Original Article
, Volume: 15( 1)Study of Physical and Chemical Properties of Cardanol Ethyl Ester for Its Suitability as Petro-Diesel
- *Correspondence:
- Saravanakumar S , AVC College of Engineering, Mayiladuthurai, Tamil Nadu, India, E-Mail: sskchowdry@gmail.com
Received: February 03, 2017; Accepted: February 24, 2017; Published: March 05, 2017
Citation: Saravanakumar Chowdry S and Sundar Raj C. Study of Physical and Chemical Properties of Cardanol Ethyl Ester for Its Suitability as Petro-Diesel. Int. J. Chem. Sci. 2017;14(1):103.
Abstract
Biodiesels can be the best renewable alternate energy source for diesel due to its economical and eco-friendly nature. The properties of the biodiesel depend on the individual fatty esters that the biodiesel is having. Important fuel properties of biodiesel influenced by the fatty acid profile of the various fatty esters are cetane number, cold flow, oxidative stability, and viscosity. In this study cardanol oil and its ethyl ester have been chosen to find out their suitability as petro-diesel. Experimental investigations were made to find out the physico-chemical properties of cardanol biofuel extracted from cashew nutshell and their ethyl esters produced through transesterification. The cardanol ethyl ester (CEE) was characterized as an alternative diesel fuel through series of ASTM standard fuel tests. A constant speed diesel engine, which develops 5.2 kW of power, was run with biodiesels and its performance was compared with diesel fuel. The CEE had 56% reduction of viscosity at 40°C and 9.2% heat value over its crude oil. Higher cetane number, specific gravity and lower flash and fire points were observed for CEE compared with neat diesel fuel. The fatty acid composition shows 10.8% increase in saturated and 23.4% decrease in unsaturated fat after tranesterification and their values were found to be within limits set by various international standards for biodiesel.
Keywords
Cardanol Ethyl Ester, Fatty acid profile, Transesterification, Physico-chemical properties, Diesel engine, Performance, and emission analysis
Introduction
The depletion nature of fossil fuels and fluctuation in crude oil prices are leading to find the alternative fuels sources to keep the energy security of a country by reducing its dependence on imported oil. India imports 64 billion dollars of crude oil in 2016 fiscal year for transport, industrial and agriculture applications. The alternative fuels must be cost effective, eco-friendly, and easily available. Biodiesel are clean sources of fuels which can reduce the emission like CO and NOx [1]. Currently, biodiesel derived from vegetable oils or animal fats is becoming popular as an environment friendly fuel [2]. The advantage of this biofuel over the conventional diesel fuel is its renewability and biodegradability. High cetane numbers, low smoke, low carbon dioxide and hydrocarbon emissions of bio diesel are some added advantages to consider for the replacement of diesel fuel [3,4]. However, high viscosity and low volatility of pure vegetable oil reduces fuel atomization and increases fuel spray penetration [5]. Researchers reported that polymerization of unsaturated fatty acids at elevated temperatures caused engine deposits and thickening of the lubricating oil. To overcome these difficulties approaches had been taken to improve the physical properties of vegetable oil by preheating, adding additives and blending the vegetable oils with other fuels etc., which had not given a long-term solution. Finally, vegetable oils in the form of alkyl esters and blends with diesel were found to be more attractive as biodiesel [6,7].
Several studies [8-13] have been carried out on preparation of biodiesel from waste cooking oil, animal fat and various vegetable oil. The fatty acid compositions of biodiesel which affects the physical and chemical properties of biodiesel derived from different sources are not same and they perform differently in diesel engine in terms of performance, emissions, and combustion. Hence, the need for standardization of biofuels physical and chemical properties has been widely recognized [14]. At present, many European countries have defined their own norms with a land mark on iodine number less than 115 which reflect the degree of unsaturation of the oil. Some biodiesel standards are ASTM D6751 (ASTM=American Society for Testing and Materials) and the European standard EN 14214, which was developed from previously existing standards. Biodiesel consists of fatty acids esters, the properties of the various individual fatty esters that comprise biodiesel determine the overall fuel properties of the biodiesel fuel. Hence, the structural features of the fatty acid and the alcohol moieties that comprise a fatty ester plays important role in the physico-chemical properties of biodiesel. Molecular chain length, degree of unsaturation, and branching of the chain are some of the structural features that influence the properties of fatty acids [15].The unsaturated fatty acid favor oxygenation to form hydro peroxides and in turn increases the risk of polymerization and acidification that may result in insoluble sediments and gums, which can lead to filter plugging and deposits in the fuel systems [16].Other important properties influenced by the fatty acid profile are cetane number and its related properties like exhaust emissions, heat of combustion, cold flow, oxidative stability, viscosity, and lubricity.
