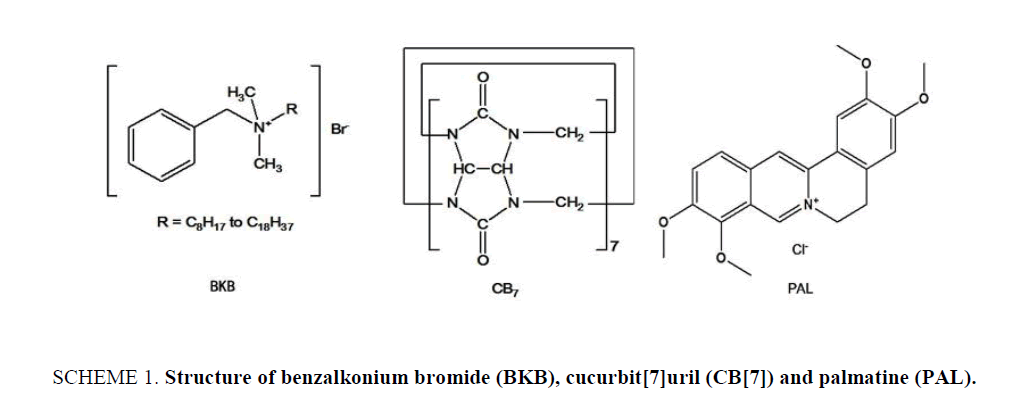
Original Article
, Volume: 16( 15)Supramolecular Interaction of Benzalkonium Bromide with Cucurbit[7]uril and Its Analytical Application
- *Correspondence:
- Li-Ming D , School of Chemistry and Material Science, Shanxi Normal University, Linfen Shanxi, P.R. China, Tel: 029-85308442; E-mail: lmd@sxnu.edu.cn
Received:August 03, 2016; Accepted: August 30, 2016;; Published: September 04, 2016
Citation: Jian HX, Shi L, Li-Ming D. Supramolecular Interaction of Benzalkonium Bromide with Cucurbit[7]uril and Its Analytical Application. Anal Chem Ind J. 2016;16(15):113.
Abstract
Benzalkonium bromide (BKB) shows weak fluorescence in aqueous solutions, which makes it difficult to determine its concentration directly using fluorescence methods. In the present study, a competitive reaction between BKB and palmatine for occupation of the cucurbit[7]uril (CB[7]) cavity was studied by spectrofluorometry. Palmatine reacted with CB[7] to form a probe with strong fluorescence. However, the presence of BKB quenched the fluorescence of the probe. The rate of fluorescence quenching was proportional to the concentration of BKB. Formation of a supramolecular complex with CB[7] provides a simple, accurate, and highly sensitive fluorescent probe for the determination of BKB in commercially available injection solutions and plasma samples. The linear range of the method was 0.004 μg mL-1 to 1.768 μg mL-1, with a detection limit of 0.002 μg mL-1. Molecular modeling of the interaction between BKB and CB[7] was established, and the results were confirmed by 1H NMR. The mechanism of fluorescence for the probe is discussed. The proposed method has potential for therapeutic monitoring, pharmacokinetics, and clinical applications.
Keywords
Benzalkonium bromide; Fluorescence probe; Cucurbit[7]uril; Palmatine
Introduction
Benzalkonium bromide (BKB; ( Scheme 1)), also known as alkyl dimethyl benzyl ammonium bromide, is a nitrogenous cationic surface-acting agent belonging to the quaternary ammonium group. It has three main categories of use: as a biocide, a cationic surfactant and a phase transfer agent in the chemical industry. BKB is generally used as the active ingredient in disinfectants [1,2], sanitizers [3-6] and preservatives [7-11], it can be used as food preservative, although this use is not permitted [12,13]. Although BKB is an effective antibacterial and antifungal agent, it has been confirmed that there is a side effect with surface epithelial cells, as determined from in vitro and in vivo studies, which is related to its long-term use for chronic diseases in particular. Many methods have been reported for the determination of BKB in biological and pharmaceutical samples, including mass spectrometry [14], high performance liquid chromatography-tandem mass spectrometry [15], ion-selective electrode membranes [16], high-performance liquid chromatography [17,18], potentiometric sensors [19], ultra-high performance liquid chromatography [20], capillary zone electrophoresis [21], and capillary electrophoresis [22,23]. However, the reported liquid chromatographic, mass spectrometric and ion-selective electrode membrane analyses generally require a complicated extraction process, time consuming operation steps, complicated and expensive equipment and labor-intensive sample preparation procedures. Though easy to operate, the capillary electrophoresis method was limited because of the low sensitivity in pharmaceutical samples. Therefore, a sensitive and simple detection method is of urgent need for the detection of BKB.
In the field of supramolecular chemistry, cucurbit[n]urils and their unique structures and properties have attracted much attention since the first report of their preparation and continuous separation in 2000 [24]. Cucurbit[7]uril (CB[7]; Scheme 1) is a large ring containing seven glycoluril units linked by 14 methylene groups. It has a hydrophobic internal cavity and an opening on either side surrounded carbonyl oxygen atoms. The two openings are the same size, and their diameter is smaller than that of the cavity. The carbonyl groups at either end of the cavity can bind cations and guest molecules through ion-dipole or hydrogen bond interactions. CB[7] can also selectively form inclusion complexes with various organic cations and metal ions [25,26]. As a molecular container or host, CB[7] can encapsulate small molecules for partial or full protection. Earlier studies have shown that CB[7] can form various supramolecular complexes with either one or two aliphatic or aromatic amines [27,28] and form coordination compounds with transition or non-transition metal salts to increase their water-solubility. There are many potential applications for CB[7] in the field of molecular recognition [29-31]. In this paper, the inclusion behavior of CB[7] with BKB has been studied using Palmatine (PAL; Scheme 1.) as a probe. It is found that PAL can form 1:1 complex with CB[7], which lead to the enhancement of fluorescence intensity of PAL. When proper amount of BKB were added in the system, the fluorescence intensity decreased regularly. Based on the linear relationship between the change of fluorescence intensity (ΔF) and the concentration of BKB, a simple, high selectivity fluorimetirc method for the determination of the BKB was developed. Possible interaction mechanism of the PAL-CB[7] complex was studied with 1H NMR and computation methods. The mechanism for the inclusion of BKB is also discussed.
