Original Article
, Volume: 15( 3)Synthesis, Characterization, Thermal, DNA binding, DFI, Antioxidant and Anticancer Studies of Bis (methylphenylmethanamine) Dichloroplatinum Complex
- *Correspondence:
- Rakesh Kumar Ameta Department of Chemistry, KADI University, Gandhinagar, Gujarat, India Tel: 9173692349; E-mail: ametarakesh40@gmail.com
Received Date: June 06, 2017 Accepted Date: June 28, 2017 Published Date: July 03, 2017
Citation: Ameta RK, Sharma NK, Sangani CB, et al. Synthesis, Characterization, Thermal, DNA binding, DFI, Antioxidant and Anticancer Studies of Bis Complex. Int J Chem Sci. 2017;15(3):152
Abstract
Bis (methylphenylmethanamine) dichloroplatinum (MMCP), a new Pt (II) complex, has been synthesized and characterized with different spectroscopic means. Thermal analysis expressed its heat absorption capacity and weight loss with respect to temperature. UV/Vis study performed in DMF and DMSO/phosphate buffer with 10, 30, 50, 70 and 90 μM that inveterate stability of the complex. The DNA binding activity studied with absorption spectroscopy, viscosity, surface tension, sound velocity, refractive index, conductivity and zeta potential which has confirmed its strong DNA interaction. The DFI study with comparison of viscosity and surface tension has been analyzed which explored its anticancer nature. The DPPH* scavenging effect was carried out with 50, 100, 150, 200, 250, 300, 350 and 400 μM which showed its antioxidant activity due to its terminating action against reactive species. In vitro anticancer results on MCF-7 cell line showed its anticancer activity.
Keywords
DSC; DFI; Viscosity; DNA interaction
Introduction
Platinum complexes have been proven effective cancer curing drugs and hence the research in platinum metal chemistry has been intensified for last many decades in treatment of several incurable diseases such as cancer. For instance, the discovery of cisplatin, a transition metal complex, has been fascinated chemists to draw attention for developing materials to be used in curing cancer [1]. Although, due to several side-effects of the cisplatin, its uses are restricted in curing disease, for instance nephrotoxicity and drug resistance [1-3]. Thereby, several organic compounds containing heteroatoms have been used in vitro and in vivo studies either directly or by coordination with various metals, especially, with platinum. The metal coordinated compounds along with their derivatives have been found to be effective for their anticancer activities and reducing cytotoxicity. The DNA binding activity either by intercalation or covalent or coordination with metal has the first and prime most aspect of anticancer compounds, because metal binding blocks the DNA replication and further cell growth. Therefore, the organic compounds with aromatic ring and amine group as side arms have been studied previously and found to be intercalator to DNA [4-5]. Several other studies have also been revealed that the DNA binding activity is enhanced by coordination with platinum through Pt-DNA base pair interactions [6-8]. In general, the DNA is always remains a primary molecular target for any anticancer drug because the DNA is polar biomolecules lone pair electrons on nitrogen bases. Also, the DNA binding of organic compounds coordinated with platinum have been extensively studied due to their potentiality in anticancer activity where the DNA structure dependent electron transfer probes and sequence-specific cleaving agents [9-10] have been favorable factors in the process. Many Pt based drug are used for specific cleaving and specific probes thereby diagnosing the DNA altered structure [8,10]. Drug-DNA interaction ascertains an extent and a mode of DNA binding as well as chemotherapeutic potential of the compounds [11]. Apart from the DNA binding, the antioxidant property has also been proven the anticancer activity of the compounds, and several studies have been reported where the antioxidant property has been found responsible for their anticancer activity [12-14]. Thereby, synthesis, DNA and free radical have been targeted to design novel antitumor agents with a low reactivity in the blood stream and in non-tumoral tissues [6-9]. With this interest, we synthesized platinum coordinated, MMCP, and its anticancer activity, DNA and antioxidant properties have studied which show its anticancer aspect. Thus, this study could be applicable in medicinal field, especially, in treatment of cancer.
Experimental
Materials and methods
Table 1 reports chemicals used in this work. Potassium tetrachloropalatinate (K2PtCl4, 99.99%, Sigma Aldrich) and methylphenylmethanamine (>99.5%, Sigma Aldrich) were used as received. The MMCP was characterized with FTIR, 1H NMR, 13C NMR, Q-TOF LC/MS and CHN elemental analyzer as per literature process. The conductance was measured with LABINDIA, PICO+model conductivity meter at 25°C for 1 × 10-3 M solution in DMF and DMSO. Aqueous 0.1 M (12.88 mS/cm), 0.01 M (1.413 mS/cm) and 0.001 M (147 μS/cm) KCl solutions were used for calibration standard for conductivity meter.
| Reactants | Source | % Purity |
|---|---|---|
| K2PtCl4 | Sigma Eldrich | 99.99 |
| Methylphenylmethanamine | Sigma Eldrich | 99.95 |
Table 1. Chemical used in this work with source and purity.
Synthesis method
Initially, K2PtCl4 and methylphenylmethanamine were dissolved separately in freshly prepared solvent of absolute ethanol (4 mL) and Milli-Q water (6 mL) using 1 MLH magnetic stirrer, REMI. Then methylphenylmethanamine solution (pH=12.01) was added drop wise in K2PtCl4 solution (pH=7.1) with a continuous stirring at room temperature. The K2PtCl4 and methylphenylmethanamine were taken in 1:2 molar ratio, after 24 h, a yellow precipitate was formed which was filtered off, washed with 25 mL water and ethanol several times and kept overnight in vacuum oven at room temperature for absolute dryness.