The cashew tree (Anacardium occidentale) a waste land crop is an evergreen tree that produces the cashew seed and the cashew apple. The shell of the cashew seed yields derivatives that can be used in many applications from lubricants to paints. Raw cashew nut shell contains around 20% oil. Cardanol, a non-edible oil obtained from cashew nut shell as a byproduct on deshelling contains more than 93% fatty acid composition can be a potential alternate for diesel engine Fatty acid methyl esters (FAME) were the first fatty acid esters to be introduced for use as biodiesel. However, there is a growing interest in the use of fatty acid ethyl esters (FAEE) in biodiesel since ethyl esters are a renewable biofuel and a greener product, than methyl esters. In addition, ethanol have superior solvency properties for vegetable oils and low toxicity compared to methanol [17]. Moreover, in the body, methanol is metabolized into formaldehyde and then formic acid whereas ethanol is metabolized in the body to acetaldehyde, which quickly converts to acetic acid and hence ethanol vapor does not cause blindness or any threatening to the respiratory system and hence is safe [18]. In this present investigation, Cardanol ethyl ester (biodiesel) was prepared from cardanol oil, all the biodiesel fuel properties were measured as per the ASTM standard and the biodiesel was tested in a diesel engine for performance and emission study.
Materials and Methods
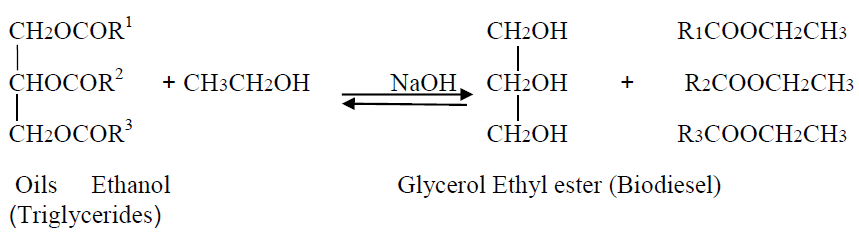
Transesterification is the process of reducing the high viscosity of triglycerides in to mono alkyl ester and glycerol with an alcohol in the presence of acidic or alkaline catalyst with reaction temperature below the boiling point of alcohol for a period of 30 minutes to 2 hours as shown in scheme 1.
A glass reactor fitted with a stirrer, an external heater and a condenser is used for transesterification process. 700 ml of good quality cardanol oil was taken in the reactor and was heated to 50ºC. An alkoxide prepared with 200 ml of 99% grade laboratory reagent type unhydrous ethyl alcohol and 4 gm of sodium hydroxide (catalyst) was then added to the reactor and the contents were again heated to 60ºC with continuous stirring for faster reaction. Reflux condenser condenses the evaporated alcohol back into the reactor. Stirring of the mixture was continued for 90 minutes at temperature between 60-65ºC at the boiling temperature of the alcohol. After two hours, the reaction was stopped and the product was allowed to settle down. The products settled in two layers and the upper layer consisted of ester and alcohol and was separated from the bottom layer of glycerin. The ester and alcohol thus obtained was distilled to remove and recover excess alcohol and the esters were washed with hot water to remove traces of glycerin and alkali. Finally, the product cardanol ethyl ester was dried for 1 hour in hot air oven at 105ºC. Various fuel properties of cardanol oil and cardanol ethyl esters were determined experimentally to ascertain their suitability as diesel fuel and subsequently used for engine test. ASTM standard test methods were used since the biodiesel standards of the ASTM specification D6751 are applicable for both fatty acid methyl esters (FAME) and FAEE, whereas the current European biodiesel standard (EN14214) is applicable only for FAME [19].
A single cylinder, four-stroke, water cooled diesel engine developing 5.2 kW as per the specifications given in Table 1 was used to find out the performance, emission, and combustion characteristics of CEE.
| Type | Vertical, Water cooled, DI four stroke |
|---|---|
| Power rating | 5.2 kW |
| Speed | 1500 rpm |
| Number of cylinder | One |
| Bore | 87.5 mm |
| Stroke | 110 mm |
| Compression ratio | 17.5:1 |
| Loading device | Eddy current |
| Injection timing | 23° before TDC |
| Injection pressure | 216 bar |
Table 1: Engine specification.
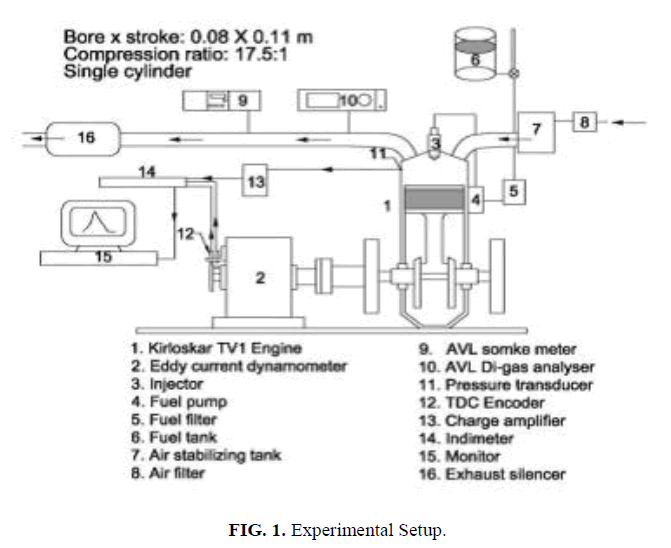
The engine speed was set constant at 1500 rpm for standard injection pressure of 216 bar. The engine was coupled to an eddy current dynamometer with control system. The time taken for fuel consumption was measured with the help of a digital stop watch. K-type thermocouple and a digital display were employed to note the exhaust gas temperature. AVL437C smoke meter was used to measure the smoke density. A non-dispersive infrared (NDIR-AVL-444) digasanalyzer was used to measure HC, CO, CO2, O2, and NOx emissions on dry basis. In cylinder pressure and heat release rate were measured by AVL combustion analyzer with 619 Indi meter hardware and indwin software version 2.2 consists of inbuilt analog to digital convertor, charge amplifier with PC interface. The schematic diagram of the experimental setup is shown in Figure. 1.