Experimental
Instrumentation
The fluorescence measurements were performed on a Cary Eclipse spectrofluorometer (America). All measurements were performed using a standard 10 mm path-length quartz cell at 25.0°C ± 0.5°C. Absorption spectra were measured on a U-3010 UV-visible spectrophotometer (Hitachi, Japan). 1H NMR spectra were obtained using a Bruker AV 600 MHz spectrometer (Switzerland) in D2O buffered with DCl (0.10 mol L-1). Molecular modeling calculations were optimized at the B3LYP/6-31 G (d) level of density functional theory with the Gaussian 09 program.
Materials and reagents
Palmatine (PAL) was obtained from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing). Benzalkonium bromide (BKB) was purchased from Toronto Research Chemicals Inc. BKB content was determined for an eye drop preparation, Tobramycin eye drops (0.01%, s.a. Alcon-Couvreurn.v.). The tested preparation contained BKB as a preservative. CB[7] was prepared and characterized according to a reported procedure [32,33]. CB[7] stock solutions of 1.0 mmol L-1 were prepared by dissolving CB[7] in a 100-mL volumetric flask with doubly distilled water. All of the stock standard solutions were stable for several weeks at room temperature. Britton-Robinson buffer solutions were prepared using 0.01 mol L-1 boric acid, acetic acid and phosphoric acid, and the pH values were adjusted using 0.05 mol L-1 sodium hydroxide or hydrochloric acid. All other chemicals were of analytical grade, and double-distilled water was used throughout the procedure.
Spectral measurement procedure
The fluorescence measurements of PAL and the complexes were carried out using excitation wavelengths of 344 nm in the absence or presence of BKB or a sample. In a 10 mL colorimetric flask, 0.8 mL of 0.1 mmol L-1 CB[7] solution, 0.8 mL of 0.1 mmol L-1 probe molecular solution (PAL), followed by 0.1 mL of 0.001 mol L-1 Britton-Robinson buffer solutions, and a given concentration of BKB standard solution or sample solution were sequentially added. The mixture was diluted to the volume with doubly distilled water and shaken for 10 min at room temperature.
Preparation of BKB in pharmaceutical preparations
A fluorescence method was used to determine BKB homologues in various pharmaceutical preparations. Commercially available tobramycin eye drops were extracted by solid-phase extraction using cartridges containing strong-acid cation exchange resin (1 cm by ø 1 cm, 001 × 7 (732)). The cartridges were conditioned with 2 × 1 mL of methanol and 2 × 1 mL of water, and then loaded with 1.0 ml of a tobramycin eye drops. After rinsing sequentially with 1 mL of water, 1 mL of methanol, and 1 mL of water, elution was performed with 1.0 mL of 0.05 mol L-1 HCl. Then, the general procedure was followed.
Preparation of BKB in human plasma samples
This method could be employed to monitor the time dependent concentration of BKB in the plasma of healthy volunteers after the oral administration of the BKB medication. The volunteers were premedicated with 80 mg of BKB, and the plasma was collected over 24 h at various times. A plasma sample (1.0 mL) was placed in a centrifuge tube, and then centrifuged at 10,000 rpm for 12 min. The clear supernatant was removed, and then loaded on the same solid phase extraction cartridges as above, which were conditioned, rinsed, and eluted in the same manner (analysis of BKB in pharmaceutical preparations). Then, the general procedure was followed.
Results and Discussion
Optimization of experimental conditions
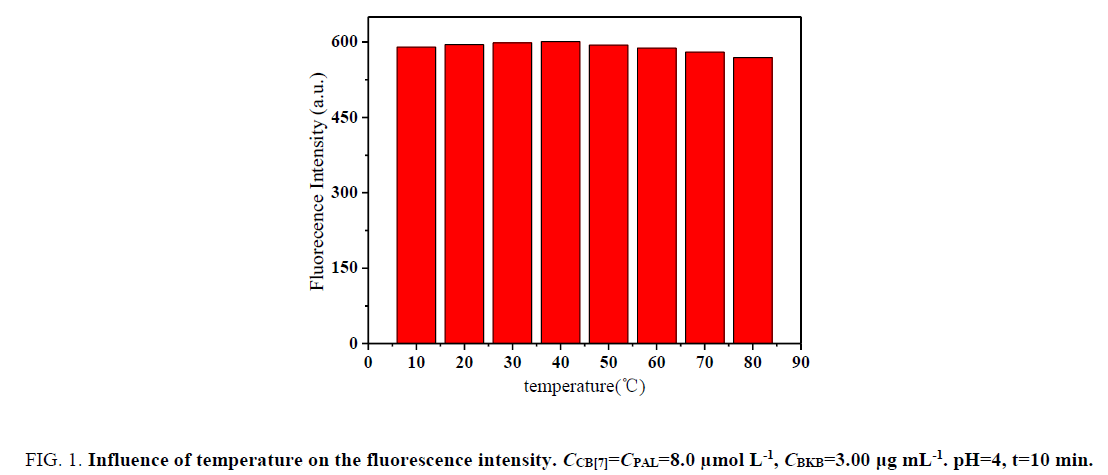
Influence of temperature and reaction time: The effect of temperature on ΔF was investigated within 10°C to 70°C. The formed complexes were stable, and ΔF was not obviously mutative below 40°C (Figure. 1). However, the fluorescence intensity greatly decreased above 40°C in the sense that the complexes were dissociated at high temperatures. Hence, all subsequent measurements were performed at room temperature.
