Research
, Volume: 12( 3) DOI: 10.37532/2277-288X.2022.12(4).192Synthesis and Antibacterial Effect of 2-(Benzylthio) Methyl-1h-Benzimidazole Derivatives on Two Bacteria of Medical Interest
- *Correspondence:
- Souleymane Coulibaly Laboratoire de Constitution et Réaction de la Matiere, UFR Sciences des Structures de la Matiere et Technologie, Université Felix Houphouet Boigny, 22 BP 582 Abidjan 22, Cote d’Ivoire Tel: +2250759544673 , E-mail: souleydestras@yahoo.fr
Received: June 29,2022, Manuscript No. tsacpi-22-67993; Published: July 28,2022.
Citation: Achi PA, Kouadio FK, Coulibali S, et al. Synthesis and Antibacterial Effect of 2-(Benzylthio) methyl-1H-Benzimidazole Derivatives on Two Bacteria of Medical Interest. Acta Chim Pharm Indica 2022;12(3):1-11.
Abstract
This work involved the synthesis of ten (10) novel 2-benzylthiomethyl-1H-benzimidazole derivatives (5a-d) and evaluation of their antibacterial activities. These new compounds were synthesized by reacting the isothiouronium-1H-benzimidazole salts (3a and 3b) with the benzyl halides (4) to ethanol reflux in the presence of a sodium hydroxyde solution. The obtained compounds were characterized by nuclear magnetic resonance 1H, 13C NMR and High-Resolution Mass Spectrometry (HRMS). These compounds were evaluated for antibacterial activity on two bacterial strains, Escherichia coli and Staphylococcus aureus. The results showed that on S. aureus, six compounds (5b, 5d, 5e, 5f, 5g and 5j) were significantly potent with antibacterial activity MIC values ranging from 140 to 320 µg/mL. On the bacterial strain E. coli, five compounds (5b, 5e, 5g, 5h and 5j) showed a significant antibacterial effect with MIC ranging from 140 to 400 µg/mL.
keywords
Isothiouronium-1H-benzimidazole, antibacterial activity, bactericidal, bacteriostatic.
Introduction
Bacteria, viruses, fungi and parasites evolve over time and are increasingly resistant to drugs [1]. This resistance becomes a major factor complicating the treatment of bacterial infections and increasing the risk of spread [2]. Recently, the rate of resistance to ciprofloxacin, an antibiotic frequently used to treat urinary tract infections, has ranged from 8.4% to 92.9%. Enterobacteria such as E.coli, Klebsiella, are resistant to carbapenems. Colistin, the only treatment of last resort for deadly infections caused by theseenterobacteria, has been found inactive on some bacteria in some parts of the world [3]. In 2019, according to a UN report on theenvironment, antibiotic-resistant infections caused nearly 5 million deaths. Thus, this report tells us that without immediate action,these infections could cause up to 10 million deaths per year by 2050 [4]. Antibiotic resistance is now one of the most seriousthreats to global health, food security and development. This problem has led several research groups to develop new antibacterialagents [5-7]. In order to contribute to this research, we looked at the benzimidazole scaffold. This compound is present in thefamily of cobalamins in the form of vitamin cyano-cobalamin [8]. The benzimidazole nucleus has been widely described literature because of its various biological properties, such as antihypertensive, antifungal, antioxidant, antiviral and topoisomerase inhibitor, anti-proliferative, anti-allergic, antitumoral, anti-kinase, anticanceral, cytotoxicity and anti-HIV-1 [9-29]. Many approved drugs have benzimidazole as the major moiety or substructure [30,31]. Also, the different modulations carried out around the benzimidazole scaffold have led to very effective drugs Omeprazole, Lansoprazole, Rabeprazole and Pantoprazole [32]. In this study, pharmacomodulation will be carried out by introducing benzylthiomethyl groups at the -2 position of the benzimidazole scaffold and then evaluate their antibacterial activities.