Characterization data
Yield: 80%. Anal. Calculated For C16H22N2Cl2Pt: C, 37.80; H, 4.36; N, 5.51%. Found: C, 37.41; H, 4.10; N, 5.12%. IR (KBr): ν (NH) 3206.1, 3177.6 cm-1, ν (Ph, C=C) 1496, 1455 cm-1, ν (mono substitute Ph) 748.59 cm-1, ν (C-N) 1063 cm-1, ν (Pt-N) 483.67 cm-1, ν (Pt-Cl) 329 cm-1. 1H NMR δH (500 MHz; DMSO-d6; Me4Si) 2.147 (1H, s, PhCH2 NHCH3), 3.653 (3H, s, PhCH2 NHCH3), 5.375 (2H, s, PhCH2 NHCH3), 7.497-7.417 (m, PhCH2 NHCH3).13C NMR δH (500 MHz; DMSO-d6; Me4Si) 136.01(C1), 130.49(C2 and C6), 131.10(C3 and C5), 128.49(C4), 57.88(C7), 40.82(C8) +veESI-MS: m/z 526.1178 [M+1+H2O] (calc. for [C16H22N2Cl2Pt]=507.08).
Thermal analysis
The thermal analysis was determined with Intracooler Perkin-Elmer DSC-6000 and TGA, thermo scientific instrument with 5 and 10°C/min heating and cooling rate. 2 mg sample was taken for measurement where platinum pen was used as reference as well as sample holder.
UV/V is study
It was performed with DMF and DMSO/phosphate buffer of pH 7.2 where buffer solution was prepared by adding 30 mL of 0.1 M aqueous NaOH solution into 70 mL of 0.1 M aqueous KH2PO4 solution. The pH of resultant buffer solution was checked with RS-232 modeled Cyber scan pH 2100, EUTECH pH meter instrument.
DNA binding activity
Solutions were prepared w/v with ± 0.01 mg using Mettler Toledo electronic Kern balance ABS 220-4. Absorption spectra were recorded with Spectro 2060 plus model UV/Vis over 200-600 nm on 1 cm path length cuvette. The CT-DNA (Sigma) was used as received (analytical grade). Tris-HCl buffer (10 M, pH=7.2) prepared in Milli-Q water, was used for DNA stock solution, absorption titration, viscosity and surface tension measurements. The DNA concentration was determined by absorption spectroscopy with molar absorptivity (6600 M −1 cm −1) at 260 nm [15,16]. The CT-DNA in buffer gave a ratio of UV absorbance at 260 and 280 nm of 1.8-1.9, indicating that the DNA was sufficiently free of protein [17-20]. Absorption titrations in Tris-buffer were performed with 10, 30, 50, 70 and 90 μM to which the DNA stock solutions (50 μM) was added, (ri=[compound]/[DNA]=0.2, 0.6, 1, 1.4, 1.8). MMCP-DNA solutions were incubated at room temperature for 15 min before taking absorption spectra and their densities were measured with ± 10-3 kg m -3 accuracy by Anton Paar Density and Sound velocity meter DSA 5000 M (DMA instruction manual; Anton Paar, Graz, Austria). The refractive index was measured with ± 10 -4 Rudolph Research analytical J series Refractometer model-57. The surface of Refractometer prism was cleaned with HPLC grade ethanol and a lens wiper in measurements. This process ensured complete cleaning with no stains or air bubbles left on the prism surface. Each measurement was repeated at least twice to check reproducibility, and their pendent drop numbers (PDN) and viscous flow times (VFT) for surface tension and viscosity measurements respectively, were measured with Borosil Mansingh Survismeter (BMS) [21,22]. Data of viscosity are presented as (η/η0)1/3 versus binding ratio, the η is viscosity of DNA with MMCP while the η0 is a viscosity of DNA alone [23]. Zeta potential of DNA solution with and without compound was measured with LABINDIA, PICO+conductivity and Microtrac Zetatrac, U2771, DLS respectively. Auto suspended solution of alumina suspension (400206-100) was used for zeta potential standard. Initially for DMSO+Tris buffer a set-zero was made for zeta potential. For both the measurements, the DNA concentration was kept constant and concentrations of MMCP were varied from 10 to 90 μM with 20 μM interval.
Antioxidant activity
The free radical scavenging effect was determined using DPPH free radical [24] where both the stock solutions of 1000 uM of the samples and 0.002% DPPH• were prepared in DMSO+water (1:1). One mL of 0.002% DPPH• solution was added in 1 mL of 50, 100, 150, 200, 250, 300, 350 and 400 μM of the MMCP separately to prepare the test samples. Both the components as reacting mixtures were thoroughly mixed by shaking the test tubes vigorously and incubated at 25°C by keeping in a water bath in dark for 60 min. An absorbance was measured at 517 nm with UV/Vis spectrophotometer. The interaction activity was identified in terms of a decrease in absorbance of DPPH [25] and calculated with following formula:
Interaction activity%=(A0 – AS/A0) × 100
AS is an absorbance of DPPH• with a tested compound and A0 is of DPPH• without a tested compound (control). The data calculated are presented as means ± SD of three determinations.
Anticancer activity
Cell lines and culture conditions: Human breast cancer cell lines (MCF7) were obtained from NCI, USA, and grown in minimal essential medium (MEM). Eagles media were supplemented with 10% heat inactivated fetal bovine serum (FBS, Sigma-Aldrich), 2 mM L-glutamine and 1 mM sodium pyruvate (Hyclone) in humidified CO2 incubators.
Assay of cytotoxicity in cancer cell lines: Cytotoxicity of MMCP was determined by SRB assay where ≈ 5,000 cells were seeded into each well of 96-well clear flat bottom polystyrene tissue culture plate and incubated for 2 h in MEM. An additional 190 μL cell suspension was added in each well containing 10 μL test sample in 10% DMSO with 10 μL Adriamycin (Doxorubicin) and cisplatin as positive drug control. Each experiment was carried out in 3 replicate wells. After an incubation of 48 h, 100 μL of 0.057% SRB solution (w/v) was added into each well. Then 200 μL of 10 mM Tris base solution (pH 10.5) was added into each well and was shaken smoothly. The cell viability was assayed by absorption at 510 nm with a micro plate reader. The experiments were repeated thrice with 5 replicates each time and 99% reproducibility was obtained.
Results and Discussion
Synthesis and characterization
Reaction for synthesis of MMCP was conducted between K2PtCl4 and methylphenylmethanamine in ethanol-aqueous solution accordingly reaction scheme as under.