Figure 1: Influence of temperature on the fluorescence intensity. CCB[7]=CPAL=8.0 μmol L-1, CBKB=3.00 μg mL-1. pH=4, t=10 min.
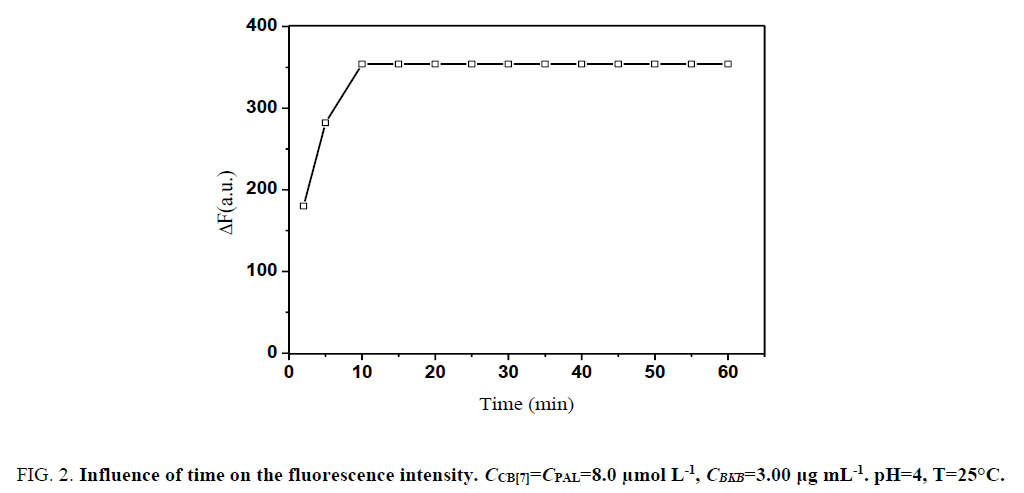
Moreover, ΔF attained a maximum within 10 min after CB[7] and PAL were added and remained constant for several hours (Figure. 2). Therefore, the measurements were determined after the mixing of reagents for 10 min at room temperature.
Figure 3: Influence of time on the fluorescence intensity. CCB[7]=CPAL=8.0 μmol L-1, CBKB=3.00 μg mL-1. pH=4, T=25°C.
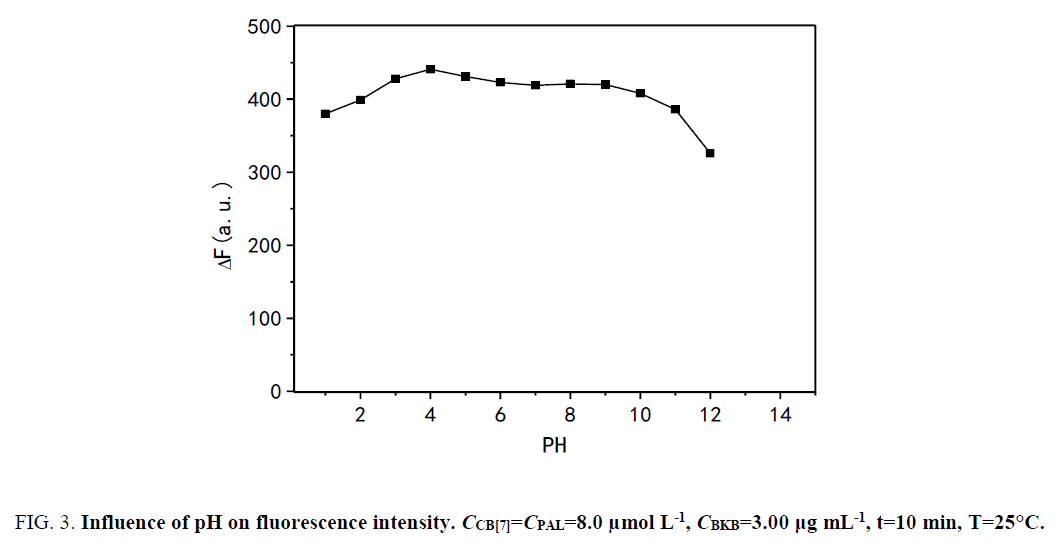
Influence of pH: The effect of pH on ΔF was studied over the pH range of 1.0 to 12.0 (Figure. 3). The results show that ΔF was maximized and changed slightly in the pH range of 1.0 to 4.0. However, ΔF markedly decreased with pH greater than 9.0 because alkali cations are readily coordinated with the carbonyl-fringed portals of CB[7] in alkaline medium. Binding of the alkali cation lowers the rate constant of the ingress of organic guests [34]. The results show that the presence of sodium salts in alkaline medium lowers not only the equilibrium constant of PAL binding with CB[7] but also the fluorescence quantum yield. Hence, by using hydrochloric acid, the pH was adjusted to 4.0, which was the desired pH for the subsequent experiments.
Figure 3: Influence of pH on fluorescence intensity. CCB[7]=CPAL=8.0 μmol L-1, CBKB=3.00 μg mL-1, t=10 min, T=25°C.