Materials
Materials of Chemistry
Unless otherwise indicated, 1H and 13C NMR spectra were recorded at 300 and 75MHz or 400 and 101 MHz or 600 and 151MHz, respectively, in CDCl3, solution. Chemical shifts are reported in ppm on the δ scale. Multiplicities are described as s (singlet), d (doublet), dd (doublet of doublet, m (multiplet) and further qualified as app (apparent), br (broad) coupling constants, J are reported in Hz. HRMS were measured in the electrospray (ESI) mode on a LC-MSD TOF mass analyzer.
Biological Materials
The microbial support consisted of clinical strains of E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria) which are resistant to Piperacillin and Erythromycin, respectively. The strains were supplied by the Laboratory of Bacteriology-Virology of the Institute Pasteur in Côte d'Ivoire. These strains are all pathogenic and multi-resistant. S. aureus strains are resistant to Erythromycin, sensitive to Cefoxitin and Clindamycin. Those of E. coli are all resistant to Piperacillin/Tazobactam, sensitive to Ceftriaxone and Ticarcillin/Clavulanic Acid. The culture medium used was Mueller-Hinton broth (Oxide) and Mueller-Hinton agar (Lab.Conda s.a). Dimethyl sulfoxide (DMSO) and distilled water were used as solvents for chemical solubilization.
Methods
Methods of Synthesis
General procedure for the synthesis of 2-(chloromethyl)-1H-benzimidazoles (1a and 1b)
To 1g (9.3mmol) of orthophenylenediamine, was added 1.5eq of chloroacetic acid and 10 mL of hydrochloric acid (4N). The mixture was let under reflux for 2 hours. Then 5% potassium hydrogenocarbonate (KHCO3) solution was added. The precipitate formed was purified on silica gel in a solvent mixture ethyl-hexane acetate (80/20).
2-(chloromethyl)-1H-benzimidazole (1a)
Yellow powder, Yield = 80%, 1H NMR (DMSO-d6) δ (ppm): 7.19-7.24 (m, 2H, HAr), 7.54-7.58(m, 2H, HAr) 4.92 (s, 2H, CH2). 13CNMR (DMSO-d6) (δ ppm): 149.57, 138.52, 122.28, 115.26, 38.29. HRMS(ESI) Calc for C8H8ClN2 (M+H) +: 167.7312,Found: 167.7316.
2-(chloromethyl)-5-nitro-1H-benzimidazole (1b)
Black powder, Yield = 88%, 1H NMR (DMSO-d6) δ (ppm): 8.39 (m, 1H, HAr), 8.09 (m, 1H, HAr), 7.66 (m, 1H, HAr), 4.64 (s, 2H,CH2). 13C NMR (DMSO-d6): 141.5, 142.7, 118.6, 116.0, 111.4, 41.8. HRMS(ESI): Calc for C8H7ClN3O2 (M+H) +: 212. 4928,Found: 212.4931
Synthesis method of 2 methyl-1H-benzamidazole thiourunium chloride salt (3a and 3b)
To a solution of 2-(chloromethyl)-1H-benzimidazole (1 eq, 57.2 mmol) in 50 mL of acetonitrile, thiourea (1 eq, 57.2 mmol) was added. The mixture was brought to reflux for 1.30 hours. After cooling to room temperature, a precipitate was formed, filtered, washed several times with ethyl acetate and then dried in the open air to afford yellow powder.
2-[(1H-benzimidazol-2-yl) methyl] isothiouronium (3a)
Yellow powder, Yield = 88%, HRMS(ESI): Calc for C9H12ClN4S (M+H) +: 243.1127, Found: 243.1132
2-[(5-nitro-1H-benzimidazol-2-yl) methyl] isothiouronium (3b)
Yellowish-orange powder, Yield = 70%, HRMS(ESI): Calc for C9H11ClN5O2S (M+H) +: 288.0411, Found: 243.0415
Synthesis method of 2-[(thiobenzyl) methyl]-1H-benzimidazole
To a solution of 2-methylbenzimidazole thiourunium chloride salt (1 eq, 2.1 mmol) in 15 mL of absolute ethanol was added (2.5 eq, 0.35N) of sodium hydroxide solution. The mixture was stirred under reflux, then benzyl chloride derivative (1.2 eq, 2.52 mmol). was added. The reaction stayed like this for two more hours. After cooling to room temperature, the mixture was diluted in dichloromethane and washed several times with water. The organic phase was dried over anhydrous Na2SO4. and the solvent was evaporated in vacuo. The residue obtained after evaporation of solvent was purified by silica column chromatography (hexane / ethyl acetate: 85 / 15) to give compound 5(a-j).