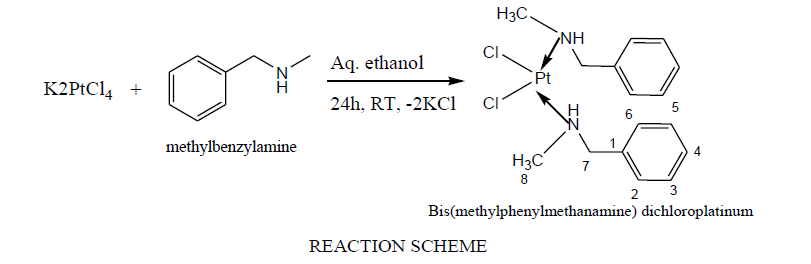
In FTIR, the -NH stretching frequency of MMCP was from 3177.6 cm-1. The effects of d orbitals of the metal make a difference in the vibration of Pt-Cl and C-N, instance, the Pt-Cl vibration band found at 329 cm-1 while the 1087 to 1026.5 cm-1 inferred the C-N bending vibration bands. The platinum nitrogen coordinate bond vibration was identified at 483.67 cm-1 [26-28]. In proton NMR, the single proton attached with nitrogen appeared with singlet having δ=2.147. The 3H of methyl groups attached on nitrogen have found at δ value of 3.653. The protons of phenyl ring have appeared with multiplate in range of 7.497-7.417 δ while 2H of methylene group attached with nitrogen and phenyl ring appeared with 5.375 δ value. The carbons of benzene ring in 13CNMR have appeared from 136.49 to 128.49 δ, while another carbon such as benzyl and nitrogen attached methyl carbon have appeared at 57.88 and 40.82 δ respectively. The ESI+ve mass spectra showed [M+1] having additive mass of H2O, and found 526.11.
Thermal analysis
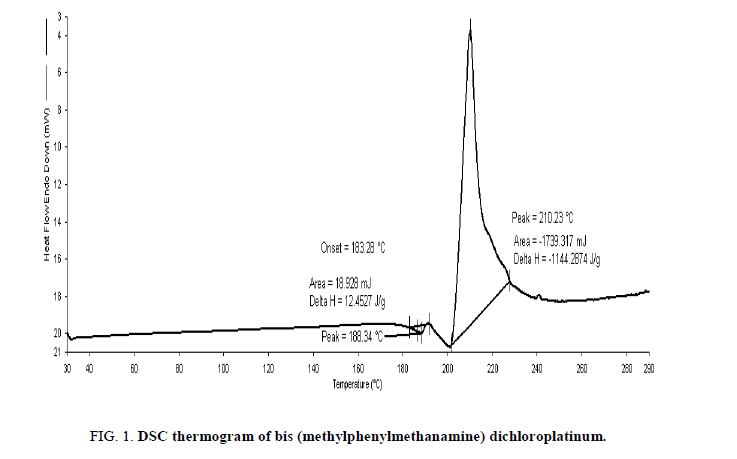
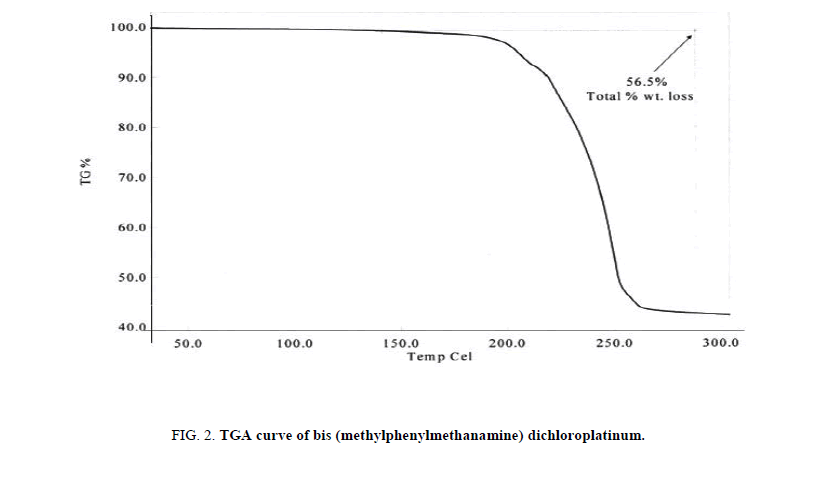
A heat quantity which is radiated or absorbed excessively on the basis of a temperature difference between the sample and reference material was studied with DSC [29,30]. The DSC, a thermal process, analyzes change in physical properties on rise of temperature with time and is widely used for medicinal purposes [31]. On a temperature change, a disruption of molecular forces occurs and depicts a nature and stabilization of the compounds [32]. The complex showed two transition states (endothermic as well as exothermic) with a rise in temperature, where it depicts an effect on onset point, peak and fusion enthalpy (Figure. 1). The endothermic transition showed an onset point at 183.28°C considered as melting point. For the transitions endothermic and exothermic, the area and delta H (enthalpy) obtained 18.928 mJ, 12.4527 J/g and -1739.317 mJ, -1144.2874 J/g respectively. As per TGA study (Figure. 2), the complex is thermally stable upto 200°C but above this it lost substantial mass in a fairly temperature range (200°C-260°C) at rate of 1.1 g/°C. This mass loss (56.5%) could be due to a loss of two molecules of C6H5CH2NH2 because these molecules were attached to the Pt (IV) by coordinative bonding which is weaker than covalent bonding. Thus, the less enthalpy is required to dissociate the legend molecules and such thermodynamic response of the Pt complex could be most fascinating in developing the wider entropy based chemical applications. For example, if the complex is designed in any thermally induced applications for developing higher entropy due to free legend molecules obtained on the dissociations and is free available in the process. It noticed that the decomposition product is stable up to 260°C. The MMCP could develop remarkable thermodynamics and kinetics for developing a desired level of the entropy on heating to 260°C where the Pt (II) and the C6H5CH2NHCH3 ligand molecules are available for developing the collisions of definite frequencies. Such remarkable development in TGA study of the complex could be used as most interesting piezoelectric materials for high sensitive applications in generating the stretching frequencies and oscillations of defense applications in coding and decoding the secret messages in strategic areas. Not only these applications be developed as an advanced science with platinum complexes but the same materials could be used as excellent sensors in case of entropy and entropy induced interactions and reactions, thin film coating, wafer for CMOS and MEM technologies, Schottky and Frenkel effects in developing the docked or blended materials to designing the electric and thermal conductivities for semiconducting materials or superconducting sciences.
