Original Article
, Volume: 16( 4) DOI: 10.21767/0972-768X.1000291Synthesis, Characterization and Antimicrobial Study of 1, 2-Disubstituted Benzimidazoles
- *Correspondence:
- Girija K , Department of Pharmaceutical Chemistry, College of Pharmacy, Mother Theresa Post graduate and Research Institute of Health Sciences (A Government of Puducherry institution), Gorimedu, Puducherry, India, Tel: +91-7338894408; E-mail: indhu_ganapathi@yahoo.com
Received: October 15, 2018; Accepted: November 19, 2018; Published: November 21, 2018
Citation: Indhumathi S, Girija K. Synthesis, Characterization and Antimicrobial Study of 1, 2-Disubstituted Benzimidazoles. Int J Chem Sci. 2018;16(4):291
Abstract
A series of novel disubstituted benzimidazole derivatives were synthesized by condensation of o-phenylenediamine with different aromatic aldehydes in presence of ceric ammonium nitrate forming 2-substituted benzimidazoles which on further condensation with secondary amine and formaldehyde forms 1, 2-disubstituted benzimidazoles. The purity of the synthesized compounds was monitored by thin layer chromatography and characterized by FT-IR, 1HNMR, MASS spectral analysis. The compound exhibited moderate antibacterial activity against Bacillus subtilis, good activity against Escherichia coli, compared to standard ciprofloxacin. The two synthesized compounds named 3a [ (2-{[2-(3-chlorophenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione)] and 5a [2-{[2-(2-chlorophenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione] were tested for the antitubercular activity showed a significant activity against Mycobacterium tuberculosis (H37Rv).
Keywords
Benzimidazoles; Primary aromatic amine; Aromatic aldehydes; Antibacterial antitubercular
Introduction
Tuberculosis is still the greatest infectious cause of mortality worldwide. It is the only disease which does not require any vector for transportation from one person to another or to cross the physical boundary of the countries. Being air born disease with no vaccine, it is the single largest disease encountered by both developing and developed countries. Despite the availability of highly potential antitubercular agents, tuberculosis remains primary cause of comparatively high mortality worldwide [1]. The statistics shows that around three million people throughout the world die annually from tuberculosis and today more people die from tuberculosis than ever before [2]. Two of the common problems associated with treatment, one is serious and life-threatening adverse effects of existing antitubercular drugs such as hepatotoxicity, neuritis, depression, asthenia, anorexia etc. which many a time forces to withdraw the treatment temporarily or change of treatment. Other one is the development of resistance due to non-completion of treatment regime by patients and hence gene mutation by organisms made its management more difficult. Another major concern is that tuberculosis is the most common HIV-related opportunistic infection and caring for patients with both the disease is a major public health challenge.
The current literature indicates that benzimidazole derivatives possess diverse pharmacological activities, such as anti-cancer, antimicrobial, analgesic, and anti-inflammatory [3] activities. In keeping in view of the potential benefits of benzimidazoles, the present work involves the synthesis of Mannich base by condensation of o-phenylenedimaine with aromatic aldehydes forming benzimidazoles. These on further treatment with formaldehyde and a secondary amine in the presence of ethanol forms Mannich base.
Experimental
Melting point of the synthesized compounds was determined using melting point apparatus in one-end open tube capillary method and is uncorrected. The purities of compounds were checked by thin layer chromatography (TLC) using readymade silica gel plates (Merck) using Methanol: chloroform (9:1) as solvent system. The spots developed visualized under UV lamp Infra-red spectra (IR) absorption spectra were recorded on Shimadzu FT-IR spectrometer using KBR pellet technique. 1H-NMR spectra were recorded at 400 MHz on a Brucker FT-NMR spectrophotometer using TMS as internal standard. Mass spectra were recorded using an ESI Mass spectrometer. The synthetic strategy to synthesize the target compounds is depicted in SCHEME 1 and 2.
