Research
, Volume: 20( 3)Synthesis of Nanostructured Conducting Polymers for Gas Sensing Application
- *Correspondence:
- Snehal Kargirwar, Department of Basic Sciences and Humanities, Fr. Conceicao Rodrigues Institute of Technology, Vashi, Navi Mumbai, India ,Tel: 8975145419; E-mail: snehalkargirwar@yahoo.co.in
Received: March 25, 2022, Manuscript No. TSIJCS-22-58441; Editor assigned: March 28, 2022, PreQC No. TSIJCS-22-58441; Reviewed: April 11, 2022, QC No. TSIJCS-22-58441; Revised: May 24, 2022, Manuscript No. TSIJCS-22-58441;Published: June 01, 2022, DOI: 10.37532/ 0972-768X.2022.20(3).430
Citation:Snehal Kargirwar. Synthesis of Nanostructured Conducting Polymers for Gas Sensing Applications. Int J Chem Sci. 2022;20 (3):430Z
Abstract
Polyaniline (PANI)–coated Polyacrylonitrile (PAN) (PANI/PAN) nanofiber was prepared with an electrospinning technique and in-situ chemical polymerization. The morphology and chemical structure of PANI/PAN nanofiber was characterized by scanning electron microscopy (SEM). SEM images shows that PANI as the shell layer was homogeneously and uniformly polymerized on the surface of PAN nanofiber. The effects of different concentrations of methanol and acetone on the responses of PANI/PAN nanofiber sensor at room temperature were investigated. The sensitivity of PANI/PAN was observed to increase with the increase in the concentration of methanol and acetone.
Keywords
Electrospinning; Gas sensing; Nanofibers; Polyacrylonitrile
Introduction
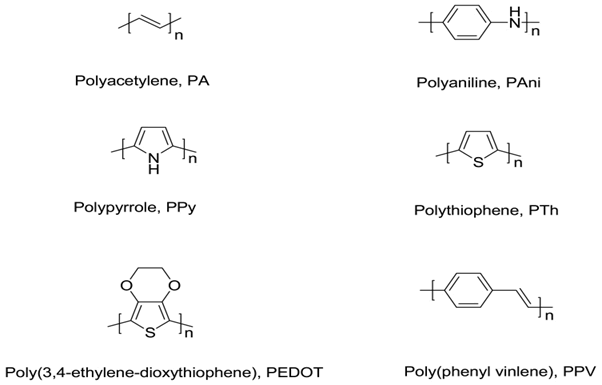
Conducting polymers, such as Polypyrrole (PPy), Polyaniline (PAni), Polythiophene (PTh) and their derivatives, have been used as the active layers of gas sensors since early 1980’s [1]. In comparison with most of the commercially available sensors, based usually on metal oxides and operated at high temperatures, the sensors made of conducting polymers have many improved characteristics. They have high sensitivities and short response time; especially, these feathers are ensured at room temperature. Conducting polymers are easy to be synthesized through chemical or electrochemical processes, and their molecular chain structure can be modified conveniently by copolymerization or structural derivations. Furthermore, conducting polymers have good mechanical properties, which allow a facile fabrication of sensors. As a result, more and more attentions have been paid to the sensors fabricated from conducting polymers, and a lot of related articles were published. There are several reviews emphasize different aspects of gas sensors [2-4], and some others discussed sensing performance of certain conducting polymers [5-7], but few of them paid special attention to summarizing gas sensors based on different conducting polymers. The conducting polymers mentioned is all referring to intrinsic conducting polymers. Their main chains consist of alternative single and double bonds, which leads to broad π-electron conjugation. Figure 1 presents several typical conducting polymers used as the active layers in gas sensors. However, the conductivity of these pure conducting polymers are rather low (<10-5 S cm-1). In order to achieve highly conductive polymers, doping process is necessary. The concept of doping is the central theme which distinguished conducting polymers from all other polymers [8]. Conducting polymers can be doped by redox reaction or protonation, in which the latter is only applicable to PAni.
Figure 1:Several typical conducting polymers used as the active layers in gas sensors.