Results and Discussions
Fuel Composition
The ability of biodiesel to meet ASTM D 6751 standard criteria is dependent on the fatty acid composition [20]. The fatty acid composition for crude cardanol oil and CEE tested as per the method IS: 548 part 3, 1976 is presented in Table 1. The molecular structure of different types of unsaturated fatty acids and saturated fatty acids influence the combustion properties of a biodiesel. Fatty acids are having carbon chain skeleton with a carboxyl group (COOH) at one end. The carbon to hydrogen bonding is complete in the case of saturated fatty acids and hence there will not be any double bond between the carbon atoms whereas, monounsaturated fatty acids have one double bond and polyunsaturated fatty acids have more than one double bond. The chemical structure of biodiesel leads to a higher cetane number compared to diesel. Biodiesel fuel with more unsaturated fatty acid composition will have more density and less viscosity and more number of double bonds is prone to have less cetane number and heating value which directly affects the ignition delay and combustion process, on the other hand it will improve the flow properties particularly in low temperatures [21]. Gopinath, et al. found that the biodiesel having equal amount of saturated and unsaturated fatty acid composition proves a better fuel for diesel engine application in terms of thermal efficiency and NOx emission [22]. The CEE is having the advantages of 58.61% saturated and 30.87% unsaturated fat as referred in Table 2. The presence of 28.69% of poly unsaturated fat is an indication of good low temperature flow properties.
| Fuel | Saturated Fat (%) | Mono Unsaturated Fat (%) | Poly Unsaturated Fat (%) | Trans Fatty Acid (%) |
|---|---|---|---|---|
| Crude Cardanol Oil | 52.9 | 16.18 | 24.1 | 5.78 |
| Cardanol Ethyl Ester | 58.61 | 2.18 | 28.69 | 0.37 |
Table 2: Saturated and Unsaturated Fatty Acid Weight Percentage in Biodiesel.
Fuel Characterization
Table 3 shows the comparison of physical and chemical properties of crude cardanol and CEE tested in a certified laboratory with ASTM/EN14214 standards and diesel fuel. It is noticed that the important properties like heating value, cetane number, density and viscosity are favorable for CEE to alternate the diesel fuel.
| Fuel Properties | Units | Diesel | Crude cardanol Oil | CEE | ASTM/ EN14214 Limits |
|---|---|---|---|---|---|
| Specific gravity@ 20°C | Kg/m3 | 0.835 | 0.9216 | 0.8979 | |
| Density@20°C | Kg/m3 | 0.85 | 0.9209 | 0.8971 | 0.86-0.9 |
| Flash Point | °C | 80 | 171 | 136 | >130 |
| Cloud Point | °C | - | 20 | 6 | |
| Viscosity@40°C | cst | 2.8 | 25.77 | 4.94 | 1.9-6 |
| Fire Point | °C | 88 | 210 | 58 | - |
| Calorific value | KJ/Kg | 42000 | 42037 | 38192 | - |
| Cetane Number | - | 50 | 57 | 49 | Min 47 |
| Acid value | mgKOH/g | - | 5.7 | 0.37 | Max 0.8 |
| Iodine Value | G12/110g | - | 86.3 | 80.2 | Max 120 |
| Saponification Value | mg/KOH/g | - | 118 | 138 | Max 120/160 |
| Carbon Residue | mol/mol | - | 0.07 | 0.03 | Max 0.05 |
Table 3: Physico-chemical properties of CEE.
Cetane Number
Cetane number (CN) is a prime indicator of fuel quality in the domain of diesel engines like the octane number used for gasoline. In general fuel having high octane number tends to have a low CN and vice versa. For diesel engine operation, fuel with higher CN is an indication of shorter ignition delay in turn with lower nitrogen oxides (NOx) exhaust emissions [23]. However, NOx emissions increase slightly when operating a diesel engine on biodiesel in spite of the high CNs of fatty compounds due to higher unsaturation and decreasing chain length [24]. It was also reported that CNs decrease with increasing unsaturation and increase with increasing chain length. Hence the cetane scale clarifies why fatty acid alkyl esters are suitable as diesel fuel. Biodiesel with branched esters are of interest because they exhibit improved low-temperature properties. The ASTM standard for conventional diesel fuel requires a minimum CN of 40 while the standards for biodiesel prescribe a minimum of 47. The CN for CEE was observed as 49 since it has 30% unsaturation [25].
