Research
, Volume: 19( 6)Synthesis Characterization of Copper Selenide Thin Films and Study of Photovoltaic Solar Applications
Kishan C Rathod*
Department of Chemistry, The New College, Kolhapur, India
*Corresponding author:
Kishan C Rathod,
Department of Chemistry, The New College, Kolhapur, India
Tel: 9067868043
E-mail: kishanchandurathod@gmail.com
Received: August 17, 2021; Accepted: August 31, 2017; Published: September 07, 2017
Citation: Kishan C Rathod. Synthesis Characterization of Copper Selenide Thin Films and Study of Photovoltaic Solar Applications. Mat Sci Ind J. 2021;19(6):157
Abstract
The photovoltaic studies on chemically deposited copper selenide thin films have been carried out to assess its suitability for use in conversion of solar energy to electrical by chemical energy. The PV cell with CuSe thin films as photoanode and sulphide-polysulphide (2M) solution as electrolyte have been constructed and investigated of the PV cell is n-Cuseâ??NaOH (2M) + S(2M) + Na2S(2M)â??C(graphite). The photoelectrochemical cell characterization of the films is carried out by studying current–voltage characteristics in dark, capacitance–voltage in dark, barrier height measurements, power output, photo response and spectral response. The study shows that CuSe thin films are n-type conductivity. The junction ideality factor, flat band potential and barrier height was found to be 2.64, 0.620 V and 0.182 eV respectively. The study of power output characteristic shows open circuit voltage, short circuit current, fill factor, efficiency, and photoesponse lighted ideality factor were found to be 290 mV, 20 μA, 49.36 % and 0.73 %, and 2.59 respectively.
Keywords: The films; Power output; Photo
Introduction
Solar generation of electricity has been an active area of research since shortly after Becquerel first discovered the photoelectric effect in 1839.Concern over fossil fuel production as well as issues of pollution and climate change have spurred intensive research into Photovoltaic Cell (PVC) as well as other renewable energy source. Solar energy is the most utilized of the green energy sources to still however it has stacked with regard to market penetration. The total solar energy adsorbed by the earth is 1 × 1022 J per day, which is enough to meet the world’s energy needs for a year sunlight is not a limitation of adoption [1]. At present the main barrier to mass adoption is the cost in comparison with hydrocarbon and coals in particular the increasing abundance of cheap natural gas. Improvements in solar cell efficiency and decreasing in manufacturing costs will be needed to reach the cost of PV solar cell energy equals that of coal and natural gas.
The copper selenide photovoltaic solar thin films are much cheaper, simple to construct and need no complicated processing steps. Copper selenide solar thin films are semiconductor-semiconductor junction in solar cells, the semiconductor-electrolyte junction in photovoltaic is readily established so that the study of junction energetics becomes easy. This is probably due to the intimate and perfect contact of liquid electrolyte with the crystalline grains. Thus, PVC cells provide an economic chemical route for trapping solar energy. Along with PV cell the semiconductor electrolyte-interface may be used for photo electrolysis, photocatalysis and photovoltaic power generation. The properties of such systems are mainly dependent on the interface formed between the semiconductor electrode and electrolyte hence from material point of view the microstructure of the photoelectrode surface is of main importance. The advantage of PV cells is simpler to make as compared to the p–n junctions which require highly pure semiconducting material.
We reported the successful deposition of crystalline copper selenide thin films by chemical bath deposition technique. Growth mechanism, structural, morphological, optical, electrical and thermoelectrically properties are studied. This paper devoted to photovoltaic performance of chemically deposited CuSe photoelectrode. I–V, C–V characteristics, barrier height measurements, power out curves, and photo response, parameters are studied [2].
Materials and Methods
Experimental details
The chemicals used for preparation of thin films were AR grade copper sulphate, tartaric acid, hydrazine hydrate, sodium sulphite and selenium. Sodium selenosulphate was prepared by the following the method reported earlier. In actual experimentation, 10 mL (0.2 M) copper sulphate was taken in 100 mL beaker. 2.5 mL (1 M) tartaric acid, 10 mL (10%) hydrazine hydrate and 10 mL (0.25 M) sodium selenosulphate were added in the reaction bath. The total volume of the reactive mixture was made up to 80 mL by adding double distilled water. The beaker containing the reactive solution was transferred to an ice bath at 278 K temperature. The pH of the resulting solution was found to be 11.80 ± 0.05. The temperature of the solution was then allowed to rise slowly to 293K. The stainless-steel plate was subsequently removed from the beaker after 6 h of deposition.