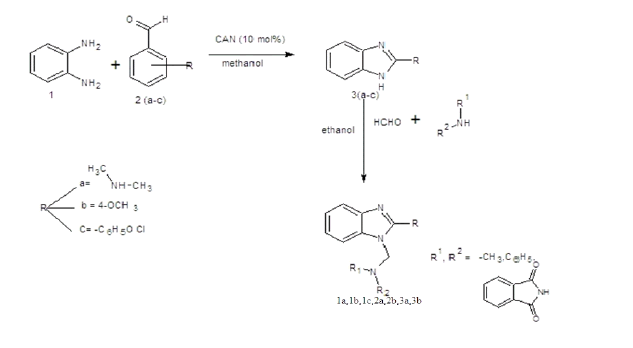
General procedure for the synthesis of 2-substituted benzimidazoles
Equimolar quantity of o-phenylene diamine (20 mmol) and aromatic aldehyde (20 mmol) in presence of 10 ml of methanol to be treated with ceric ammonium nitrate (CAN) 10 mol% the reaction mixture is to be stirred at room temperature overnight and the solid formed is collected by filtration and recrystallized the crude sample from absolute ethanol [4].
General procedure for the synthesis of Mannich bases
Formaldehyde (5 mmol) was added slowly to 5 mmol of 2-subsituted benzimidazole and 5 mmol of amino compounds in 15 ml of ethanol, with continuous stirring for 1 hour, and refrigerated overnight (TABLES 1-3). The product was filtered and recrystallised using absolute alcohol [5].
| Code | R | R1 | % yield | Melting point (°C) | Rf value | Log p | Elemental analysis |
|---|---|---|---|---|---|---|---|
| 1a | 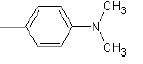 |
 |
40 | 115-118 | 0.76 | 5.78 | C (72.71%), H (5.08%) N (14.13%), O (8.07%) |
| 1b | 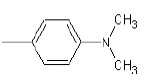 |
 |
38.5 | 118-120 | 0.67 | 4.29 | C (73.44%), H (7.53%) N (19.03%) |
| 1c | 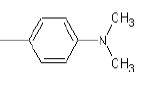 |
 |
37.5 | 150-153 | 0.81 | 7.69 | C (80.35%), H (6.26%) N (13.39%) |
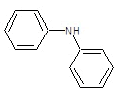
| 2a | 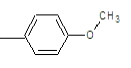 |
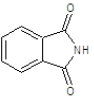 |
50.31 | 135-138 | 0.61 | 5.53 | C (72.05%), H (4.47%) N (10.96%), O (12.52%) |
| 2b | 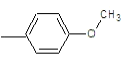 |
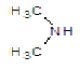 |
30.1 | 132-133 | 0.78 | 4.04 | C (72.57%), H (6.81%) N (14.94%), O (5.69%) |
| 3a | 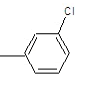 |
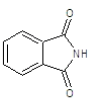 |
51.93 | 125-127 | 0.72 | 6.16 | C (68.13%), H (3.64%) Cl (9.14%), N (10.84%) O (8.25%) |
| 3b | 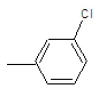 |
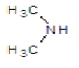 |
45 | 120-124 | 0.87 | 4.67 | C (67.25%), H (5.64%) Cl (12.41%), N (14.7%) |
| 3c | 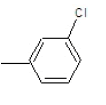 |
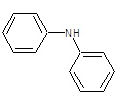 |
62.3 | 122-124 | 0.72 | 8.06 | C (76.18%), H(4.92%) Cl (8.65%), N (10.25%) |
| 4a | 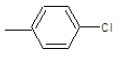 |
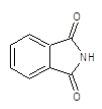 |
70.2 | 130-132 | 0.76 | 6.13 | C (68.13%), H (3.64%) Cl (9.14%), N (10.84%) O (8.25%) |
| 5a | 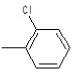 |
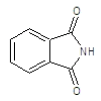 |
75.5 | 134-136 | 0.69 | 5.54 | C (68.13%), H (3.64%) Cl (9.14%), N (10.04%) O (8.25%) |
Table 1: Physicochemical properties of the synthesized compound.
| Compound Code | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Standard | Bacillus subtilis | Standard | Escherichia coli | |||||
| 50 µg | 150 µg | 250 µg | 50 µg | 150 µg | 250 µg | |||
| 1b | 38 mm | 10 mm | 12 mm | 20 mm | 32 mm | 20 mm | 25 mm | 28 mm |
| 2a | 38 mm | 16 mm | 10 mm | 23 mm | 35 mm | 25 mm | 27 mm | 29 mm |
| 3a | 18 mm | - | 14 mm | 15 mm | 33 mm | 20 mm | 25 mm | 29 mm |
| 4a | 24 mm | 12 mm | 14 mm | 20 mm | 30 mm | 14 mm | 20 mm | 21 mm |
Table 2: Antibacterial activity of the synthesized compounds.
| Compound code | 200 μg | 400 μg | 800 μg | 1600 μg | 3200 μg |
|---|---|---|---|---|---|
| 3a | R | R | R | S | S |
| 5a | R | R | S | S | S |
Table 3: Antitubercular activity of the synthesized compounds.