Higher Calorific Value
Higher calorific value is another fuel property to indicate the suitability of fatty compounds as diesel fuel. The heats of combustion of fatty esters and triacyclglycerols depends on the chain length [26]. The tested value of higher calorific value for CEE was found to be 38192 KJ/kg which was in agreement with the value of many of the biodiesel in practice.
Kinematic Viscosity
Viscosity is a property which affects the atomization of a fuel upon injection into the combustion chamber and in turn the spray characteristics and forms engine deposits. High viscosity is the reason why neat vegetable oils have not been used as alternative for diesel fuel. Transesterification decreases the viscosity by 56% for cardanol oil. Kinematic viscosity for CEE was found to be 4.94 mm2/s@40°C which is in accordance with the ASTM standards for biodiesel (1.9-6.0 mm2/s)
Specific gravity and density
The fuel consumption to generate a fixed power output depends on its density or specific gravity. These values are measured using capillary stopper relative specific gravity bottle and capillary stopper relative density bottle of 50 ml capacity. The value of density for CEE was observed as 0.8971 which is in accordance with the biodiesel standards.
Acid Number
The acid number is a property provides the measure of acidic substance in the oil and is expressed in KOH/g of a sample used to monitor the degradation of the oil in storage. Table 2 indicates that the acid value of crude and esterified cardanol oil is 5.7 mg KOH/g and 0.37 mg KOH/g of oil respectively. The value is higher in crude oil due to the presence of 5.78% of trans fatty acid while it is less for CEE because of the catalyst used in the esterification of the crude oil to neutralize some of the free fatty acid present in it. The result agrees with the range (less than 0.5 mg KOH/g) mentioned in ASTM standards.
Iodine value
Iodine value of oil is the indication of the presence of unsaturation, an accepted parameter for expressing the degree of carbon to carbon unsaturation. Rancidity of an oil may be predicted using this parameter as rancidity of triacyl glycerols depends on unsaturation. It is defined as g of iodine absorbed by 100 g of oil. The iodine value of CEE was found to be 80.2 which agrees with the limits mentioned in the ASTM standards for biodiesel.
Saponification value
Refluxing a known quantity of oil with an excess amount of alcoholic KOH is saponification. The excess NaOH available in the oil after saponification is known as saponification value. The saponification value of CEE was found to be 138 mg/KOH/g which agrees with the ASTM standards for biodiesel.
Cloud Point
The unsaturated fatty acid favour polymerization and acidification that may result in insoluble sediments and gums. The cloud point of crude cardanol and CEE was measured as 20°Cand 6°Crespectively as crude cardanol is having 9.6 wt% higher unsaturated fat than CEE.
Flash Point
The flash point of CEE was observed as 136°C which is in agreement with the ASTM standards for biodiesel.
Performance Analysis
CEE is less compressible than diesel fuel since it has more density than diesel. Therefore the pressure developed in the fuel line will be more for CEE and the fuel will be injected into the combustion chamber earlier in the compression cycle hence, advancement in dynamic injection time is expected.
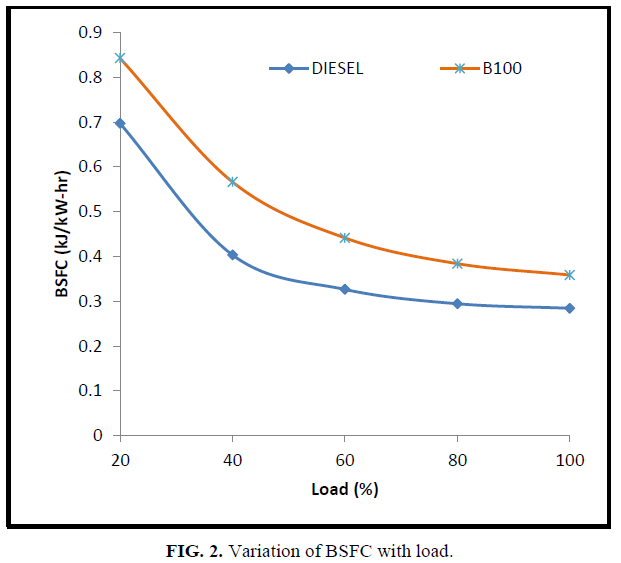
Figure 2 shows the variation of BSFC with brake power for CEE and diesel fuels. It can be observed that the specific fuel consumption for CEE at all loads is higher than diesel fuel. This may be attributed to the lower heating value and higher density of biodiesel fuels.