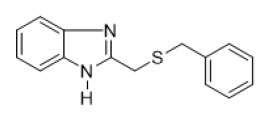
2-[(benzylthio) methyl]-1H-benzimidazole (FIG.1)
Yellow powder, Yield = 53%, 1H NMR (400 MHz, CDCl3) δ (ppm): 7.65–7.47 (m, 2H, HAr), 7.34–7.17 (m, 7H, HAr), 3.93 (s, 2H,CH2), 3.71 (s, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ (ppm): 151.52, 137.45, 128.99, 128.65, 127.30, 122.65, 36.72, 29.49.HRMS(ESI): Calc for C15H15N2S (M+H) +: 255.1685, Found: 255.1689
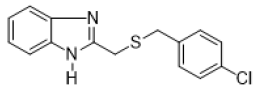
2-{[(4-chlorobenzyl) thio] methyl}-1H-benzimidazole (FIG.2)
Yellow powder, Yield = 59%, 1H NMR (400 MHz, CDCl3) δ (ppm): 7.60–7.54 (m, 2H, HAr), 7.30–7.15 (m, 6H, HAr), 3.92 (s, 2H,CH2), 3.66 (s, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ (ppm): 130.28, 128.70, 123.13, 115.10, 35.50, 29.20, HRMS(ESI): Calc forC15H14ClN2S (M+H) +: 289. 1127, Found: 289.1131
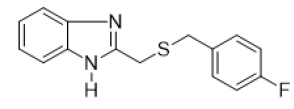
2-{[(4-fluorobenzyl) thio] methyl} -1H-benzimidazole (FIG.3)
Oil, Yield = 59%, 1H NMR (600 MHz, CDCl3) δ (ppm): 10.74 (s, 1H, NH), 7.65 – 7.56 (m, 2H, HAr), 7.33 – 7.26 (m, 2H, HAr),7.20 – 7.14 (m, 2H, HAr), 6.92 – 6.83 (m, 2H, HAr), 3.93 (s,2H, CH2), 3.66 (s, 2H, CH2).13C NMR (151 MHz, CDCl3) δ (ppm) :162.73, 161.10, 151.54, 138.52, 132.92, 130.50, 115.46, 115.32, 115.05, 35.94, 29.13, HRMS(ESI): Calc for C15H13FN2S (M+H) +:373.1328, Found: 373.1332
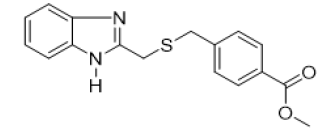
Benzoate, 4-methyl{[(1H-benzimidazol-2-yl) methyl] thio} methyl (FIG.4)
Oil, Yield = 61%, 1H NMR (400 MHz, CDCl3) δ (ppm):8.47 (s, 1H, NH), 7.88 (dd, J = 8,3, 3,5 Hz, 2H, HAr), 7.60 – 7.54 (m, 2H,HAr), 7.33 – 7.24 (m, 4H, HAr), 3.92 (s, 2H, CH2), 3.90 (s, 3H, CH3-O), 3.73 (s, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ (ppm):166.67, 151.16, 142.84, 129.82, 128.96, 123.19, 115.26, 52.12, 36.01, 29.03, HRMS(ESI): Calc for C17H17N2 O2S (M+H) +:313.1224, Found: 313.1227
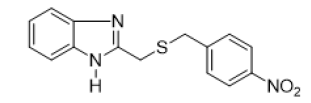
2-{[(4-nitrobenzyl) thio] methyl} -1H-benzimidazole (FIG.5)
Brown powder, Yield = 55%, 1H NMR (300 MHz, CDCl3) δ (ppm): 8.07–8.00 (m, 2H, HAr), 7.60–7.52 (m, 2H, HAr), 7.44–7.35 (m,2H, HAr), 7.32–7.22 (m, 2H, HAr), 3.93 (s, 2H, CH2), 3.76 (s, 2H, CH2). 13C NMR (75 MHz, CDCl3) δ (ppm): 150.56, 146.88,145.07, 129.77, 123.66, 123.06, 35.60, 29.16, HRMS(ESI): Calc for C15H14N3O2S (M+H) +: 300.1366, Found: 300.1369