Several typical conducting polymers
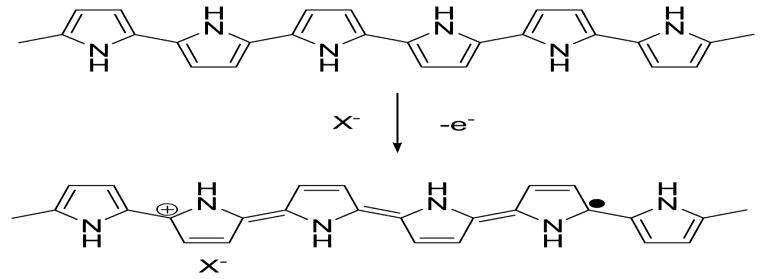
Figure 2 demonstrates the oxidation process of PPy. Some electrons are removed from PPy backbones by chemical or electrochemical oxidations, leaving positive charges on them. The resulted cation radicals are called polarons, acting as the charge carriers. Counter ions, X−, are also induced close to the polymer chains to balance the positive charges. Doped conducting polymers are semiconductors or conductors (σ∼100-105 S cm-1). This doping process is reversible, that is, the doped PPy can be turned into its undoped state by chemical or electrochemical reductions.
Oxidation doping of Ppy
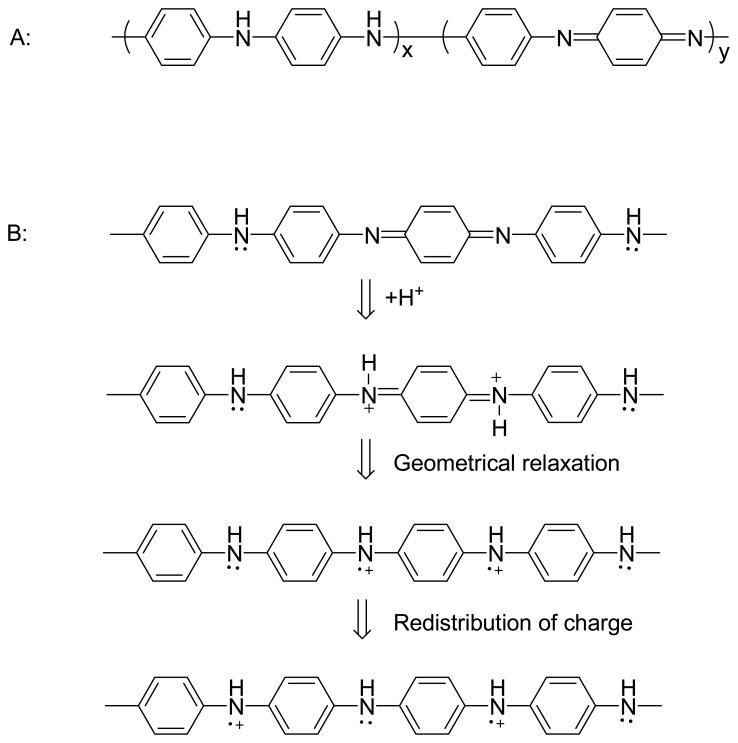
For PAni, the doping process is different. PAni structure shown in Figure 1 is in a totally reduced state. Generally, Pani Chains consist of two types of structural units: Quinoid and Benzenoid Figure 3. These two units can be transformed into each other by redox. Not all types of doped PAni are conductors; the protonated PAni is electrically conductive only when x: y= l: l (Benzenoid: Quinoid=3: l). The protonic acid doping process is illustrated in Scheme 3B. Doping and undoping play key roles in the sensing mechanism of conducting polymer based sensors.