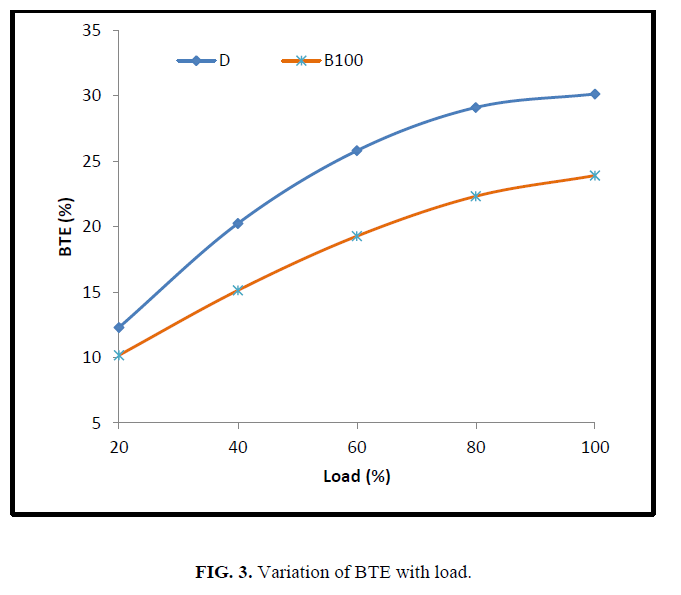
Figure 3 shows the variation of brake thermal efficiency of CEE at different loading conditions. From the figure, it can be noticed that the brake thermal efficiency is lesser than that of diesel fuel at all loads. The difference is low at low loads and is gradually increased as the load increases. This may be attributed to the polymerization of poly unsaturated fat at higher temperature in the presence of oxygen.
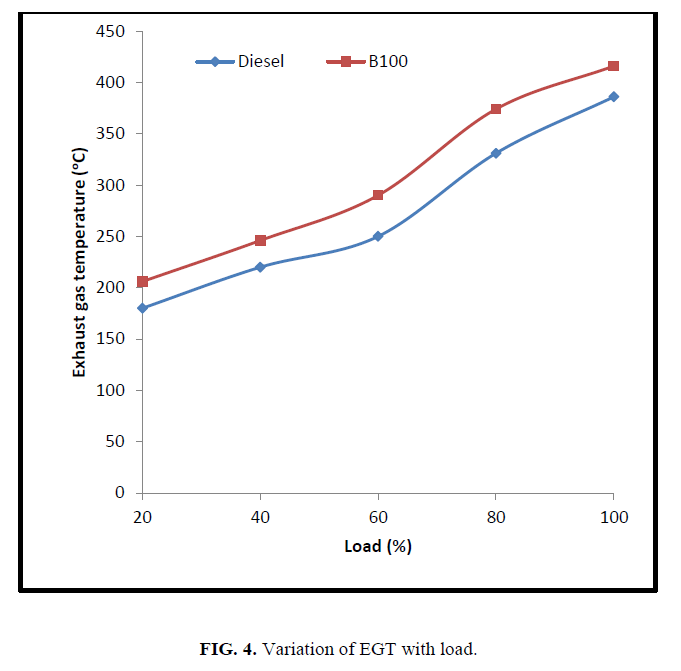
Figure 4 shows the exhaust gas temperature of CEE and diesel fuel with load. The exhaust gas temperature of CEE is higher than diesel fuel. The poly unsaturated fatty acid esters have higher ignition delay and shorter premixed combustion period which increases the diffusion combustion period and the exhaust gas temperature.
Emission Analysis
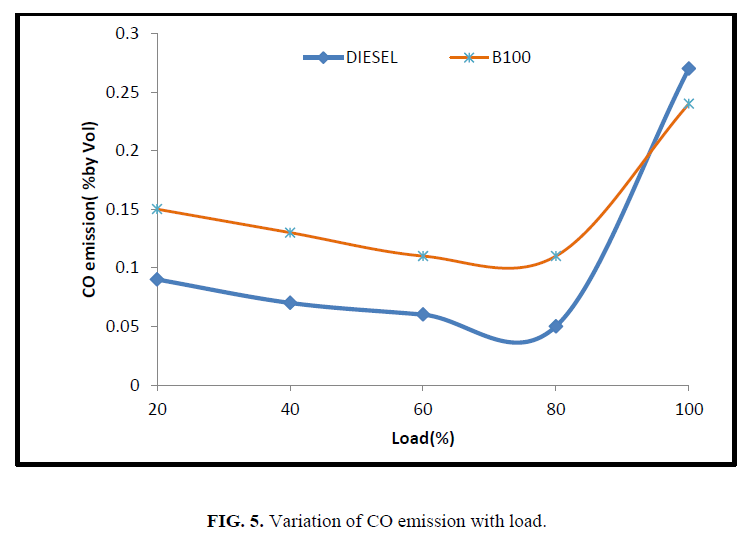
Carbon monoxide is an instable compound formed during the intermediate combustion stages of hydrocarbon fuels and having grater affinity with oxygen to complete the combustion by oxidation to form CO2. Lack of oxygen in the fuel or low gas temperature is the reason for the existence of CO in the flue gas. Figure 5 depicts the variation of CO emission for CEE and diesel fuel. From the figure, it’s observed that, an increase in load, results in lower CO emissions because of the increased gas temperature and oxidation reactions. However, due to low oxidants concentration and shorter reaction time, the increase in fuel-air ratio beyond a certain limit may reduce the elimination reactions, in spite of the increase in temperature results in higher CO emissions at peak load. CO emission for CEE was found to be higher than that of neat diesel at low and medium loads. This may be due to relatively poor atomization and lower volatility of biodiesel.