UV/Vis analysis
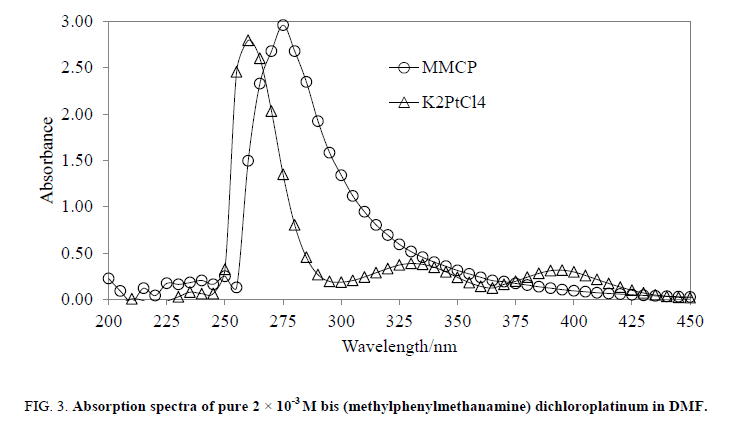
UV/Vis spectra were recorded in DMF for 2 × 10-3 molar solution over 200nm-600 nm wavelengths. For making comparison, the UV/Vis spectra for both the MMCP and K2PtCl4 have obtained with the same concentration and solvent. The pure K2PtCl4 exhibited three λmax at 260 nm, 335 nm and 400 nm (Figure. 3) whereas MMCP showed only one λmax at 275 nm. This is may be, due to, in pure K2PtCl4, empty d orbitals are available for transition while in MMCP these empty orbitals are filled by electron pairs of the ligands, and are not available for transition. One λmax for MMCP has showed that only one transition occurred due to ligand to metal electron pair transfer. And also, the λmax (260 nm K2PtCl4) shifted (275 nm, compound) which showed formation of methylphenylmethanamine coordinated platinum transitions complex, MMCP.
Figure 3: Absorption spectra of pure 2 × 10-3 M bis (methylphenylmethanamine) dichloroplatinum in DMF.
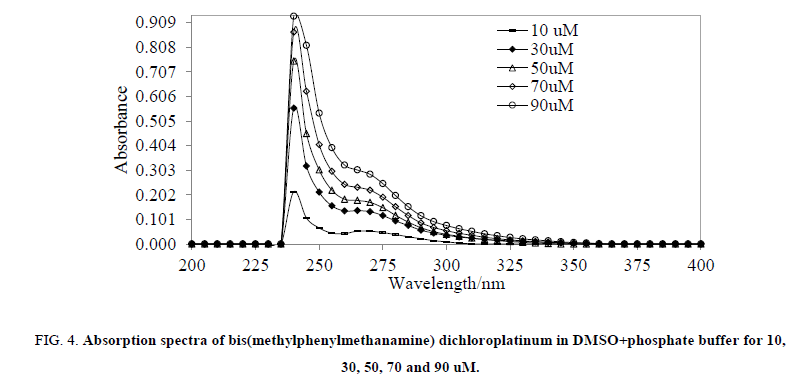
The UV-Vis spectral behavior was also investigated in DMSO/phosphate buffer (pH=7.2) for 10 to 90 uM with 20 uM interval (Figure. 4). The MMCP showed λmax=270 nm in both the solutions which confirmed its stability, in support, the low molar conductivities in DMF and DMSO showed its greater stability. Substitution of chloride from methylphenylmethanamine ligand which has less trans directing character than chloride [33], and due to absence of vacant π or π* orbitals to accept electron from platinum, its attachment occurred at cis position of the platinum according to trans effect of the square planer complexes by replacing the chloride [33]. Also, the obtained platinum chloride vibration at 329 cm-1 in comparison with other reported cis Pt complexes, in those it was found within 340 cm-1-320 cm-1 [34-36] shows that the obtained product is cis.
Figure 4: Absorption spectra of bis(methylphenylmethanamine) dichloroplatinum in DMSO+phosphate buffer for 10, 30, 50, 70 and 90 uM.
DNA binding activity
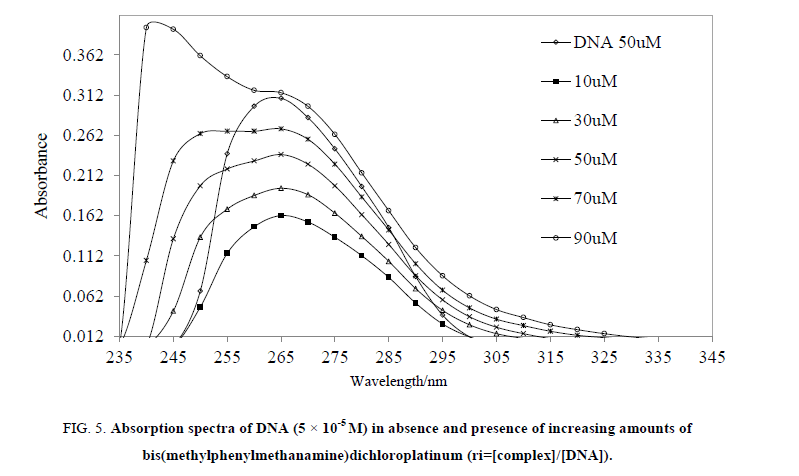
Absorption spectroscopy: DNA binding is an introducing mechanism for a concept of structure activity relationship of the complex where its absorption spectral study analyzes this ability. Thus, the synthesized MMCP has treated with CT DNA to measure its binding strength. Generally, an extent of hypochromism reveals intercalative binding strength which could be attributed to the interaction being developed with the DNA bases, and considered as a characteristic for intercalation [37,38]. Similarly, a hyperchromic effect might be ascribed to an external contact or to a partial uncoiling of DNA structure being developed may be due to exposing to more bases of the DNA with an electrostatic binding [39,40]. A significant hypochromic effect was observed in absorption titration of interacted DNA with MMCP which exposed an intercalation phenomenon between compound and base pairs of DNA [37,38] (Figure. 5). In spectrophotometric titration, the intercalating strengths depend on size and electron density of interacting aromatic rings including –NH-, an amine group as well as due to a combined effect of hydrophobic and hydrophilic interactions [37-40]. Thereby, the hydrophobic (benzyl group) and hydrophilic (an amine) part in compound, showed an intercalation with DNA and confirmed in spectrophotometric analysis.