Materials and Methods
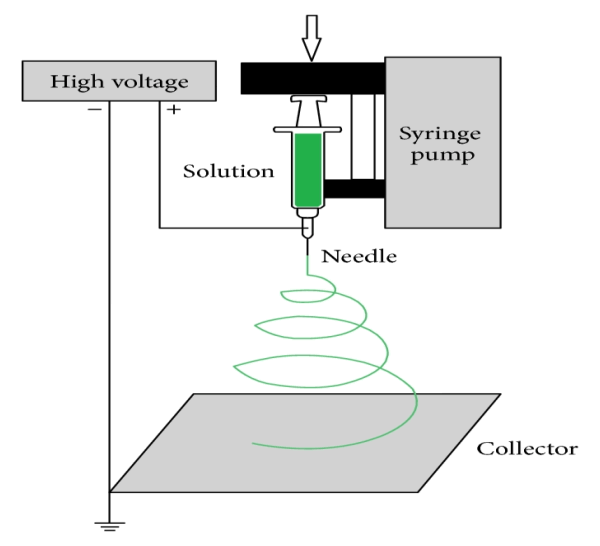
Electrospinning is an efficient, relatively simple and low cost way to produce polymer and composite fibers with diameters ranging from several nanometers to a few micrometers by applying a high voltage to a polymer solution or melt ejected from a micro-syringe pump [9–11]. The ultrafine fibers produced via electrospinning are assembled as a three dimensional structured fibrous membrane with controllable pore structure and high specific surface area. A schematic of the electrospinning process and a typical SEM image of electro spun fibers are shown in Figure 4. Electrospinning is a special case of the electrospray process which uses an electrostatic field to form and accelerate liquid jets from the tip of a needle [12-13]. The surface of a hemispherical liquid drop suspended in equilibrium will be distorted into a conical shape in the presence of an external electric field. This distortion is caused by a balancing of the repulsive force resulting from the induced charge distribution on surface of drop with the surface tension of liquid [14]. A stable jet of liquid can be ejected and accelerated if the applied voltage exceeds this critical voltage. The jet breaks up into droplets as a result of the longitudinal Rayleigh instability caused by surface tension in the case of low viscosity liquids. This process is known as electrospray for applications requiring aerosols composed of sub-micron droplets with narrow distribution [15-16]. For high viscosity liquids, polymer solutions or melts, the jet does not break up, but travels as a jet to the grounded target. A transverse instability or splaying of the jet into two or more smaller jets is observed due to the radial charge repulsion. This process is termed as “electrospinning” and it produces the polymer and composite fibers with diameters on the sub-micron scale 1.
1.2 g of Polyacrylonitrile was dissolved in 10 ml Dimethyl formamide and magnetically stirred for 1 hour to get homogeneous solution. Polyacrylonitrile solution was then loaded into 5 ml disposable plastic syringe with the metallic needle of 0.5 mm diameter for electro spinning. During electrospinning process at room temperature in air, the solution was fed to the tip using a computer controlled syringe pump at a flow rate of 0.2 ml h-1. Positive voltage of 20 KV was applied to the needle of the syringe containing solution and the metallic plate wrapped with aluminium foil was grounded and kept at a distance of 15 cm from the needle of the syringe. The electro spun nanofibers were collected on the conducting foil (aluminium). After electrospinning, the nonwoven membranes of PAN nanofibers were dried at 600C. Further as prepared Polyacrylonitrile nanofibers were used for coating PANI to form PAN/PANI blend nanofibers by using dip coating in- situ chemical oxidative polymerization process.
In the typical process of polymerization, 0.4 M HCl was added to 100 ml deionized water and stirred for about 30 min. It was divided into two equal parts of 50 ml each. 0.4 M aniline was added to one part and stirred thoroughly to form a stable solution ‘A’. 0.4 M ammonium persulfate was added to second part and stirred thoroughly to get the solution ‘B’. The dried Polyacrylonitrile fibers were immersed in the solution ‘A’ in a beaker and the solution ‘B’ was added drop wise from the burette with constant stirring to form the solution ‘C’ which was kept stable at room temperature for 18 hours. The solution ‘C’ turned dark green. Polyacrylonitrile fibers were also coated with green coloured PANI. The fibers were then removed from the solution, washed thoroughly with distilled water before drying at 80oC.
Results and Discussion
SEM
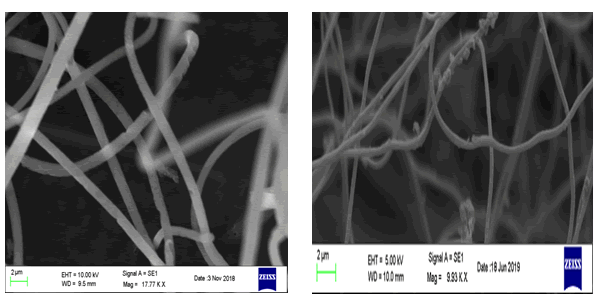
SEM images of pure PAN and PAN/PANI blend nanofibers are shown in Figure 5 a and b. Pure PAN exhibits long and uniformly thin nanofibers compared to that of PAN/PANI blend nanofibers. White coloured clusters of PANI can be seen coated on the PAN nanofibers confirming the formation of PAN/PANI with increased in diameter compared to that of pure PAN.