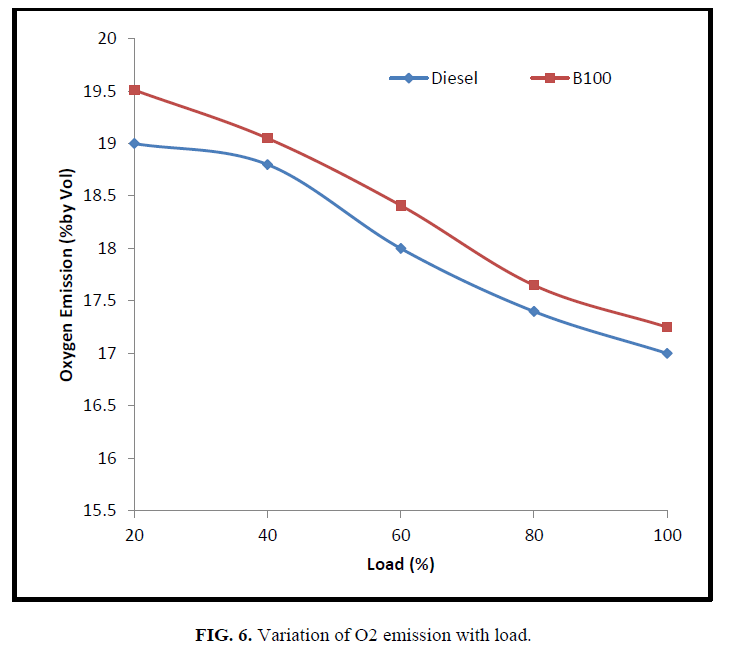
Figure 6 indicates the oxygen present in the exhaust gas is higher for CEE than diesel fuel. The stoichiometric air requirement for biodiesel is lesser for biodiesel compared to diesel fuel as the biodiesel is having oxygen molecule in the fuel itself. Hence ester based fuel demands lesser oxygen compared to diesel fuel for complete combustion. Whereas, the engine is designed in such a way that the intake air is constant for all fuels, leading to higher O2% in the exhaust gas for CEE than the diesel fuel. As load increases, the fuel-air ratio increases and hence O2% in the exhaust decreases for both the fuels.
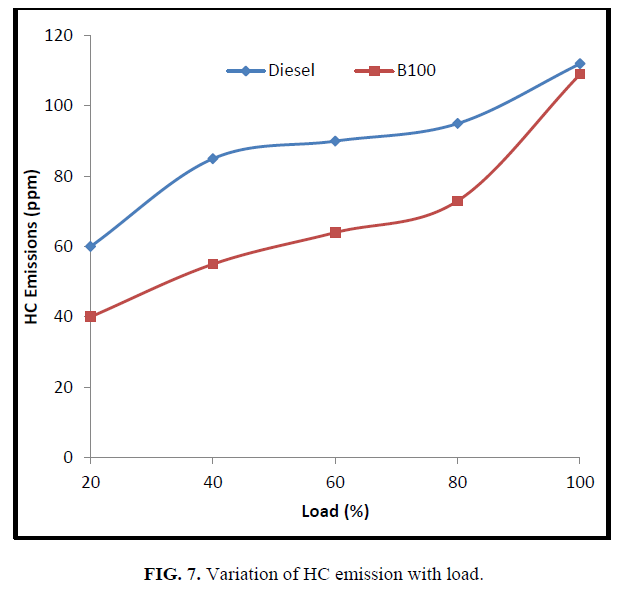
It was observed from the Figure. 7 that the magnitude of HC emission increased when the load was increased for both the fuels as more amount of fuel is injected at high load to maintain engine speed constant. The diesel fuel produced more hydrocarbons as compared with CEE, due to the lack of the oxygen content.
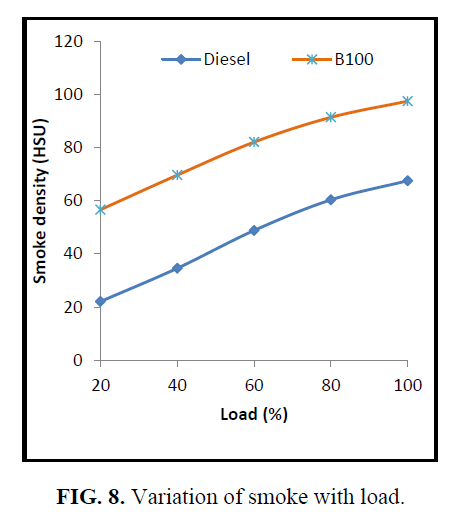
The variation of smoke density for different blends is depicted in Figure. 8. Smoke density for CEE blends is noticed to be higher than that of the diesel oil. This is due to the heavier molecular structure, poor atomization, presence of high carbon residue in the CEE.