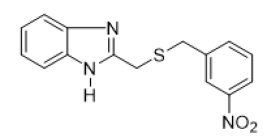
2-{[(3-nitrobenzyl) thio] methyl} -1H-benzimidazole (FIG.6)
Brown powder, Yield = 68%, 1H NMR (400 MHz, CDCl3) δ (ppm): 8.12 (s, 1H, HAr), 7.96 (d, J = 7.4 Hz, 1H, HAr), 7.55 (d, J =6.6 Hz, 3H, HAr), 7.28 (dd, J = 10.7, 8.8 Hz, 3H, HAr), 3.95 (s, 2H, CH2), 3.79 (s, 2H, CH2).13C NMR (101 MHz, CDCl3) δ (ppm):150.78, 148.09, 139.55, 135.06, 129.28, 123.66, 122.90, 122.09, 35.55, 29.33. HRMS(ESI): Calc for C15H14N3O2S (M+H) +:300.1366, Found: 300.1369
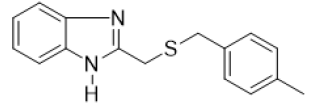
2-{[(4-methylbenzyl) thio] methyl} -1H-benzimidazole (FIG.7)
Yellow powder, Yield = 89%, 1H NMR (300 MHz, CDCl3) δ (ppm): 10.24 (s, 1H, NH), 7.52 (d, J = 7.4 Hz, 2H, HAr), 7.30–7.23(m, 2H, HAr), 7.10 (dd, J = 35.6, 7.9 Hz, 4H, HAr), 3.93 (s, 2H, CH2), 3.68 (s, 2H, CH2), 2.30 (s, 3H, CH3). 13C NMR (101 MHz,CDCl3) δ (ppm): 151.76, 137.04, 134.37, 129.33, 128.88, 36.71, 29.49, 20.79. HRMS(ESI): Calc for C16H17N2S (M+H) +:369.1763, Found: 369.1770
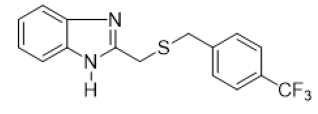
2-{[(4-(trifluoromethyl) benzyl) thio] methyl}-1H-benzimidazole (FIG.8)
Yellow powder, Yield = 64%, 1H NMR (400 MHz, CDCl3) δ (ppm): 10.00 (s, 1H, NH), 7.6–7.56 (m, 2H, HAr), 7.45 (d, J = 8.1 Hz,2H, HAr), 7.33 (d, J = 8.1 Hz, 2H, HAr), 7.31–7.26 (m, 2H, HAr), 3.93 (s, 2H, CH2), 3.73 (s, 2H, CH2). 13C NMR (101MHz, CDCl3)δ (ppm): 151.04, 141.45, 138.30, 129.22, 125.45, 125.41, 122.95, 115.01, 35.74, 29.07. HRMS(ESI): Calc for C16H14F3N2S (M+H)+: 323.1131, Found: 323.1134
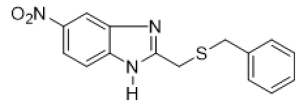
2-[(benzylthio)methyl]-6-nitro-1H-benzimidazole (FIG.9)
Oil, Yield = 68%, 1H NMR (400 MHz, CDCl3) δ (ppm): 8.20 (dd, J = 8.9, 2.2 Hz, 1H, HAr), 7.31–7.15 (m, 7H, HAr), 3.97 (s, 2H,CH2), 3.76 (s, 2H, CH2). 13C NMR (75 MHz, CDCl3): 143.77, 137.30, 128.96, 128.77, 127.52, 36.85, 29.18, HRMS(ESI): Calc for C15H14N3O2S (M+H) +: 300. 1366, Found: 300.1369
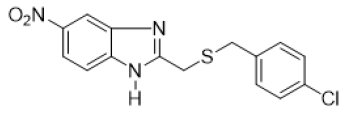
2-{[(4-chlorobenzyl) thio] methyl} -6-nitro-1H-benzimidazole (FIG.10)
Orange powder, Yield = 68%, 1H NMR (400 MHz, CDCl3) δ (ppm): 8.51 (d, J = 1.9 Hz, 1H, HAr), 8.22 (dd, J = 8.9, 2.2 Hz, 1H,HAr), 7.60 (d, J = 8.9 Hz, 1H, HAr), 7.20 (s, 4H, HAr), 3.95 (s, 2H, CH2), 3.71 (s, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ (ppm):155.70, 143.81, 135.55, 133.29, 130.25, 128.75, 118.83, 35.84, 28.98. HRMS(ESI): Calc for C15H13 ClN3O2S (M+H)+: 334.0531 ,Found : 334.0534