Figure 5: Absorption spectra of DNA (5 × 10-5 M) in absence and presence of increasing amounts of bis(methylphenylmethanamine)dichloroplatinum (ri=[complex]/[DNA]).
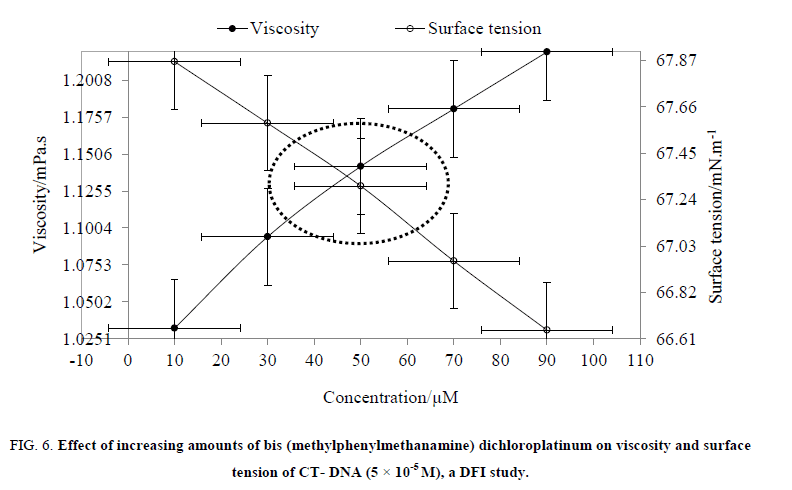
Physicochemical analysis: Binding mode of compound with DNA was also studied with physicochemical properties (Table 2). The density, sound velocity and refractive index of MMCP-DNA solution have decreased due to interaction between DNA and MMCP. A decrease in these values inferred that the DNA structure got compressed and produced compactness to the DNA-MMCP system with stronger intermolecular forces. It also inferred that MMCP completely interacted with the DNA due to aromatic ring at side arm. For extreme support a DNA binding mode of compound, the spectroscopic data are not sufficient and need support of other data, such as viscosity which is least ambiguous and most critical tests for a classical intercalation [28]. The viscosity is a classical intercalative mode where with the interaction of compound, it is of DNA solution increased due to increase in overall DNA length and decreased due to decrease in overall DNA length [41]. But, a partial, non-classical intercalation produces bend or kink to the DNA helix and reduces its effective length concomitantly [42]. For example, the interaction with cisplatin causes a decrease in viscosity due to reducing its axil length. Thus, partial intercalator decreases relative viscosity by reducing the axial length of DNA, whereas the classical organic intercalator such as ethidium bromide increases a relative viscosity by increasing an axial length of the DNA [43,44]. To observe a covalent binding or classical organic interaction, the values of relative specific viscosity (η/η0)1/3 (η0 and η are specific viscosity contributions of DNA with and without MMCP) were plotted against 1/R (R=[DNA]/[compound]=0.2, 0.6, 1.0, 1.4, 1.8) (Figure. 6). It was observed that an increase in MMCP concentration has led to an increase of DNA viscosity (Figure. 6). Thus, we may deduce that the MMCP is a DNA intercalator. For ethidium bromide, the relative viscosity of DNA is increased with a slope between 0 and 0.94 [45], whereas, with a slope of 0.0023, the relative viscosity of DNA increased for the MMCP. A decrease in slope value may be, due to, an interaction of MMCP with DNA made DNA longer which reasonably believed that may be other interaction between DNA and MMCP occurred. The obtained value of binding constant is lower than values of other platinum complex reported such as 2.0 × 104 M −1 [46], 1.37 × 104 M −1 and 9.62 × 103 M −1 [47], 0.71, 0.53, 3.50, 1.38 [48], which may be attributed due to hydrogen bonding and there is no covalent bonding. Since MMCP showed low binding constant but also showed stronger interaction with DNA that has been proved by viscosity data analysis, thus this complex is of much better uses than others. Apart from the viscosity, the surface tension is interaction probing thermodynamic indicator where it tells about disruption of intramolecular forces of DNA which are exist between nitrogen bases and act as an evidence for stronger interaction having higher viscosity [28]. This force disruption is measured with a decrease in surface tension, because surface tension is a cohesive property, and when it is decreased from its initial value then means a force disruption has happened. Therefore, we have studied surface tension of DNA with addition of increasing amounts of MMCP (1/R=0.2, 0.6, 1.0, 1.4, 1.8) (Figure. 6). The resultant surface tension of DNA has confirmed that cohesivity or intramolecular interaction of DNA molecules have destroyed on interaction with MMCP.
Figure 6: Effect of increasing amounts of bis (methylphenylmethanamine) dichloroplatinum on viscosity and surface tension of CT- DNA (5 × 10-5 M), a DFI study.
| Conc. uM | 103.ρ kg m-3 | u ms-1 | uri | ? mPa.s | ?r | γ mN.m-1 |
|---|---|---|---|---|---|---|
| 10 | 1.016486 | 1566.90 | 1.3524 | 1.0323 | 1.0383 | 67.87 |
| 30 | 1.019587 | 1578.00 | 1.3556 | 1.0944 | 1.0587 | 67.59 |
| 50 | 1.021997 | 1585.84 | 1.3580 | 1.1422 | 1.0739 | 67.30 |
| 70 | 1.024802 | 1597.11 | 1.3606 | 1.1813 | 1.0860 | 66.96 |
| 90 | 1.026957 | 1609.85 | 1.3630 | 1.2200 | 1.0978 | 66.65 |
Estimated uncertainties in density, speed of sound, refractive index, viscosity and surface tension measurements were less than ± 1 × 10-6 g. cm -3, ± 0.01 m. s-1, ± 1. 10-1, ± 10 -4 mPa.s and ± 10-2 mN/m respectively. The uncertainties in temperature are ± 0.01°C.