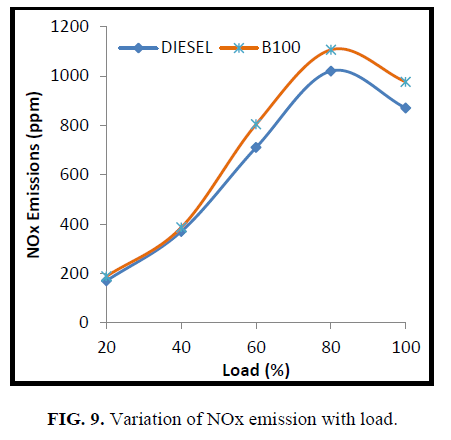
The variation of NOx emission at different loading conditions is shown in Figure 6. With biodiesel, depending on its ignition quality and premixed-burn fraction either increase or decrease in NOx emission can be observed [19]. Earlier start of combustion of biodiesel increased in-cylinder temperature and the rate of NOx formation may increase, on the other hand low volatility, higher viscosity and higher density will decrease the fuel atomization and combustion and the net result was slightly higher NOx emissions when engine was running with biodiesel.
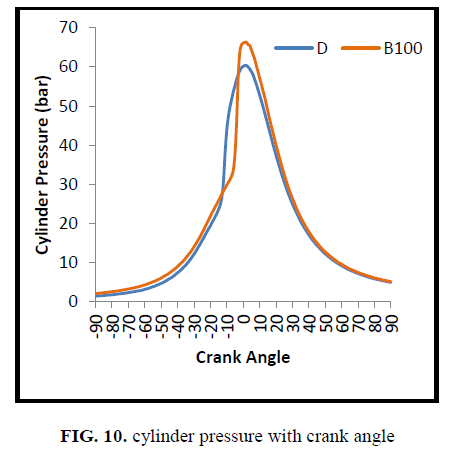
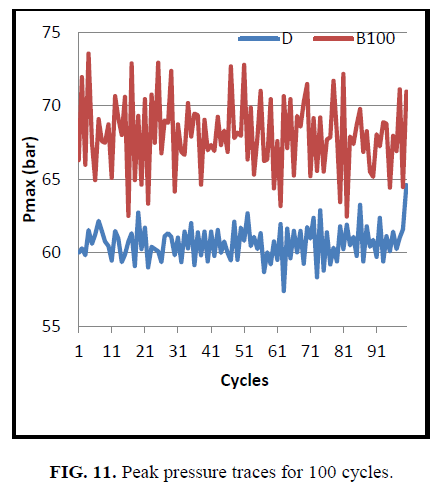
Figure. 10 and Figure. 11 shows the in cylinder pressure with crank angle and maximum pressure rise for 100 cycles respectively. Cylinder pressure and heat release rate depends on the fraction of fuel burned during the premixed burning phase. The cylinder pressure for CEE was found to be higher compared to diesel at all crank angles. It could be attributed to the increased ignition delay that shortened the diffusion combustion and quickens the initiation of combustion [25]. Higher Ignition delay leads to higher combustion duration and gathering of more fuel in the premixed combustion phase that causes faster combustion and higher peak pressure.
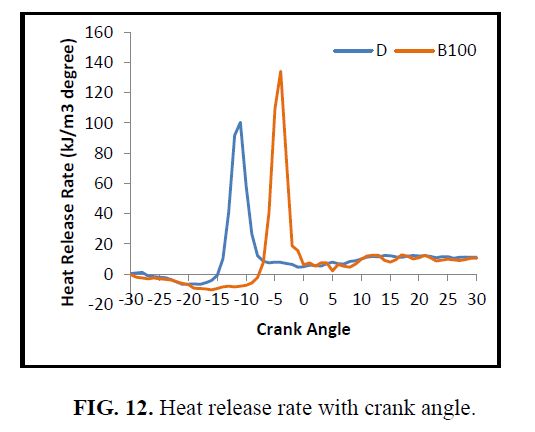
The maximum heat release rate with crank the variation of crank angle to read its effect on the premixed and diffusion combustion zone is shown in Figure. 12. Due to the presence of unsaturated fatty ester CEE has a longer ignition delay, higher maximum burn rate, and a longer premixed burn fraction. The reason for longer ignition delay may be due to higher specific heat of CEE fuel which needs more heat energy to evaporate inside the cylinder by absorbing heat from the combustion chamber. As a result, the heat release rate for CEE is higher than diesel fuel and is in agreement with the higher exhaust gas temperature.