Biological Methods
The liquid micro dilution method was used to determine the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (BMC). A colony isolated from an 18 hours’ bacterial culture was collected and homogenized in 10 mL of 0.9% NaCl and incubated for 3 hours at 37°C. From this bacterial suspension, 0.1 mL was added to 10 mL of 0.9 NaCl solution. This prepared bacterial suspension constituted the starting bacterial inoculum. To do this, the bacterial inoculum was homogenized and diluted from 10 to 10 until dilution 10-4. Four successive dilutions were obtained from 10-1 to 10-4. The initial bacterial inoculum and the 4 successive dilutions were inoculated with a 2 μL calibrated loop in Muller-Hinton agar boxes with 5 cm long streaks. This preparation represents Box (A) that will help to determine the BMC. Antibacterial testing was conducted using the liquid micro dilution method [33]. Final concentrations ranging from 100 to 3.12 mg/mL were achieved. In a series of 5 test tubes, a growth control tube and a sterility control tube, a volume of one milliliter of an extract known concentration from the concentration range was added to the test tubes. The growth control tube received 0.5 mL of sterile distilled water while all test tubes received 0.5 mL of bacterial inoculum. The sterility control tube received 1 mL of 0.9% NaCl solution. The tubes were incubated for 24 hours at 37°C. The MIC is the lowest concentration of extract for which no bacterial growth is observed. The contents of the tubes in which there was no visible growth were used to seed the Muller-Hinton agar on 5 cm ridges using a 2 μL calibrated loop. This Petri dish is called B. Analysis of the results after 24 hours of incubation allowed to calculate the CMB which corresponds to the lowest concentration that kills 99.99% of the bacteria in culture.
Results and Discussion
Chemistry
The orthophenylenediamine derivatives were condensed with 2-chloroacetic acid under reflux in a HCl (4N) solution following the Phillips reaction Yielded to 2-chloromethyl-1H-benzimidazole derivatives (1a and 1b) (Scheme 1) FIG.11 [34].