Gas sensing
A gas sensor is a device which detects the presence of different gases in an area, especially those gases which might be harmful to humans or animals. The development of gas sensor technology has received considerable attention in recent years for monitoring environmental pollution. It is well-known that chemical gas sensor performance features such as sensitivity, selectivity, time response, stability, durability, reproducibility, and reversibility are largely influenced by the properties of the sensing materials used [17–19]. Many kinds of materials such as polymers [20,21], semiconductors [22,23], carbon graphites [24,25], and organic/inorganic composites [26,27] have been used as sensing materials to detect the targeted gases based on various sensing techniques and principles. It is worth noting that the sensitivity of chemical gas sensors is strongly affected by the specific surface of sensing materials [28,29]. A higher specific surface of a sensing material leads to a higher sensor sensitivity, therefore many techniques [30-32] have been adopted to increase the specific surface of sensing films with fine structures, especially to form the nanostructures, taking advantage of the large specific surface of nanostructured materials.
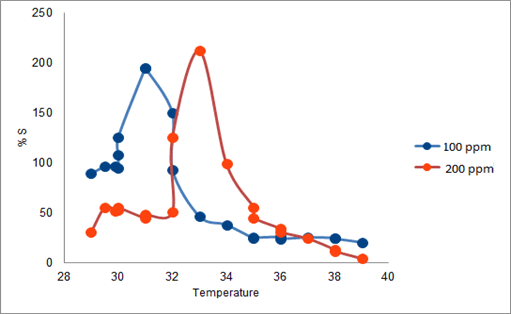
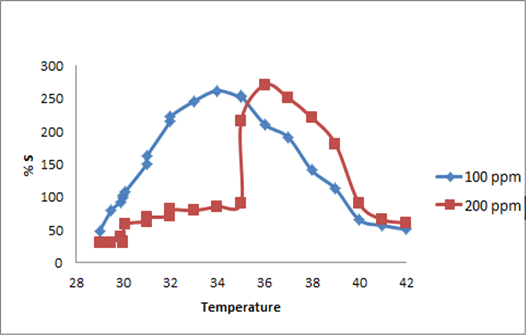
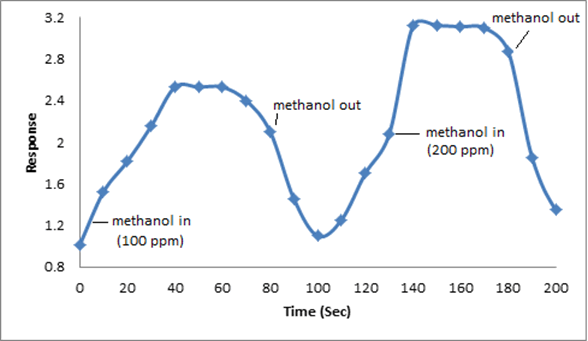
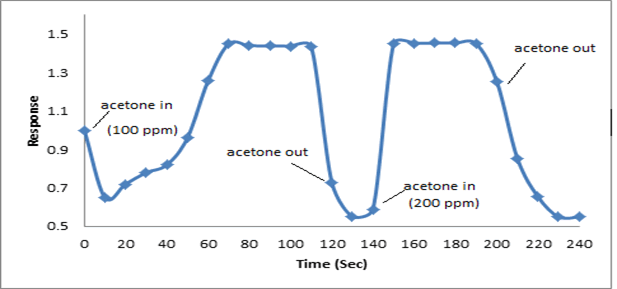
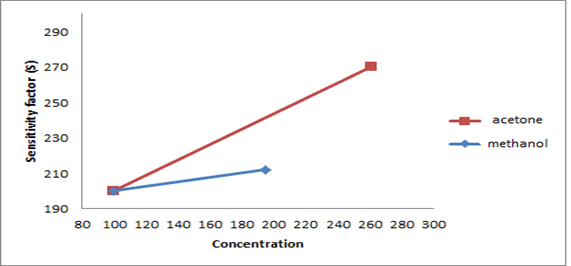
The synthesized PAN/PANI blend nanofibers were used as the receptor for analyte gas molecules. Exposure of the fabricated PAN/PANI sensor showed an increase in the resistance with increasing temperature was observed for methanol and acetone. The PANI layer in the blend acts as the active sensing layer and shows changes in the resistivity on adsorption of the analyte gas molecules. The sensitivity of PAN/ PANI sensor calculated for both methanol and acetone gas for different concentrations are shown in Figure 6 a and b respectively. It was observed that the sensitivity of the sensor increases for higher concentration of methanol and acetone. It is worth mentioning that the sensitivity is highest at near room temperature although the working temperature slightly differs with concentrations. Also, the sensitivity of the sensor increases with increasing concentration of methanol and acetone. The sensing mechanism is found to be governed by protonation and deprotonation phenomena. The resistivity of the blend increases in the presence of methanol due to the reduction or undoping of charge carriers by adsorption of methanol on the surface of blend. The sensitivity factor was found to be 195 & 212 for 100 ppm and 200 ppm of methanol respectively at temperatures of 310C and 330C respectively. For acetone, the sensitivity factor was obtained as 261 and 270 for 100 ppm and 200 ppm at temperatures of 34°C and 36°C respectively. A plot of response (Rg/Ra) and recovery of the sensor for the analyte gases methanol and acetone with respect to time is shown in Figure 7, respectively. It can be seen that the response of the sensor increases for increasing concentration of methanol as well as acetone with the highest response for 200 ppm of acetone. A plot of response versus concentration of the two gases is shown in Figures 8-10 (Tables 1 and 2).