Antimicrobial activity
The antimicrobial activity of the synthesized compounds was determined by Agar disc diffusion method against gram positive organism Bacillus subtilis and gram-negative organism Escherichia coli at 50 mcg/ml, 150 mcg/ml, 250 mcg/ml concentration of sample compounds against Ciprofloxacin as standard [6].
Antitubercular activity
The antitubercular screening of the synthesized compounds against H37Rv strain at 200 mcg/ml, 400 mcg/ml, 800 mcg/ml, 1600 mcg/ml, 3200 mcg/ml. LJ medium containing standard drugs as well as control. The medium was inoculated with Mycobacterium tuberculosis of H37Rv strain. The inoculated medium was incubated for 37°C for 6 weeks the growth of Mycobacterium tuberculosis was read [7-11].
Analysis data of the synthesized compounds
Compound 1a: (2-({2-[4-(dimethylamino) phenyl]-1H-benzimidazol-1-yl} methyl)-1H isoindole-1, 3(2H)-dione) IR cm-1: (C-H-aromatic stretch)-3486.67, (C=C aromatic)-1607.38, 1207.72, 1523.49 (C-N stretch), 1771.3(C=O), 1394.28 (CH2 bend).
Compound 1b: (4-{1-[(dimethylamino) methyl]-1H-benzimidazol-2-yl}-N, N-dimethylaniline): IR cm-1: C-H (aromatic stretch)-3433.64, (C=C aromatic)-1607.38, (C-N)-1031.12, 1214.93, (CH2 bend)-1465.63 1H NMR δ ppm (Ar-H, m): 7.394-7.861, (S, 2H-CH2): 3-3.05, (s-6H, (CH3)2): 2.7-2.75, mass: m/z: 294.75790.
Compound 1c: (4-{1-[(dimethylamino) methyl]-1H-benzimidazol-2-yl}-N, N-diphenylaniline): IR cm-1: C=C (aromatic)-1604.41, (C-N)-1213.01, 1283.39, C-H (aromatic stretch)-3418.21, CH2 bend- 1382.71, 1H NMR δ ppm (Ar-8H, m): 7.059-7.220, (Ar-10H, m): 7.411-7.904, (s, CH2-2H)-3.071, (s-(CH2)): 1.3, m/z: 418.1200.
Compound 2a: (2-{[2-(4-methoxyphenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione): IR cm-1: C=C (aromatic)-1658.48, C-H (aromatic stretch)-3486.67, (C=O)-1771.3, C-N (Aryl) 1145.51, (OCH3)-2962.48.
Compound 2b: (1-[2-(4-methoxyphenyl)-1H-benzimidazol-1-yl]-N, N-dimethylmethanamine): IR cm-1: C-N (Alkyl)-1107.9, C=N (Aryl)-1280.25, C-H(aromatic stretch)-3423.99 (C=C)-1657.52, (C-O-C)-1280.25, 1H NMR δ ppm (Ar-H, m): 7.070-7.759, (S-2H-CH2): 2.918, (m-(CH3)2): 1.148-1.387, (3H-OCH3): 3.330, m/z: 291.1568.
Compound 3a: (2-{[2-(3-chlorophenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione): IR cm-1: C-H (aromatic stretch) - 3427.85, C=C (aromatic)-1658.48, C-N (Aryl)-1386.52, C-Cl-712.569, 1H NMR δ ppm (m, Ar-H): 7.499-7.903, (s, 2H-CH2): 3.327, m/z: 387.3137.
Compound 3b: (1-[2-(3-chlorophenyl)-1H-benzimidazol-1-yl]-N, N-dimethylmethanamine): IR cm-1: C-N (Aryl) - 1278.75, C-N (akyl)-1278.75, C-Cl- 742.46, C-H (aromatic stretch)-3424.06, C=C (aromatic)-1655.68.
Compound 4a: 2-{[2-(4-chlorophenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione: IR cm-1: (C-H aromatic stretch)-2910.88, (C=C)-3053.42, (C-N)-1273.06, (–C=O)-1600.97, –(CH2 bend) 1471.74 , (-C-Cl)-740.25.