Figure 11: Reaction conditions: 1(i) Thiourea (2), MeCN (reflux 2h); 3(ii) Benzyl chloride (4), NaOH, EtOH/ H2O (reflux 2h). Scheme 1: Synthesis method of 2-((thiobenzyl)methyl)-1H-benzimidazole (5)
These two derivatives were condensed in acetonitrile (CH3CN) under reflux with thiourea by adopting the protocol used by Farid et /> al., allowing the synthesis of the isothiouronium-1H-benzimidazole salts (3a and 3b). The reactivity of these isothiouronium salts /> was studied by Oleynk et al. [35-36]. Thus, using the protocol, isothiouronium-1H-benzimidazole salts (3a and 3b) were put in /> reaction with the benzyl halides (4) of under reflux in ethanol with the presence of sodium hydroxide to lead to the formation of 2- /> benzylthiomethyl-1H-benzimidazole derivatives FIG. (5a-j). Structures of these compounds FIG. (5a-d) were confirmed by 1H, /> 13C NMR spectroscopy and HRMS. 1H NMR spectra of FIG. 5a-j compounds indicate presence of a singlet in the vicinity of 3.9 /> ppm corresponding to the methylene protons (C2-CH2-S) located between the C2 carbon of the benzimidazole scaffold and the /> sulfur. Another singlet occurs around 3.7 ppm due to sulfur bound to benzyl methylene hydrogen (-S-CH2-?). Analysis of 13C /> NMR spectra of the synthesized compounds FIG. (5a-j) confirms the presence of the methylenic carbon’s (C2-CH2-S) around 27 /> ppm. Methylene carbon bound to benzyl (-S-CH2-?) was also observed around 35 ppm.
Biology
The Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (BMC) and ratio (BMC/MIC) of each compound tested using the liquid micro dilution method were reported in (TABLE 1) below.
TABLE 1. Bactericidal and Bacteriostatic effects of obtained compounds 3a-l on bacteria strains E. coli and S. aureus determined by MIC and BMC values.
| S. aureus 2275C2021 | E. coli 2279C2021 | |||||||
|---|---|---|---|---|---|---|---|---|
| MIC | BMC | BMC/ | Effect | MIC | BMC | BMC/ | Effect | |
| Compounds | (µg/mL) | (µg/mL) | MIC | (µg/mL) | (µg/mL) | MIC | ||
| 5a | - | - | - | - | - | - | - | - |
| 5b | 300 | 610 | 2 | bc | 150 | 610 | 4 | bs |
| 5c | - | - | - | - | - | - | - | - |
| 5d | 320 | 650 | 2 | bc | - | - | - | - |
| 5e | 180 | 740 | 4 | bs | 180 | 740 | 4 | bs |
| 5f | 140 | 590 | 4 | bs | - | - | - | - |
| 5g | 280 | 570 | 2 | bc | 140 | 570 | 4 | bs |
| 5h | - | - | - | - | 150 | 630 | 4 | bs |
| 5i | - | - | - | - | - | - | - | - |
| 5j | 200 | 830 | 4 | bs | 400 | 830 | 2 | bc |
|
Non-determined. bc means bactericidal, bs means bacteriostatic |
||||||||
On S. aureus 2275C2021 six compounds including FIG. 5b, 5d, 5e, 5f, 5g and 5j showed significant antibacterial activity with MIC ranging from 140 to 320 μg/mL. Of these six compounds, FIG. 5b, 5d and 5g showed bactericidal potency. Compounds FIG. 5e, 5f, and 5j showed bacteriostatic properties. Compounds FIG. 5e and 5f possessing the nitro group (NO2) on the benzyl were more potent with respective MICs of 180 and 140 μg/mL. On the bacterial strain E. coli 2279C2021, the compounds FIG. 5b, 5e, 5g, 5h and 5j showed significant antibacterial effect with MIC ranging from 140 to 400 μg/mL. Compounds FIG. 5b and 5g with Electro-Donating Groups (EDG) such as methyl (CH3) by inductive effect and chlorine (Cl) by mesomeric effect gave the best MICs (140 and 150 μg/mL). Compound 5j with the nitro (NO2) group on the 5-position of the benzimidazole scaffold yielded a MIC of 400 μg/mL and represents the only compound with bactericidal potency. Others tested compounds showed bacteriostatic potency.