Table 2. Physicochemical properties: density (ρ), sound velocity (u), refractive index (uri), viscosity (η), relative viscosity (ηr) and surface tension (γ) of bis (methylphenylmethanamine) dichloroplatinum+DNA solution.
DFI study
DFI is known as drug-friccohesity-interaction as a correlation between surface tension and viscosity of compound-DNA, reported elsewhere [28]. This study reveals about mechanism of interaction of drug or complex with DNA which leads to anticancer effect of the drug. Therefore, in this context, a critical and comparative analysis of surface tension and viscosity of DNA-MMCP solution have conducted (Figure. 6). It also proves efficacy of interacting activity of MMCP with its fluidity as well as with adsorptivity. We found a converse relation between surface tension and viscosity where the MMCP developed higher viscosities and lower surface tensions after interaction with DNA solution in compare to pure DNA (Figure. 6). Since, surface tension is decreased due to disruption of intermolecular force, therefore, MMCP, initially, disrupted hydrogen bonding of DNA nitrogen bases (distortion of intermolecular forces). On the other hand, the viscosity is increased due to interaction between dissimilar molecules, so, secondly, the MMCP has intercalated between nitrogen bases of DNA, and increased viscosity. Thus, the analysis of diminution in surface tension and increase in viscosity of resultant MMCP-DNA solution than pure DNA solution inferred that MMCP first broke the DNA geometry and then made new interaction bond with DNA base pairs. Thus, the comparative analysis of surface tension and viscosity of MMCP-DNA has explained DFI mechanism, and proved its anticancer nature. Since, to bind with DNA is a first characteristic of an anticancer complex, and DFI study suggests that MMCP has strong binding affinity with DNA, thus it could be applicable in treatment of cancer.
Conductivity and zeta potential analysis
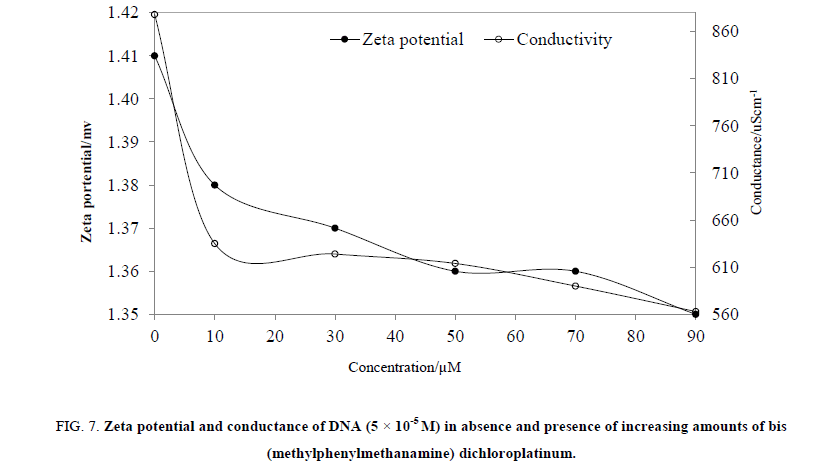
Conductivity and zeta potential for MMCP-DNA has been analyzed and reported in Table 3. The DNA solution of 50 μM had showed 878 μS/cm conductivity and with increasing amount of the MMCP, the same has decreased whereas the viscosity increased (Figure. 7). The DNA molecule is a negatively charged due to phosphate groups, but by interaction, the negative charge density is decreased due to a positively charged metal that balances the charge density. Thus, the interaction of MMCP with DNA interferes in movement of charged DNA with a lower conductivity. Similarly, the zeta potential has decreased of resultant MMCP-DNA (Figure. 7), due to increase in Coulombic interaction. Both the data of conductivities and zeta potential confirmed that the DNA is structurally modified as a result of MMCP. Since, strong interaction of MMCP with DNA confirmed with decrease in conductivities and zeta potential, and DFI also suggested about stronger interaction of MMCP with DNA. Thus, the DFI model is also supported by the conductivities and zeta potential data of the similar mixtures.
Figure 7: Zeta potential and conductance of DNA (5 × 10-5 M) in absence and presence of increasing amounts of bis (methylphenylmethanamine) dichloroplatinum.
| Concentration uM |
Zeta Potential mv |
Conductance uScm-1 |
|---|---|---|
| 10 | 1.38 | 635 |
| 30 | 1.37 | 624 |
| 50 | 1.36 | 614 |
| 70 | 1.36 | 590 |
| 90 | 1.35 | 563 |
| Pure DNA (50 uM) | 1.41 | 878 |
Table 3. Conductance and zeta potential of pure DNA and DNA+compound.
Antioxidant activity
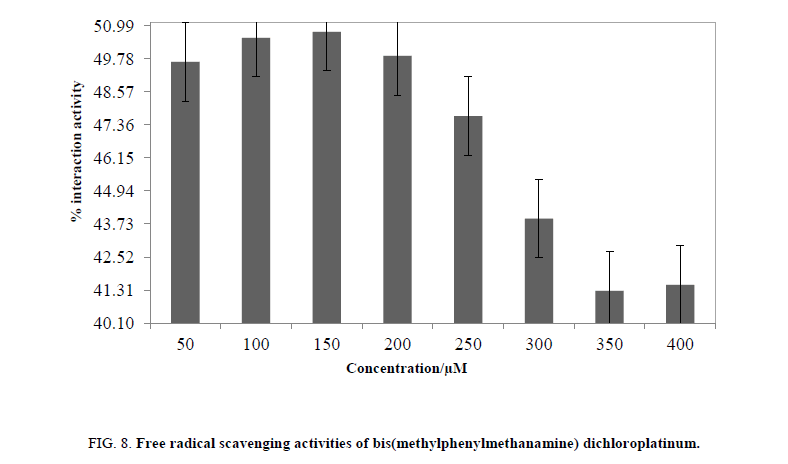
Generally, the antioxidant nature of compound is attributed for anticancer activity up to certain extent. Thus, the antioxidant activity for supporting the anticancer nature of MMCP studied, and was analyzed by ascertaining the scavenging effect of a stable free radical 1, 1-2, 5 diphenyl-2-picrylhydrazyl (DPPH) as per literature procedure [24,25]. A percent scavenging activity was determined in a concentration-dependent mode with a comparison of the DPPH free radical’s absorption at 517 nm [24,25]. The DPPH free radical’s absorption at 517 nm in DMSO+water was 0.440 to 0.445, a maximum. 50 to 400 μM at an interval of 50 μM, have shown a decrease in absorption, which inferred their antioxidant activities [24]. The decrease in absorbance from absorbance of pure DPPH radical was encouraging factor between the scavenging effect of a stable DPPH and the chosen data. The complex expressed antioxidant activity may be, due to that it formed radical by removing hydrogen and stabilized DPPH free radical. As per Meyerstein's manuscript [49], it may also that as the MMCP was dissolved in DMSO, clearly DPPH radical reacted with the DMSO and not with MMCP but antioxidant activity of MMCP is due to its role in inhibition and termination of the radical chain process. At 100 and 150 μM MMCP showed maximum% activity<50% (Figure. 1).