PAL binding to CB[7] in aqueous solution
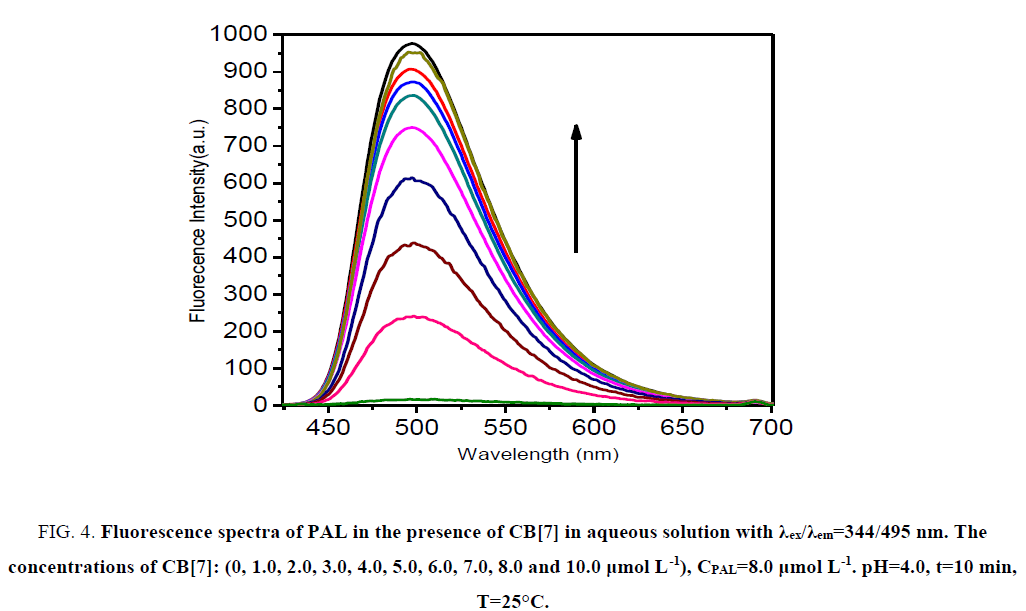
CB[7] is a non-fluorescent substance in aqueous solution, and PAL can emit only feeblish fluorescence. Remarkably, the fluorescence intensity was increased evidently when CB[7] was added to the PAL solution (Figure. 4). The factor for the increase of fluorescence intensity was that CB[7] changed the conjugate plane rigid structure of PAL, which formed a complex substance of CB[7]-PAL. The concentration of PAL and CB[7] from 1.0 μmol L-1 to 13 μmol L-1 was tested at 495 nm, and the result shows that the complex substance of CB[7]-PAL can engender the maximum of the fluorescence intensity when 8 μmol L-1 CB[7] was added into 8 μmol L-1 PAL in aqueous solution.
Figure 4: Fluorescence spectra of PAL in the presence of CB[7] in aqueous solution with χex/χem=344/495 nm. The concentrations of CB[7]: (0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0 and 10.0 μg mL-1). CPAL=8.0 μmol L-1. pH=4.0, t=10 min, T=25°C.
Effect of BKB on the formation of CB[7]-PAL complex
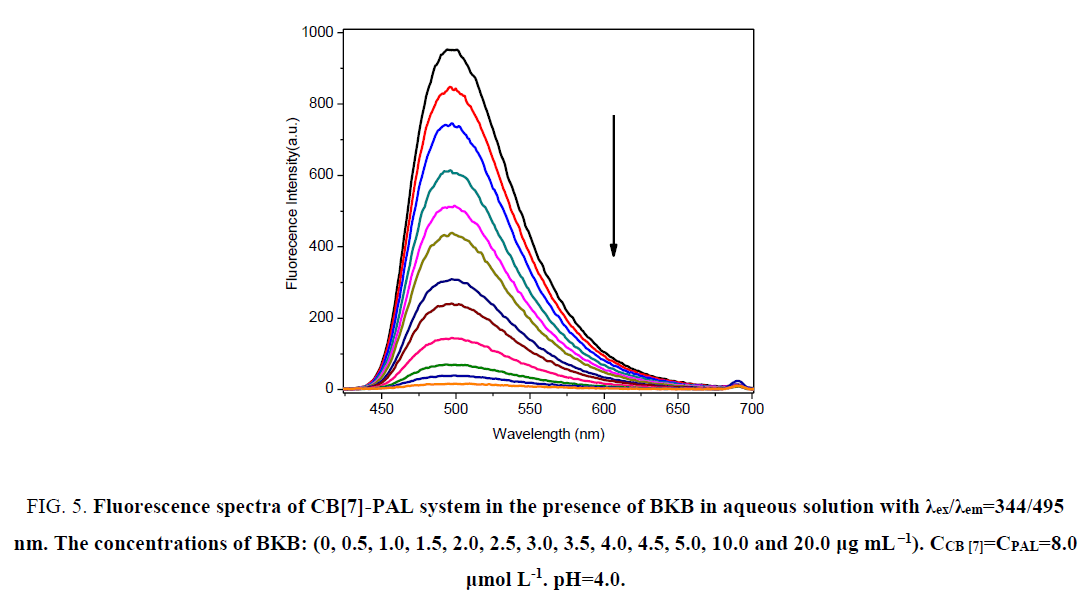
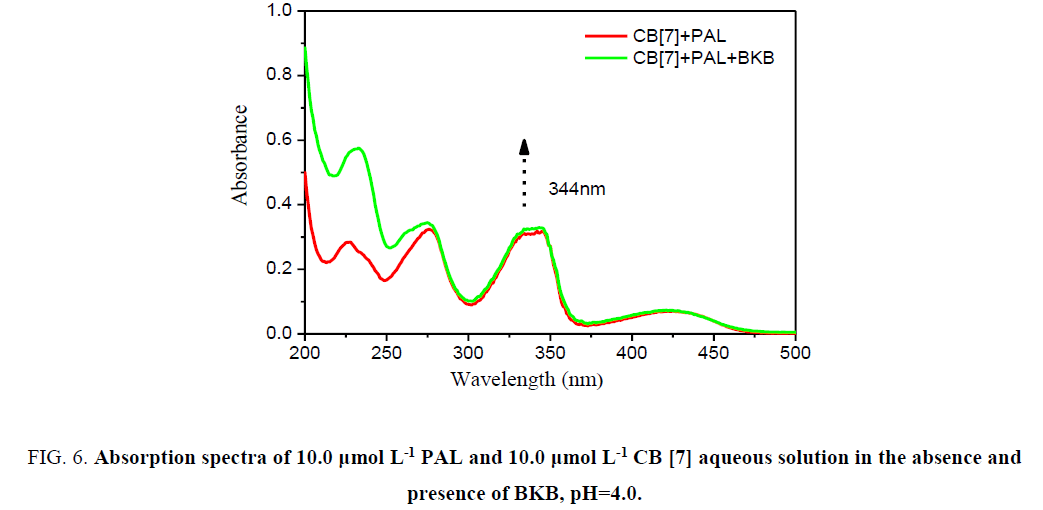
In acidic aqueous solutions, PAL and BKB showed weak fluorescence emission, and CB[7] did not fluoresce. However, for PAL in the presence of CB[7], the fluorescence intensity greatly increased. Interestingly, when BKB was added to the PALCB[ 7] complex, the fluorescence was obviously quenched (Figure. 5). There was a clear relationship between the amount of BKB and the ΔF. As the concentration of BKB increased, the fluorescence quenching became more obvious. The effect of BKB on the PAL-CB[7] complex was evaluated using an ultraviolet spectrophotometer (Figure. 6). The spectrum of the PAL-CB[7] complex blue shifted to 344 nm in the presence of BKB. The strong fluorescence of PAL with CB[7] could be explained by inclusion of PAL in the CB[7] cavity, showing PAL had a coplanar structure. Addition of BKB resulted in fluorescence quenching because of a competitive reaction between BKB and PAL. The interaction between BKB and CB[7] was stronger than that between PAL and CB[7], which resulted in removal of PAL from the CB[7] cavity. The UV spectrophotometry results were used to confirm this.