Conclusion
This work resulted in the synthesis of ten (10) new derivatives of 2-benzylthiomethyl-1H-benzimidazole (compound 5a-j) with yields between 72 and 79 %. Structures of all compounds were confirmed by the results of spectroscopic 1H, 13C NMR analyses and High-Resolution Mass Spectroscopy (HRMS). Antibacterial tests of the ten (10) compounds were explored on two bacterial strains of E. coli and S. aureus. Compounds FIG. 5b, 5d and 5g showed bactericidal potency on S. aureus while compounds FIG. 5e, 5f, and 5j showed bacteriostatic potency on the same strain. On the bacterial strain E. coli, the compound FIG. 5j showed antibacterial activity with bactericidal potency and FIG. 5b, 5e, 5g and 5h were active with bacteriostatic potency.
Acknowledgements
for helpful discussions. Also we thank the Laboratory of Synthesis and Natural Products of the University of Quebec in Montreal (Canada) and the Laboratory LG2A of Jules Verne Picardie University (France) for providing us the chemical reagents and material for the spectroscopic analyzes.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
REFERENCES
- Nicole M., Mediachimie Jul. 2020 [Google Scholar] [Crossref]
- Yala D, Merad AS, Mohamedi D, et al. Résistance bactérienne aux antibiotiques. Méd du Maghreb. 2001;91.
[Google Scholar] [Crossref]
- Résistance bactérienne aux antibiotiques
- UN Environment Program. How Drug-Resistant Pathogens in Water Could Trigger a New Pandemic. 2022. [Google Scholar] [Crossref]
- Alasmary F, Snelling A, Zain M, et al. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules. 2015;20(8):15206-23.
- Anand K, Wakode S. Synthesis, characterization and biological evaluation of benzimidazole derivatives. Int J Pharm Sci Res. 2018;9(2):617-24
- Yoon Y, Ali M, Wei A, et al. Synthesis, characterization, and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors. Bioorg Chem. 2013;49:33-9
- Asif M. Green Synthesis of Benzimidazole Derivatives: an Overview on Green Chemistry and Its Applications. Chem Methodol. 2019;3(6):620-31.
- Xu JY, Zeng Y, Ran Q, et al. Synthesis and biological activity of 2-alkylbenzimidazoles bearing a N-phenylpyrrole moiety as novel angiotensin II AT1 receptor antagonists. Bioorg Med Chem Lett. 2007;17(10):2921-6.
- Kerimov I, Ayhan-Kilcigil G, Can-Eke B, et al. Synthesis, antifungal and antioxidant screening of some novel benzimidazole derivatives, J Enzyme Inhib Med Chem.. 2007;22(6):696-701. [Google Scholar]
[Crossref]
- Gurer-Orhan H, Orhan H, Suzen S, et al. Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J Enzyme Inhib Med Chem. 2006;21(2):241-7.
- Patel PD, Patel MR, Kaushik-Basu N, et al. 3D QSAR and Molecular Docking Studies of Benzimidazole Derivatives as Hepatitis C Virus NS5B Polymerase Inhibitors. J Chem Inf Model. 2008;48(1):42-5.
- Oksuzoglu E, Tekiner-Gulbas B, Alpher S, et al. Some benzoxazoles and benzimidazoles as DNA topoisomerase I and II inhibitors. J Enzyme Inhib Med Chem. 2008; 23(1):37-42.
- Klimesova V, Koci J, Pour M, et al. Synthesis and preliminary evaluation of benzimidazole derivatives as antimicrobial agents. Eur J Med Chem. 2002;37(5):409-18.
- Garuti L, Roberti M, Malagoli M, et al. Synthesis and antiproliferative activity of some benzimidazole-4,7-dione derivatives. Bioorg Med Chem Lett. 2000;10(19):2193-5.
- Settimo AD, Settimo AF, Marini AM, et al. Synthesis, DNA binding and in vitro antiproliferative activity of purinoquinazoline, pyridopyrimidopurine and pyridopyrimidobenzimidazole derivatives as potential antitumor agents. Eur J Med Chem. 1998;33(9):685-96.
- Beaulieu C, Wang Z, Denis D, et al. Benzimidazoles as new potent and selective DP antagonists for the treatment of allergic rhinitis. Bioorg Med Chem Lett. 2004;14 (22):3195-9.