Anticancer activity
The complex was tested in vitro against MCF7 human breast tumor cell line in comparison to Adriamycin (ADR) and cisplatin by colorimetric micro culture 2-(3-diethylamino-6-diethylazaniumylidene-xanthen-9-yl)-5-sulfo-benzenesulfonate (SRB) assay [50]. The MCF7 interaction activity for 10, 20, 40 and 80 μg/mL MMCP was estimated the GI50, TGI and LC50 in μg/mL (Table 4) which inferred a 50% growth inhibition, resulted total growth inhibition and a net loss of 50% cells after treatment respectively. The less than 10 μg/mL of GI50 depicts anticancer activity with respect to ADR and Cisplatin. Data analysis for MMCP (GI50<10) has shown anticancer activity as compared to standard ADR and Cisplatin. This analysis has inferred that anticancer nature of MMCP associated with the methyl substitution of benzylamine. Several platinum complexes have been synthesized for treatment of the cancer but our focus was to investigate the anticancer activity of synthesized platinum (II) complexes with methyl substituted benzylamine as ligands. Thus, the complex most probably could have bright prospects to be effective anticancer drug, especially, against solid tumors.
| Entry | Complexes | LC50 | TGI | GI50 |
|---|---|---|---|---|
| 1 | MMCP | >80 | 40.8 | <10 |
| 2 | ADR (std) | 62.4 | 30.6 | <10 |
| 3 | Cisplatin (std) | >30 | >30 | <10 |
Table 4. Anticancer activity analysis data for MMCP.
Conclusion
Synthesis, thermal study, DNA binding, antioxidant and anticancer activities of MMCP have been reported. Thermal studies depicted and confirmed the structural changes with its stability respect to heating. The surface tension decreased from 5 to 7% and viscosity increased from 11% to 32% for pure DNA after binding with MMCP with the concentration. Therefore, the DNA binding study confirmed its anticancer nature and for further support the DFI study has also confirmed the same. For additional support in analyzing the anticancer nature, the antioxidant activities have been determined where the complex has exhibited an effective antioxidant activity, and the scavenging effect was noted from 41% to 50%. Thus, the DNA binding and antioxidant activities have confirmed its anticancer nature. Such things have been proven with its anticancer analysis where it showed anticancer activity. Also, further experiments in vitro for determining its role on apoptotic and proliferate pathways in tumor cell lines are in progress.
Acknowledgment
Authors are thankful to Central University of Gujarat, Gandhinagar, for financial, infrastructural support and experimental facilities.
References
- Rosenberg B. A spectrophotometric method for analysing mixtures of cis- and trans-diamminedichloroplatinum (II) in solution. Interdiscip Sci Rev. 1978;3:134-47.
- Dwyer PJ, Stevenson JP, Johnson SW. Clinical pharmacokinetics and administration of established platinum drugs. Drugs. 2000;59:19-26.
- Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma, Drugs. 2000;59:1.
- Reedijk J, Teuben JM, Lippert B, et al. Chemistry and biochemistry of a leading anticancer drug. Chimica Acta. 1999;pp:339-62.
- Reedijk J. Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell. Chem Rev. 1999;99:2499.
- Farrel N, Ha TTB, Souchard JP, et al. Towards antitumor active trans-platinum compounds. J Med Chem. 1989;32:2240-41.
- Natile G, Coluccia M. Current status of trans-platinum compounds in cancer therapy. Coord Chem Rev. 2001;216:383-410.
- Kalinowska-Lis U. Ochocki J, Matlawska-Wasowska K. Trans geometry in antitumor platinum Complexes. Coor Chem Rev. 2008;252:1328-45.
- Erkkila KE, Odom DT, Barton JK. Recognition and reaction of metallointercalators with DNA. Chem Rev. 1999;99:2777-95.
- Lincoln HP, Suh D, Norden B. Interaction of DELTA and LAMBDA [Ru(phen) 2DPPZ] 2+with DNA: A calorimetric and equilibrium binding study. J Am Chem Society. 1995;117: 4788-96.
- Metcalfe C, Thomas JA. Kinetically inert transition metal complexes that reversibly bind to DNA. Chem Soc Rev. 2003;32:215-24.
- Jianguo C, Xian X, Xuefei C, et al. Flavonoids in tropical citrus species. Food and Chem Toxicol. 2013;51:242-50.
- Loguercio C, Festi D. Silybin and the liver: From basic research to clinical practice. World J Gastroenterol. 2011;17:2288.
- Sanaa Shanab MM, Soha Mostafa SM, Emad Shalaby A. et al. Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. J Trop Biomed. 2012;2:608-15.
- Zhao P, Xu LC, Huang JW, et al. Interaction of a tricationic meso-substituted porphyrin with guanine-containing polyribonucleotides of various structures. J Biophys Chem. 2008;135:102-9.
- Zhao P, Xu LC, Huang JW, et al. Molecular fluorescence, phosphorescence, and chemiluminescence spectrometry, spectrochim. Acta A Mol Biomol Spectroscopy. 2008;71:1216-23.