Conclusion
In this study, cardanol oil biodiesel was obtained alkaline catalysis esterification with 6/1 molar ratio of ethanol to oil at 55ºC and its fatty acid profile was analyzed. Fuel properties including CN, calorific value, kinematic viscosity, specific gravity, density, flash point, cloud point, acid value, iodine value, free and saponification value etc. were characterized. The biodiesel produced was tested in as single cylinder diesel engine for performance, combustion and flue gas analysis. It was found that all the specifications were completely met and exceeded ASTM D 6751 standard. The experimental investigations indicated slight reduction in BTE compared with diesel fuel with comparable emission including slight reduction of HC. Further investigations to compensate the power loss by blending the fuel with diesel and additives can make this feedstock for biodiesel production will expand the utilization and make the biodiesel industry more viable.
References
- Krawczyk T. Biodiesel-Alternative fuel makes inroads but hurdle remains. INFORM 7. 1996;pp:801-15.
- Chand N. Plant oils-Fuel of the future. J Sci Ind Res. 2002;61:7-16.
- Jaecker-Voirol A, Delfort B, Montagne X, et al. Glycerol derivatives for diesel fuel reformulation. SAE Paper 2005;1:2203.
- Atadashi IM, ArouaMK, AzizAA. High quality biodiesel and its diesel engine application: A review. Renewable and Sustainable Energy Reviews 2010;14:1999-2008.
- Vellguth G. Performance of vegetable oils and their monsters as fuels for Diesel Engines. SAE Paper No-831358. 1983.
- Murayama T, Young T, Miyamoto N, et al. Low carbon Flower buildup. Low smoke, and Efficient Diesel Operation with vegetable Oils by Conversion to Mono-Esters and Blending with Diesel Oil or Alcohols.1984.
- Staat F, Gateau P. The effects of rapeseed oil methyl ester on diesel engine performance exhaust emissions and long- term behavior- A summary of three years of experimentation. SAE Paper No-950053. 1995.
- Tashtoush G, Al-Widyan M, Al-Shyouck A. Combustion performance and emissions of ethyl ester of a waste vegetable oil in a water-cooled furnace. Appl Therm Eng 2002;23:285-93.
- Wu F, Wang J, Chen W. A study on emission performance of a diesel engine fueled with five typical methyl ester biodiesels. Atmos Environ. 2009;43:148-5.
- Pramanik K. Properties and use of jatrophacurcas oil and diesel fuel blend in compression ignition engine. Renewable Energy 2003;28:239-48.
- Gerhard K, Steidley RK. Kinematic viscosity of biodiesel fuel component and related compounds. Influence of compound structure and comparison to petro-diesel fuel components. Fuel 2005;84:1059-65.
- Muragesan A, Umarani C, Chinnusamy TR, et al. Production and analysis of biodiesel from non-edible oils: A Review. Renewable and Sustainable Energy Reviews 2009;13:825-34.
- Ved K, Padam K. Study of Physical and Chemical Properties of Biodiesel from Sorghum Oil. Research Journal of Chemical Sciences 2013;3:64-8.
- Casetra G. Utilization of vegetable oils and their derivatives as diesel fuel. Italy: Proceedings of Seminar on Vegetable Oils as Transport Fuels. 1993;137-43.
- Gerhard K. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology 2005;86:1059-70.
- SukumarP, Saravanan N,Nagarajan G, et al. Effect of biodiesel unsaturated fatty acid on combustion characteristics of a DI compression ignition engine, biomass and bio energy.2010;34:1079-88.
- Abbaszaadeh A, Ghobadian B, Omidkhah MR, et al. Current biodiesel production technologies: a comparative review. Energy Conversion and Management2012;63:138-48.
- Firdaus M, Yusoff M, Xu X. Comparison of Fatty Acid Methyl and Ethyl Esters as Biodiesel Base Stock: a Review on Processing and Production Requirements. J Am Oil ChemSoc 2014;91:525-31.
- European Commission. White paper on internationally compatible biofuel standards. 2007.
- Andrew K, Cuevas JA, Mark F, et al. Potential of Jatrophacurcas as a source of renewable oil and animal feed. Journal of Experimental Botany 2009;60:2897-2905.
- Keith O. A review of Jatrophacurcas: an oil plant of unfulfilled promise. Biomass and Bioenergy 2000;19:1-15.
- Gopinath A, Puhan S, Nagarajan G. Effect of unsaturated fatty acid esters of biodiesel fuels on combustion, performance and emission characteristics of a DI diesel engine. International Journal of Energy and Environment 2010;1:411-30.
- Ladommatos N, Parsi M, Knowles A. The effect of fuel cetane improver on diesel pollutant emissions. Fuel1996;75:8-14.
- RL McCormick, MS Graboski, Alleman TL, et al. Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environmental Science &Technology 35:1742-7.
- Vairamuthu G, Sundarapandian S, Kailasanathan C, et al. Experimental investigation on the effects of cerium oxide nanoparticle on Calophylluminophyllum (Punnai) biodiesel blended with diesel fuel inDI diesel engine modified by nozzle geometry. J Energy Inst. 2015;1-15.