Compound 5a: 2-{[2-(2-chlorophenyl)-1H-benzimidazol-1-yl] methyl}-1H-isoindole-1, 3(2H)-dione: IR cm-1 :(-C-H aromatic stretch)-2926.11, (C=C) - 3155.65, (C-N)-1107.18, (C=O)-1606.76 (CH2 bend)-1464.02 (C-Cl) -617.24 cm-1.
Results and Discussion
In the present study ten novel 1, 2 disubstituted benzimidazoles were synthesized by condensation of o-Phenylenediamine and different aromatic aldehydes which on further treatment with primary aromatic or heteryl amine (dimethlyamine, diphenylamine, phthalimide) and formaldehyde to produce respective title compounds. Melting point of the synthesized compounds was determined by using melting point determination apparatus. IR, 1H-NMR, and MASS spectral data of the titled compounds was in correlation with the expected structure. Synthesized compounds 1b, 2a, 3a, 4a were screened for the antibacterial activity against gram positive bacteria Bacillus subtilis, and gram-negative bacteria E. coli. The compound exhibited moderate to good activity. Compounds 3a and 5a were screened for in vitro antitubercular activity against Mycobacterium tuberculosis (H37Rv), exhibited significant activity.
Conclusion
Synthesized compounds produced a moderate yield, in addition low cost and environmentally less hazardous method. Compounds 1b, 2a, 3a, 4a were screened for the antibacterial activity against Bacillus subtilis, all the compounds exhibited moderate activity. Compounds 1b, 3a exhibited good activity against E. coli. Compounds 3a and 5a were screened for in vitro antitubercular activity against Mycobacterium tuberculosis (H37Rv), exhibited significant activity. It is concludeD that the compound substituted with pthalimide at 1st position to nitrogen exhibited maximum antibacterial, antifungal, antitubercular activity.
Acknowlegement
The authors are also thankful to the Dean Dr. R. Murali, Mother Theresa Post Graduate and Research Institute of Health Sciences, for providing necessary laboratory facilities. The authors are also thankful to Indian Institute of Technology-Chennai for carrying out the spectral studies for the synthesized compounds. The authors are also thankful to Dr. Muthuraj M.Sc., M.Phil., Ph.d, Head of the department, Department of Microbiology, Government hospital for chest diseases, Gorimedu, Government of Pondicherry for helping to carry out my antitubercular activity.
References
- World Academy of Science. Engineering and Technology 31, 2009.
- Kale SC, Kale Biyani MK. Synthesis of some new benzimidazole acetic acid derivatives and evaluation for their antimicrobial and antitubercular activities Int J Biomed Adv Res. 2013;4(5):322-7.
- Singh A. Benzimidazole: A short review of their antimicrobial activities. Int Current Pharm J. 2012;1(5):119-27.
- Camacho J, Barazarte A. Synthesis and biological evaluation of benzimidazole 5-carbohyrazide derivatives as antimalarial, cytotoxic and antitubercular agents. Bioorg and Med Chem. 2011;19:2023-9.
- Araujo DML, Maste MM. Synthesis, antitubercular evaluation and docking studies of novel benzimidazole analogues. Int J Pharma Sci and Res. 2018:9(9):3696-04.
- Manju PT, Anton smith A, Padmaja V. In silico design, synthesis and in vitro antitubercular and anti-microbial screening of novel benzimidazole derivatives. Int J Pharma Sci and Res. 2018;9(9):3705-11.
- Alam F, Kumar B. Synthesis, antimicrobial and anthelmintic activity of some novel benzimidazole derivatives. Int J Drug Res and Technol. 2014;4(3):31-8.
- Sadek KU, Al-Qalaf F, Mekheimer RA, et al. Cerium (IV) ammonium nitrate-mediated reactions: Simple route to benzimidazole derivatives. Arabian J Chem. 2012;5:63-6.
- Messary MA, Elarfi MG, Mohammed R. Synthesis and spectral studies of Mannich bases derived from 2-substituted benzimidazoles. Int J Chem Res. 2010;2:1714-6.
- Xiangming Ha, Huiquiang M. A simple and efficient synthesis of 2-aryl-substituted benzimidazoles. Springer. 2008;44(6):872-4.
- Kale MK, Biyani KR. Synthesis of some new benzimidazole acetic acid derivatives and evaluation for their antimicrobial and antitubercular activities. Int J Biomed and Adv Res. 2013;4(5):322-7.