Figure 5: Fluorescence spectra of CB[7]-PAL system in the presence of BKB in aqueous solution with χex/χem=344/495 nm. The concentrations of BKB: (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 10.0 and 20.0 μg mL-1). CCB [7]=CPAL=8.0 μmol L-1. pH=4.0.
Figure 6:Absorption spectra of 10.0 µmol L-1 PAL and 10.0 µmol L-1 CB [7] aqueous solution in the absence and presence of BKB, pH=4.0.
The response mechanism of the host-guest complexation
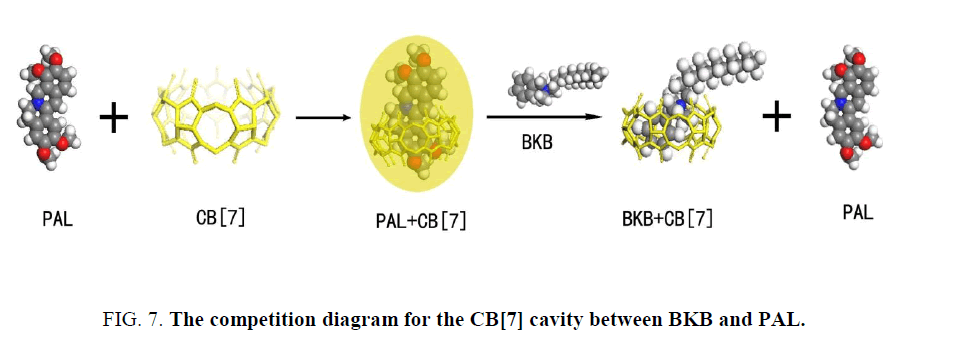
The PAL molecule contains an isoquinoline ring and a substituted benzene ring. Outwardly, PAL ought to have a strong fluorescence emission because of the large conjugated system. However, between the isoquinoline and the substituted benzene ring in PAL is a bridge of a hexatomic ring, so the two rings are not in the same plane. Thus, the PAL molecule cannot form a conjugated system, leading to the result of weak fluorescence. When PAL was mixed with CB[7], the apolar isoquinoline ring part of PAL infixed the CB[7] cavity, inducing the electrostatic attraction between the positive charge of the heterocyclic nitrogen of PAL and the high electron density of the carbonyl oxygens of the macrocycle. As a result, the dihedral angle became small between the isoquinoline and the substituted benzene ring. This change resulted in a closer resemblance to the conjugate plane rigid structure in the structure of energy minimization. The degree of freedom of motion of the PAL molecule is attenuated, thus also reducing the probability of radiationless transition. The change of PAL structure induced by CB[7] is the primary factor of fluorescence enhancement. When BKB is added to the PAL-CB[7] system, BKB will compete with PAL to occupy the CB[7] cavity. Some of the PAL molecules will be forced out of the CB[7] cavity because of the introduction of BKB (Figure. 7). Outside of the hydrophobic cavity of CB[7], PAL is subject to the local microenvironment, and this results in quenching of the PAL fluorescence.