- Nakano H, Inoue T, Kawasaki N, et al. Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg Med Chem. 2000;8(2):373-80.
- White AW, Curtin NJ, Eastman BW, et al. Potentiation of cytotoxic drug activity in human tumour cell lines, by amine-substituted 2-arylbenzimidazole-4-carboxamide PARP-1 inhibitors. Bioorg Med Chem Lett. 2004;14(10):2433-7.
- Lukevics E, Arsenyan P, Shestakova I, et al. Synthesis and antitumour activity of trimethylsilylpropyl substituted benzimidazoles. Eur J Med Chem. 2001;36(6):507-15.
- Sondhi SM, Singh N, Lahoti AM et al. Synthesis of acridinyl-thiazolino derivatives and their evaluation for anti-inflammatory, analgesic and kinase inhibition activities. Bioorg Med Chem. 2005;13(13):4291-9.
- Sondhi SM, Singh N, Kumar A, et al. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg Med Chem. 2006;14(11):3758-65.
- Demirayak S, Usama AM, Ahmet C K. Synthesis and anticancer and anti-HIV testing of some pyrazino[1,2-a]benzimidazole derivatives. Eur J Med Chem. 2002;37(3):255-60.
- Huang ST, Hsei IJ, Chen C. Synthesis and anticancer evaluation of bis(benzimidazoles), bis(benzoxazoles), and benzothiazoles. Bioorg Med Chem. 2006;14(17):6106-19.
- Kumar CC, Malkowski M, Yin Z, et al. Inhibition of Angiogenesis and Tumor Growth by SCH221153, a Dual αvβ3 and αvβ5 Integrin Receptor Antagonist. Cancer Res. 2001;61(5):2232-38.
[Google Scholar] [Crossref]
- White AW, Almassy R, Calvert A.H, et al. Resistance-Modifying Agents. 9. 1 Synthesis and Biological Properties of Benzimidazole Inhibitors of the DNA Repair Enzyme Poly(ADP-ribose) Polymerase. J Med Chem. 2000;43(22):4084-97.
- Horton DA, Bourne GT, Smythe ML. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem Rev. 2003;103(3):893-930.
- Evans TM, Gardiner JM, Mahmood N, et al. Structure-activity relationships of anti-HIV-1 N-alkoxy- and N-allyloxy-benzimidazoles. Bioorg Med Chem Lett. 1997;7(4):409-12.
- Middleton T, Lim HB, Montgomery D, et al. Inhibition of human immunodeficiency virus type I integrase by naphthamidines and 2-aminobenzimidazoles. Antivir Res. 2004;64(1):35-45.
- Tahlan S, Kumar S, Narasimhan B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: a review. BMC Chem. 2019;13(1):1-21.
- Ajani OO, Aderohunmu DV, Ikpo CO, et al. Functionalized Benzimidazole Scaffolds: Privileged Heterocycle for Drug Design in Therapeutic Medicine. Archiv der Pharmazie - Chemistry in Life Sciences. 2016;349 (7):475-506.
- Patil A, Ganguly S, Surana S. A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J Chem. 2008; 1(3):447-60.
[Google Scholar] [Crossref]
- Dosso M, Faye-Kette H. Antibiogram quality control in current practice: Experience of the bacteriology laboratory of the Pasteur Institute of Côte d'Ivoire. Bactériolo int, n special. 2000:53.
[Google Scholar] [Crossref]
- Phillips M A. CCCXVII.—The formation of 2-substituted benziminazoles. J Chem Soc. 1928; 2393-9.
- Farid MI, Stanislav AIS, Alexander GS. Paper Synthesis, 2001;3:419-22. [Google Scholar] [Crossref]
- Oleynik AS, Pevneva NY, Kandalintseva NV et al. Synthesis and Biological Activity of Hydrophilic Alkylphenols Chem sustain Dev.2008;16:559-64.
[Google Scholar] [Crossref]