- Zhao P, Xu LC, Huang JW, et al. DNA-Binding and Photocleavage Properties of Cationic Porphyrin-Anthraquinone Hybrids with Different Lengths of Links. Bioorg Chem. 2008;36:278-87.
- Kurita N, Kobayashi K. Further quantum mechanical evidence that difluorotoluene does not hydrogen bond. Comput Chem. 2000;24:351-55.
- Lu JZ, Du YF, Guo HW. Two oxovanadium complexes incorporating thiosemicarbazones: synthesis, characterization and DNA-binding studies. J Coord Chem. 2011;64:1229-39.
- Du YF, Lu JZ, Guo HW, et al. DNA binding and photocleavage properties of two mixed-ligand oxovanadium complexes. Trans Met Chem. 2010;35:859-64.
- Singh M. Survismeter-type I and II for surface tension, viscosity measurements of liquids for academic and research and development studies. J Biophy and Biophys Methods. 2006;67:151-61.
- Singh M. Survismeter, 2-in-1 for viscosity and surface tension measurement, an excellent invention for industrial proliferation of surface forces in liquids. Surf Rev and Letters. 2007;14:978-83.
- Vinay Kumar B, Bhojya Naika HS, Girija D, et al. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. Spectrochemica Acta Part A. 2012;94:192-9.
- Tahaa Z, Abdulaziz Ajlounia M, Waleed Momanib A, et al. Spectrochem Acta Part A. 2011;81:570-7.
- Rajiv T, Sadanala Bhavya D, Lingamallu G, et al. Synthesis, crystal structure, electronic spectroscopy, electrochemistry and biological studies of ferrocene-carbohydrate conjugates. Eur J Inorg Chemistry. 2012;pp:2267-77.
- Yanyan S, Shaohua G, Runting Y, et al. Synthesis, antiproliferative activity and DNA binding study of mixed ammine/cyclohexylamine platinum (II) complexes with 1-(substituted benzyl) azetidine-3, 3-dicarboxylates. Eur J Med Chem. 2011;46:5146-53.
- Allen AD, Senott CV. Characterization and lymphocyte proliferation activity of an oligosaccharide degraded from Astragalus polysaccharide. Can J Chem. 1968;45:1337.
- Ameta RK, Singh M, Kale RK. Synthesis and structure-activity relationship of benzylamine supported platinum (IV) complexes. New J Chemistry. 2013;37:1501-8.
- Haines PJ, Reading M, Wilburn FW. Crystallization features of the chiral drug timolol precursor. Netherlands: Elsevier Science BV. 1998;1:279-361.
- Hohne G, Hemminger W, Flammersheim HJ. Chemical thermodynamics of materials: Macroscopic and microscopic Aspects, Berlin, Germany: Springer-Verlag. 1996.
- Privalov PL, Potekhin SA. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:44-51.
- Haynie DT. Biological Thermodynamics. Cambridge, UK: Cambridge University Press. 2008.
- Coe BJ, Glenwright S. Trans-effects in octahedral transition metalcomplexes. J Coord Chem Rev. 2000;203:55-80.
- Engelter C, Hatton AT, Thornton DA. The infrared spectra (500-150 CM-1) of the complexes cis-and trans- [Pt (pyridine) 2X2] (X=Cl, Br, I, SCN). J Mol Structure. 1978;44:23.
- Boschi T, Deganello G, Carturan G. The trans-influence: Its measurement and significance. J Inorg Nucl Chem. 1969;31:2423.
- Van Kralingen CG, De Ridder JK, Reedijk J. Coordination compounds of platinum(II) with 2-methylimidazole and 1,2-dimethylimidazole. Inorg Chim Acta. 1979;36:69.
- Barton JK, Dennenberg JJ, Chaires JB. Tris (phenanthroline)ruthenium(II) Enantiomer Interactions with DNA: Mode and Specificity of Binding 1. Biochem. 1993;32:2573-84.
- Wang BD, Yang ZY, Crewdson P, et al. DNA interaction studies of a new Platinum (II) complex containing different aromatic dinitrogen ligands. J Inorg Biochem. 2007;101:1492-1504.
- Pasternack RF, Gibbs EJ, Villafranca JJ. Interactions of water soluble porphyrins with Z-poly(dG-dC). Biochem. 1983;22:2406-14.
- Pratviel G, Bernadou J, Meunier B. DNA and RNA cleavage by metal complexes. Adv in Inorg Chem. 1998;45:251-312.
- Lu J, Guo H, Zeng X, et al. Biological impact of Pd (II) complexes: Synthesis, spectral characterization, In Vitro anticancer, CT-DNA binding, and antioxidant activities. J Inorg Biochem. 2012;112:39-48.
- Kapicak L, Gabbay EJ. Simple total synthesis of (±)-perhydrohistrionicotoxin. J Amer Chem Soc. 1975;97:403-8.
- Long EC, Barton JK. On demonstrating DNA intercalation. Acc Chem Res. 1990;23:271-3.
- Suh D, Chaires JB. Criteria for the mode of binding of DNA binding agents. Bioorg and Med Chem. 1995;3:723-8.
- Li FH, Zhao GH, Wu HX. Criteria for the mode of binding of DNA binding agents. J Inorg Biochem. 2006;100:36-43.
- Shahabadi N, Mohammadi S, Alizadeh R. Synthesis characterization and DNA Interaction studies of a new Zn (II) Complex Containing Different Dinitrogen Aromatic ligands. Bioinorg Chem and App. 2011.
- Navarro M, Castro W, Higuera-Padilla AR, et al. Synthesis, characterization and biological activity of trans-platinum (II) complexes with chloroquine. J Inorg Biochem. 2011;105:1684-91.
- Lu J, Guo H, Zeng X, et al. Synthesis and characterization of unsymmetrical oxidovanadium complexes: DNA-binding, cleavage studies and antitumor activities. J Inorg Biochem. 2012;112:39-48.
- Hua ZL, Na WW, Yuan W, et al. Supramolecular systems constructed from crown phthalocyaninates. J Coord Chem. 2013;66:227.
- Skehan P. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Indt. 1990;82:1107-12.