Theoretical calculation
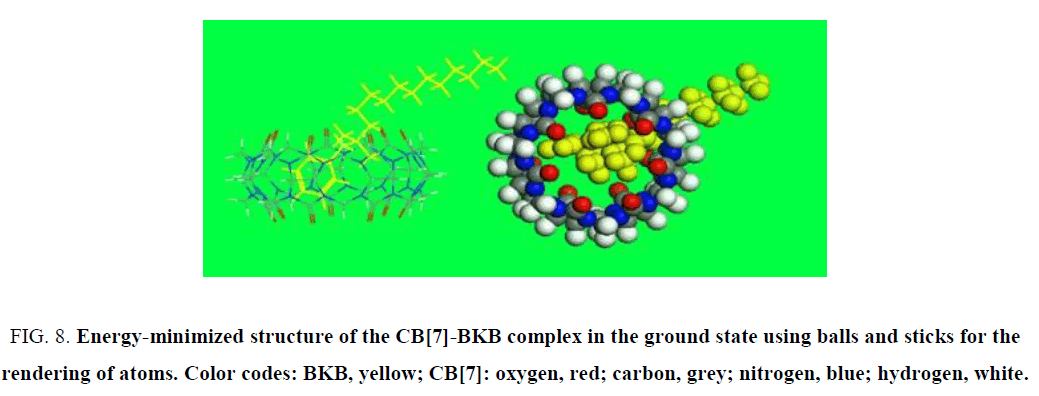
Molecular modeling density functional theory calculations were optimized at the B3LYP/6-31G (d) level [35-37] using the Gaussian 09 program (Gaussian Inc., Wallingford, CT). The molecular modeling results confirmed that the α-oxygen of the isoquinoline ring of the PAL molecule was located in the cavity of CB[7]. In a mixture of PAL, CB[7], and BKB, BKB showed much stronger binding ability for CB[7] than PAL, and its nitrogen interacted with a carbonyl group at the opening of CB[7]. Consequently, the PAL molecule was forced out of the CB[7] cavity (Figure. 8). Then, the benzyl group of BKB entered the CB[7] cavity, which quenched the fluorescence.
Figure 8: Energy-minimized structure of the CB[7]-BKB complex in the ground state using balls and sticks for the rendering of atoms. Color codes: BKB, yellow; CB[7]: oxygen, red; carbon, grey; nitrogen, blue; hydrogen, white.
1H NMR
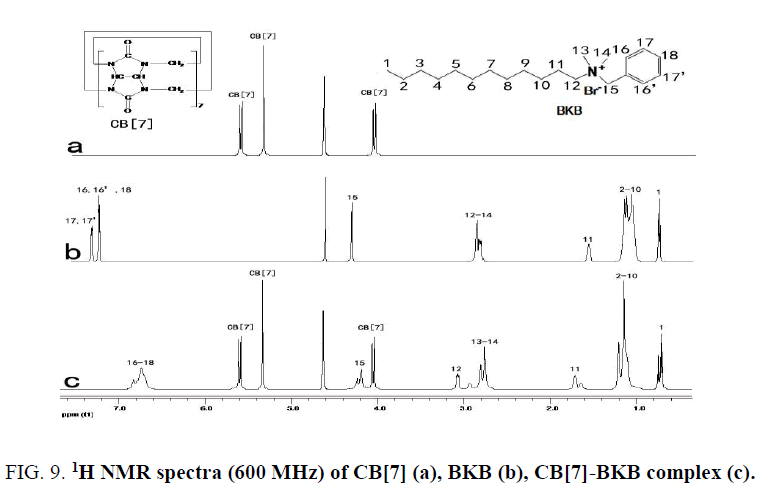
The 1H NMR spectra of the CB[7]-PAL was confirmed [38]. Figure. 9 exhibits the 1H NMR spectra of CB[7], BKB and CB[7]-BKB complex. The results of the 1H NMR experiments are consistent with the theoretical structures of the complex. Chemical shifts of compound relied on the highly sensitive microenvironment. The signals of H15, H16, H16', H17, H17' and H18 protons of the bound BKB are shifted to a higher field relative to those of the free BKB. These findings indicated that the substituted benzyl group moiety of the BKB molecule were encapsulated in the CB[7] cavity. The aforementioned discussion was consistent with this.
Figure 9: 1H NMR spectra (600 MHz) of CB[7] (a), BKB (b), CB[7]-BKB complex (c).
Effect of interfering substances
Prior to the determination of the real samples via the proposed fluorescent probe method, the interference substances (commonly used tablet excipients) on the determination of 0.2 μg mL-1 of BKB were evaluated under optimal experimental conditions. A 3000-fold mass in excess of each interference substance over BKB was first tested. When interference occurred, the ratio was progressively reduced until the interference ceased. The criterion for interference was that the variable value of fluorescence intensity transcended ± 5% before and after the interference substances. The results show that the usual excipients to determination were nearly free of interference. During the trials of real samples, no other interfering substances with similar structure to BKB in excipients were noticed, which indicated good selectivity in testing BKB in pharmaceutical formulations. However, the components in the human plasma samples (K+, Na+, Ca2+, valine, alanine, phenylalanine, cysteine and so on) may quench the fluorescence intensity of CB[7]-PAL to a certain degree. Thus, it should be abandoned from plasma samples before the assay. Moreover, it was eliminated from plasma samples by extraction with dichloromethane in this work.
Calibration graph and sensitivity
Under the optimal experimental conditions, the standard calibration curve was drawn by plotting ΔF versus the concentration of BKB, with ΔF representing the difference of fluorescence intensity between CB[7]-PAL and CB[7]-PAL-BKB. In the solution of 8.0 μg moL-1 CB[7]-PAL complexes, the plot of fluorescence intensity versus the concentration showed a series of linear relationships between 0.004 μg mL-1 to 1.768 μg mL-1. The linear regression equation was ΔF=371.32C+39.21 (C denotes the concentration in μg mL-1). The correlation coefficient was 0.9979 for BKB and the detection limits were 0.002 μg mL-1 at 3σ for the same order, indicating good linearity and sensitivity. The proposed method proved to have higher sensitivity than any other reported method for determining BKB, and the result was satisfactory.
