sonochemical assisted synthesis and spectroscopic characterization of Fe3+ doped ZnO diluted magnetic semiconductor, J Mater Sci. Mater Electron. 2014;25:4179.
- Thirumala Rao G, Babu B, Joyce Stella R, et al. Synthesis and characterization of VO2+ doped ZnO–CdS composite nanopowder, J Mol Struct 2015;1081:254.
- Mansur HS, Sadahira CM, Souza AN, et al. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde, Mater Sci Eng. 2008;28:539.
- Nakamoto K. Infrared Raman Spectra of Inorganic and Coordination Com-pounds, Part-A and B, John Wiley and Sons, New York, USA; 1997.