Figure 10:A plot of sensitivity versus concentration of PAN/PANI blend sensor for methanol and acetone.
Table 1. Comparison of the sensitivity, response time, recovery time and operating temperature of as- synthesized PAN/PANI blend nanofibers for methanol.
| Sensing materials | Operatingtemperature (°C) | Sensitivity(S%) | Responsetime (s) | Recoverytime (s) |
|---|---|---|---|---|
| PAN/PANI Blend | 33oC | 212 | 10 sec | 50 sec |
Table 2. Comparison of the sensitivity, response time, recovery time and operating temperature of as-synthesized PAN/PANI blend nanofibres for acetone.
| Sensing materials | Operatingtemperature (°C) | Sensitivity(S%) | Responsetime (s) | Recoverytime (s) |
|---|---|---|---|---|
| PAN/PANI Blend | 36oC | 270 | 20 sec | 20 sec |
Sensing mechanism
Since HCL is used as a dopant during polymerization in this work, PAni is obtained in the form of emeraldine salt. When exposed to methanol during sensing, the HCL molecule donates H+ ions to the PAni Chain, thus increasing the number of positively charged carriers in the material. The process is termed as protonation and gives bipolarons due to excess doping of PAni and results in decrease in the electrical resistivity of the sensor. With the increasing concentration of the analyte methanol, the sensitivity is increased which is attributed to the diffusion of more methanol molecules into the fibre at higher concentration. On the other hand, when the sensor is exposed to the acetone, deprotonation takes place. Acetone molecules of gas take up the hydrogen atom from the PANI Chain and form NH4+ ammonium ions. The emeraldine salt form of the PANI thus changes into the emeraldine base form with decrease in the number of carriers. This leads to the decrease in the number of polarons and the electrical resistivity of the sensor increases. Here too the sensitivity is found to increase with the increasing concentration of the acetone which indicates that more acetone molecules diffuse into the fibre at higher concentration. The response and recovery time are defined as the time to reach 90% of the resistance change during exposure and on removal of gas respectively.
Conclusion
PAN/PANI blend nanofibres have been synthesized successfully by electrospinning and dip-coating polymerization. The formation of PAN/PANI blend is confirmed by SEM analysis. The studies of PAN/ PANI blend as a sensor for methanol and acetone show sensitivity which increases with the increasing concentration of both the gases. The operating temperature of PAN/PANI blend for methanol as well as acetone gas sensor was found to be near room temperature. These results, along with low response time, confirmed the possible use of the prepared material as a potential candidate for the sensing of methanol and acetone in the environmental monitoring safety systems, chemical industry, automotive industry and medical application areas.
Acknowledgement
Author is thankful to VNIT for SEM images and also thankful to Institute of Science, Mumbai for sensing properties.
References
- Nylabder C, Armgrath M, Lundstrom I, et al. An ammonia detector based on a conducting polymer. Int Meet Chem Sens. 1983;203–207.
- Dubbe A. Fundamentals of solid state ionic micro gas sensors. Sens. Actuators B. 2003;88:138–148.
- Zakrzewska K. Mixed oxides as gas sensors. Thin Solid Films. 2001;391:229–238.