Analytical application
Analysis of pharmaceutical preparations: The analytical results of pharmaceutical preparations are presented in Table 1. The relative standard deviations of the proposed method were less than 2.0%. In addition, t-tests show the satisfaction of accuracy and precision. To confirm the availability of the proposed method, BKB was added to the drugs tested via the standard addition method and the recoveries were in the range of 97.25% ± 0.89% to 104.00% ± 0.45%.
| Sample | Lable claim (mg mL-1) | Amount added (mg mL-1) | Determination value (mg mL-1) | Amount found (mg mL-1) | Recovery (%) ± R.S.D.a |
|---|---|---|---|---|---|
| Tobramycin | 0.1 | 0.05 | 0.152 | 0.052 | 104.00±0.45 |
| 0.10 | 0.202 | 0.102 | 102.00± 1.16 | ||
| 0.20 | 0.297 | 0.197 | 98.50±0.36 | ||
| 0.40 | 0.489 | 0.389 | 97.25±0.89 | ||
| 0.80 | 0.885 | 0.785 | 98.13 ± 1.08 |
Table 1: Determination of BKB in pharmaceutical formulation (n=5, t0.05,4=2.78).
Analysis of BKB in human plasma
The fluorescent probe was also applied to the determination of BKB in human plasma samples. The method accuracy was assessed by investigating the recovery of BKB at three concentration levels. The concentration of BKB in the plasma increased with the metabolic time from 0 h to 4 h, and reached a maximum in a period of 4 h to 4.5 h. Subsequently, the concentration of BKB in the plasma decreased. Five replicate measurements were performed for each concentration. The percentage recoveries Table 2 were between 97.07% ± 1.53% and 100.56% ± 0.46%. The relative standard deviations were all less than 2.00%. The result indicated that this method was promising as a cost effective, sensitive, and selective technique for study of the pharmacokinetics of BKB.
| Sampling time after oral administration (h) | Concentration of concentration of BKB in the plasma (μg mL-1) | Added (μg mL-1) | Amount found (μg mL-1) | Recovery (%) ± R.S.DA | ||
|---|---|---|---|---|---|---|
| Subject A | Subject B | Subject C | Subject B | Subject B | Subject B | |
| 1 | 0.89 | 1.05 | 1.33 | 1 | 1.99 | 97.07 ± 1.53 |
| 4 | 3.99 | 4.21 | 4.23 | 4 | 8.17 | 99.51 ± 0.25 |
| 8 | 2.34 | 2.87 | 3.11 | 2.5 | 5.4 | 100.56 ± 0.46 |
| 12 | 1.35 | 1.66 | 1.59 | 1.5 | 3.12 | 98.73 ± 1.13 |
| 24 | 0.23 | 0.45 | 0.44 | 0.5 | 0.93 | 97.89 ± 1.32 |
A: Average of five determinations.
Table 2: Fluorimetric determination of BKB in human plasma (n=5).
Conclusions
The supramolecular interactions of CB[7] with BKB and PAL provides a simple, rapid, accurate, and sensitive fluorescent method for the determination of BKB. PAL is an excellent fluorescent probe for the investigation of the host-guest complexation of BKB with CB[7] in aqueous solution because the fluorescence intensity of PAL changed significantly when PAL was expelled from the CB[7] cavity into the aqueous phase. The complexation behaviour was investigated by absorption, spectrofluorimetry, 1H NMR, and molecular modelling theoretical calculations. The combined results of these techniques allowed us to conclude that CB[7] formed a stable 1:1 inclusion complex with BKB in aqueous solution. This method has high sensitivity and good selectivity, and can be applied to the analysis of BKB in pharmaceutical preparations and plasma. Common excipients do not interfere with the measurement of BKB. This CB[7]-based fluorescence method will be useful for monitoring the metabolism of BKB in pharmaceutical studies. Related studies are in progress in our laboratory.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81402895). Helpful suggestions by anonymous referees are also gratefully acknowledged.
References
- Martínez Carballo E, Sitka A, Gonzalez Barreiro C, et al. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ Pollut. 2007;145(2):489-96.
- Van DVA, Lorgeoux C, Gromaire MC, et al. Analysis of quaternary ammonium compounds in urban stormwater samples. Environ Pollut. 2012;164:150-7.
- Ortiz S, Lopez V, Martinez-Suarez JV. The influence of subminimal inhibitory concentrations of benzalkonium chloride on biofilm formation by Listeria monocytogenes. Int J Food Microbiol. 2014;189:106-12.
- Tavlarakis P, Greyling J, Snow NH. Simultaneous determination of pramoxine HCl and benzalkonium chloride in wound care solutions by HPLC. J Anal Methods. 2010;2(6):722-7.
- Tamburro M, Ripabelli G, Vitullo M, et al. Gene expression in Listeria monocytogenes exposed to sublethal concentration of benzalkonium chloride. Comp Immunol Microbiol Infect Dis. 2015;40:31-9.
- Carbajo JB, Petre AL, Rosal R, et al. Ozonation as pre-treatment of activated sludge process of a wastewater containing benzalkonium chloride and NiO nanoparticles. Chem Eng J. 2016;28:740-9.
- Iwasawa A, Ayaki M, Niwano Y. Cell viability score (CVS) as a good indicator of critical concentration of benzalkonium chloride for toxicity in cultured ocular surface cell lines. RegToxicol Pharmacol. 2013;66(2):177-83.
- Galletti JG, Gabelloni ML, Morande PE, et al. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 2012;6(1)24:24-34.