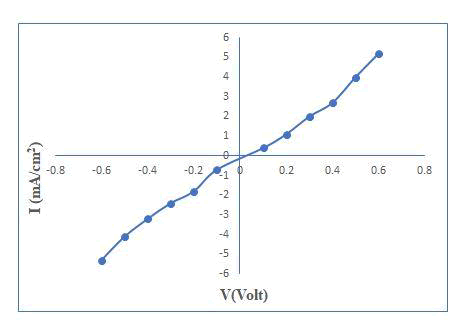
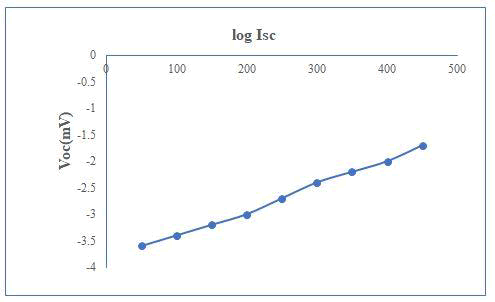
The current–voltage (I–V) characteristic in dark has been plotted. The junction ideality factor has been determined by plotting the graph of log I versus V. The illuminated area of electrode was 3.0 cm2. The fill factor and power conversion efficiency of the cell is calculated from photovoltaic power output characteristics. The fill factor and power conversion efficiency of the cell is calculated from photovoltaic power output characteristics. The power output characteristic has been obtained for a PV cell at a constant illumination of 30 mW/cm2. Light ideality factor was measured from photo response. The Mott–Schottky plot is used to determine the flat band potential one-kilohertz frequency is used to determine the flat band potential.
Fabrication of PV solar cell
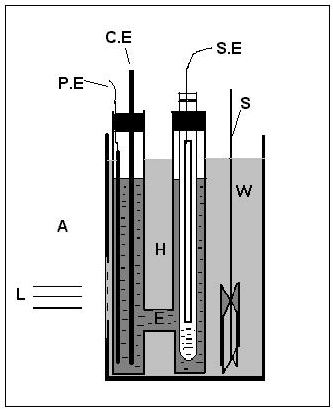
The PV cell effect is commercially utilized for electricity generation and as photosensors. In this experiment set up photovoltaic cell is consisting in three electrode configurations. CuSe as photoanode, CoS-treated graphite rod as a counter electrode and this electrode is called as a photocathode [3]. The reference electrode and sulphide–polysulphide as electrolyte is named as a calomel electrode is shown in (Figures 1-3).
Results and Discussion
Photovoltaic cell solar conductivity
A photovoltaic cell was created with relationship as n-CuSe?NaOH (2 M) +S(2 M)+Na2S (2 M)?C(graphite). PV cell reflects dark voltage with dark current still in the dark [4]. It is observed that the dark voltages polarity towards semiconductor electrode was negative. The photovoltaic investigation of CuSe thin film shows negative sign hence it is of n-type semiconductor and which was confirmed by TEP measurement study.
The study of Photovoltaic cell in dark at 300 K as a characteristic of Current–voltage (I–V). Due to non-symmetrical characteristics it reflects formation of rectifying type junction. The series of resistance as well as structural imperfection was found to be dominance owing to higher value of nd and also shows average transfer across the semiconductor electrolyte interface with significant contribution from surface states and deep traps.
The information of comparative site of the fermi levels in photoelectrode as well as effect of electrolyte and charge transfer process across the junction was observed by flat band potential (Vfb) of a semiconductor. The measurement of maximum open circuit voltage (Voc) is essential which was obtained from a cell. Precise capacitance is summation of capacitance appropriate to diminution layers and Helmholtz layer in electrolyte which is neglected by assuming high ionic concentration [5]. Under such circumstances, Vfb can be obtained using Mott–Schottky relation by standardizing with saturated calomel electrode.
Conclusion
Copper selenide photoelectrode was fabricated on stainless steel plate by using copper sulphate tartaric acid, and hydrazine hydrate and sodium selenosulphate. The photoelectrochemical cell was prepared by using CuSe photoanode, sulphide-polysulphide as electrolyte with CoS-treated graphite rod as a counter electrode. Reference electrode was used as saturated calomel electrode. The various performance parameters were determined for CuSe photoelectrode.
References
- Thirumavalavan S, Mani K, Sagadevan S. Investigation of the structural, optical and electrical properties of copper selenide thin films. Mat Res. 2015;18:1000-7.
- Hussain RA, Hussain I. Copper selenide thin films from growth to applications. Sciences. 2020;100:106101.
- Li J, Jiang L, Wang B, et al. Electrodeposition and characterization of copper bismuth selenide semiconductor thin films. Electrochimica acta. 2013;1;87:153-7.
- Tiwari KJ, Vinod V, Subrahmanyam A, et al. Growth and characterization of chalcostibite CuSbSe2 thin films for photovoltaic application. Science. 2017;418:216-24.
- Yadav AA. Photoelectrochemical studies on spray deposited copper selenide thin films. J Mat Sci. 2014;25(7):3096-102.