- Timmer B, Olthuis W, van den Berg A, et al. Ammonia sensors and their applications-a review. Sens Actuators B. 2005;107:666–677.
- Nicolas-Debarnot D, Poncin-Epaillard F. Polyaniline as a new sensitive layer for gas sensors. Anal Chim Acta. 2003;475:1–15.
- Maksymiuk K. Chemical reactivity of polypyrrole and its relevance to polypyrrole based electrochemical sensors. Electroanalysis. 2006;18:1537–1551.
[Crossref]
- Ameer Q, Adeloju SB. Polypyrrole-based electronic noses for environmental and industrial analysis. Sens Actuators B. 2005;106:541–552.
- MacDiarmid AG. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew Chem Int Edit. 2001;40:2581–2590.
- Reneker D, Chun I. Nanometer diameter fibers of polymer, produced by electrospinning. Nanotechnol. 1996;7:216–223.
- Doshi J, Reneker D. Electrospinning process and applications of electrospun fibers. J Electrost. 1995;35:151–160.
- Fong H, Chun I, Reneker D, et al. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–4592.
- Reneker D, Yarin A, Fong H, et al. Koombhongse S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys. 2000;87:4531–4547.
- Shin Y, Hohman M, Brenner M, et al. Experimental characterization of electrospinning: the electrically forced jet and instabilities.Polymer. 2001;42:9955–9967.
- Deitzel JM, Kleinmeyer J, Harris D, et al. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272.
- Dejuan L, Delamora J. Charge and size distributions of electrospray drops. J. Colloid Interface Sci. 1997;186:280–293.
- Chen D, Pui D. Experimental investigation of scaling laws for electrospraying: dielectric constant effect. Aerosol Sci Tech. 1997;27:367–380.
- Fundamentals of solid state ionic micro gas sensors.
- Gopel W, Schierbaum K. SnO2sensors: current status and future prospects. Sensor Actuator B-Chem. 1995;26:1–12.
- Yamada Y, Seno Y, Masuoka Y, et al. Nitrogen oxides sensing characteristics of Zn2SnO4thin film. Sensor Actuator B-Chem. 1998;49:248–252.
- Slater J, Watt E, Freeman N, et al. Gas and vapor detection with poly (pyrrole) gas sensors. Analyst. 1992;117:1265–1270.
- Slater J, Paynter J, Watt E, et al. Multilayer conducting polymer gas sensor arrays for olfactory sensing.Analyst. 1993;118:379–384.
- Schierbaum K, Weimar U, Gopel W, et al. Comparison of ceramic, thick-film and thin-film chemical sensors based upon SnO2. Sens Actuat B-Chem. 1992;7:709–716.
[Crossref]
- Savage N, Chwierogh B, Ginwalla A, et al. Composite n-p semiconducting titanium oxides as gas sensors.Sens. Actuat. B-Chem. 2001;79:17–27.
- Varghese O, Kichambre P, Gong D, et al. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuat B-Chem. 2001;81:32–41.
- Lukaszewicz J. Carbon materials for chemical sensors: a review. Sens Lett. 2006;4:53–98.
[Crossref]
- Lonergan M, Severin E, Doleman B, et al. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistor.Chem Mater. 1996;8:2298–2312.
- Unde S, Ganu J, Radhakrishnan S. Conducting polymer-based chemical sensor: characteristics and evaluation of polyaniline composite films. Adv Funct Mater. 1996;6:151–157.
- Gopel W. Supramolecular and polymeric structures for gas sensors. Sens Actuat B-Chem. 1995;24:17–32.
- Lee D, Han S, Huh J, et al. Nitrogen oxides-sensing characteristics of WO3-based nanocrystalline thick film gas sensor. Sens Actuat B-Chem. 1999;60:57–63.
- Jin Z, Zhou H, Savinell R, et al. Application of nano-crystalline porous tin oxide thin film for CO sensing. Sens Actuat B-Chem. 1998;52:188–194.
- Dong L, Cui Z, Zhang Z, et al. Gas sensing properties of nano-ZnO prepared by arc plasma method. Nanostruct. Mater. 1997;8:815–823.
-
Matsumiya M., Shin W, Izu N, et al. Nano-structured thin-film Pt catalyst for thermoelectric hydrogen gas sensor. Sens Actuat B-Chem. 2003;93:309–315.