- Georgiev GA, Yokoi N, Ivanova S, et al. Surface chemistry study of the interactions of hyaluronic acid and benzalkonium chloride with meibomian and corneal cell lipids. Soft Matter. 2013;9:10841-56.
- Kitazawa Y, Smith P, Sasaki N, et al. Travoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubled-masked comparison of safety and efficacy. Eye (Lond). 2011;25(9):1161-9.
- Mohapatra R, Senapati S, Sahoo C, et al. Transcorneal permeation of diclofenac as a function of temperature from film formulation in presence of triethanolamine and benzalkonium chloride. Colloids Surf B Biointerfaces. 2014;123:170-80.
- Takeoka GR, Dao LT, Wong RY, et al. Identification of benzalkonium chloride in commercial grapefruit seed extracts. J Agric Food Chem. 2005;53(19):7630-6.
- Kröckel L, Jira W, Wild D. Identification of benzalkonium chloride in food additives and its inefficacy against bacteria in minced meat and raw sausage batters. Technol. 2003;216:402.
- Kawakami S, Callicott RH, Zhang N. Trace analysis of benzalkonium chloride on skin by flow injection ionspray mass spectrometry-mass spectrometry. Analyst. 1998;123(3):489-91.
- Cao H, Kang M, Chen Z, et al. Determination of five quaternary ammonium compounds in foodstuffs using high performance liquid chromatography-tandem mass spectrometry. Anal Methods. 2014;6:4790-6.
- Abu Shawish HM, Khedr AM, Abed-Almonem KI, et al. A comparative study of solid and liquid inner contact benzalkonium chloride ion-selective electrode membranes. Talanta. 2012;101:211-9.
- Xue Y, Hieda Y, Kimura K, et al. Sensitive determination of benzalkonium chloride in blood and tissues using high-performance liquid chromatography with solid-phase extraction. Leg Med (Tokyo). 2002;4(4):232-8.
- Mehta J, Patel V, Kshatri N, et al.A versatile LC method for the simultaneous quantification of Latanoprost, Timolol and Benzalkonium chloride and related substances in the presence of their degradation products in ophthalmic solution. Anal Methods. 2010:11(2):1737-44.
- Gaber M, Abu Shawish HM, Khedr AM, et al. Determination of benzalkonium chloride preservative in pharmaceutical formulation of eye and ear drops using new potentiometric sensors. Mater Sci EngC. 2012;32(8):2299-305.
- Mallik R, Raman S, Liang X, et al. Development and validation of a rapid ultra-high performance liquid chromatography method for the assay of benzalkonium chloride using a quality-by-design approach. J Chromatogr A. 2015;1413:22-32.
- Hou YH, Wu CY, Ding WH. Development and validation of a capillary zone electrophoresis method for the determination of benzalkonium chlorides in ophthalmic solutions. J Chromatogr A. 2002;976(1-2):207-13.
- Taylor RB, Toasaksiri S, Reid RG. Determination of antibacterial quaternary ammonium compounds in lozenges by capillary electrophoresis. J Chromatogr A. 1998;798(1-2):335-43.
- Altria KD, Elgey J, Howells JS. Validated capillary electrophoretic method for the quantitative analysis of histamine acid phosphate and/or benzalkonium chloride. J Chromatogr B Biomed Appl. 1996;686(1):111-7.
- Wang R, Macartney DH. Molecular Dynamics of Methyl Viologen-Cucurbit[n]uril Complexes in Aqueous Solution. Tetrahedron Lett. 2008;49:311-4.
- Moghaddam S, Yang C, Rekharsky M, et al. New ultrahigh affinity host-guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: thermodynamic analysis and evaluation of M2 affinity calculations. J Am Chem Soc. 2011;133(10):3570-81.
- Cao L, Åšekutor M, Zavalij PY, et al. Cucurbit[7]uril guest pair with an attomolar dissociation constant. Angew Chem Int Ed Engl. 2014;53(4):988-93.
- Hart SL, Haines RI, Decken A, et al. Determination of ethambutol by a sensitive fluorescent probe. Inorg Chim Acta. 2009;36:4145-51.
- Lu LB, Yu DH, Zhang YQ, et al. Supramolecular assemblies based on some new methylsubstituted cucurbit[5]urils through hydrogen bonding. J Mol Struct. 2008;885:70-5.
- Zhou YY, Yang J, Liu M, et al. A novel fluorometric determination of melamine using cucurbit[7]uril. J Lumin. 2010;130:817-20.
- Miskolczy Z, Biczók L, Görner H. Tautomerization of lumichrome promoted by supramolecular complex formation with cucurbit [7] uril. J Photochem Photobiol A Chem. 2009;207(1):47-51.
- Wei F, Feng YQ. Rapid determination of aristolochic acid I and II in medicinal plants with high sensitivity by cucurbit[7]uril-modifier capillary zone electrophoresis. Talanta. 2008;74(4):619-24.
- Kim J, Jung IS, Kim SY, et al. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J Am Chem Soc. 2000;122:540-1.
- Day A, Arnold AP, Blanch RJ, et al. Controlling factors in the synthesis of cucurbituril and its homologues. J Org Chem. 2001;66(24):8094-100.
- Megyesi M, Biczók L, Jablonkai I. Highly sensitive fluorescence response to inclusion complex formation of berberine alkaloid with cucurbit [7] uril. J Phys Chem C. 2008;112(9):3410-6.
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785.
- Becke AD. A multicenter numerical integration scheme for polyatomic molecules. J Chem Phys. 1988;88:2547.
- Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A Gen Phys. 1988;38(6):3098-100.
- Wu WY, Yang JY, Du LM, et al. Determination of ethambutol by a sensitive fluorescent probe. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79(3):418-22.