Original Article
, Volume: 9( 6)Study of Molecular Interactions in Aqueous 2-Amino-5-Nitrothiazole-NiCl2 Solution at Different Temperatures and Concentrations
- *Correspondence:
- Thakare AR , Shankarlal Agrawal Science College, Salekasa, District Gondia (M.S) 441916 India, Tel: 07180-244355; E-mail: avinashrthakare@yahoo.com
Received: November 28, 2016; Accepted: December 09, 2016; Published: December 12, 2016
Citation: Thakare AR, Naik AB. Study of Molecular Interactions in Aqueous 2-Amino-5-Nitrothiazole-NiCl2 Solution at Different Temperatures and Concentrations. Chemxpress. 2016;9(6):110.
Abstract
Ultrasonic velocity and density of aqueous 2-amino-5-nitrothiazole – NiCl2 solution was guarded at different temperatures (303.15 K, 308.15 K, 313.15 K and 318.15 K) and concentrations, so as to know about the molecular interaction between solute and solvent. In these studies acoustical parameters such as intermolecular free length, isentropic compressibility, specific acoustic impedance, relative association and ultrasonic velocity number were used to interpret types of molecular interactions present in the aqueous 2-amino-5-nitrothiazole -NiCl2 solution of different concentrations at different temperatures. Measured values indicate the presence of effective correlation in the form of solute - solvent interactions and metal-ligand interactions for different concentration and temperature.
Keywords
Molecular interactions; 2-amino-5-nitrothiazole; Nicl2; Acoustical parameters
Introduction
During isolation, purification, modification and application, cellulose undergoes the action of various liquids. As a result of interaction, cellulose sorbs a liquid that is accompanied by diverse changes in structure and properties of this biopolymer depending on nature of the liquid. Negligible sorption of non-and low polar liquids (e.g. hydrocarbons, higher alcohols) has a small effect on mechanical properties [1] and reactivity [2] of cellulose. On the other hand, increased sorption of water and other highly polar liquids by cellulose reduces mechanical properties [2,3], lowers glass temperature and increases reactivity of the biopolymer [4].
As is known, sorption process of various liquids by cellulose samples is accompanied by an exothermic heat effect of interaction, which depends on the structure of cellulose and nature of the liquid [5-9]. It is also found that for the same sample the exothermic heat effect of interaction depends on nature of the liquid, and increases in the following order of the liquids: hydrocarbons < higher alcohols < lower alcohols < water < polar organic solvents [9].
Since the heat effect of interaction is caused by sorption, these two physicochemical characteristics should be correlated with each other. Unfortunately, a relationship between sorption value and heat of interaction has been investigated in a very limited extent, mainly for cellulose-water system [9,10]. Therefore, the purpose of this research was to study the dependence of maximum equilibrium sorption on heat of interaction using liquids of various nature and cellulose samples with different structural features. Another purpose was to use the obtained relationship to predict the sorption value and its impact on various properties of cellulose and cellulose materials.
Materials and Methods
The solvents i.e. water used in this investigation were purified by using KMnO4 and NaOH through double distillation. The ligand 2-amino-5-nitrothiazole (Hi-MEDIA, minimum wt. 97%), used are of synthesis grade. The ligand and metal solutions in water were prepared by dissolving an accurate amount in an aqueous solvent in standard flask with airtight caps and the mass measurement were performed using high precision digital balance (Adair Datta of accuracy ± 0.01 mg). The ultrasonic velocities 2-amino-5-nitrothiazole-NiCl2 aqueous solutions of different concentration were measured by ultrasonic interferometer (Mittal enterprises, model F-81s) at 2 MHz having accuracy ± 1 m·s−1 in velocity. It consists of high frequency generator and a measuring cell. The densities of experimental solutions measured by using digital density meter (Anton Paar DMA 35 of accuracy ± 0.001). A thermostatically controlled well-stirred water bath whose temperature was controlled to ± 0.1 K was used for all the measurements.
Experimental Theory
Numerous methods are available in the literature for measuring ultrasonic velocity in solid and liquids. The ultrasonic interferometer is considered as more reliable and precise instrument. The expression used to determine ultrasonic velocity using ultrasonic interferometer is:
u = vλ (1)
Where u is ultrasonic velocity and λ is wavelength.
The isentropic compressibility βs was calculated from following equation:
βs = 1/ρu2 (2)
Where ρ is density of solution and u is speed of ultrasonic velocity.
The intermolecular free length Lf is calculated by using the standard expression:
Lf = K βs1/2 (3)
Where K is temperature dependent constant known as a Jacobson constant [5].
The acoustic impedance Z is obtained by equation:
Z = uρ (4)
The relative association RA was calculated by the following equation:
RA = (ρ/ρo) (uo/u) 1/3 (5)
Where ρo is density of solvent and uo is velocity of solvent. Also the sound velocity number is calculated from following equation;
[U] = u-uo/uoc (6)
[U] is sound velocity number; c is concentration of the solute.
[U] is sound velocity number; c is concentration of the solute.
Results and Discussion
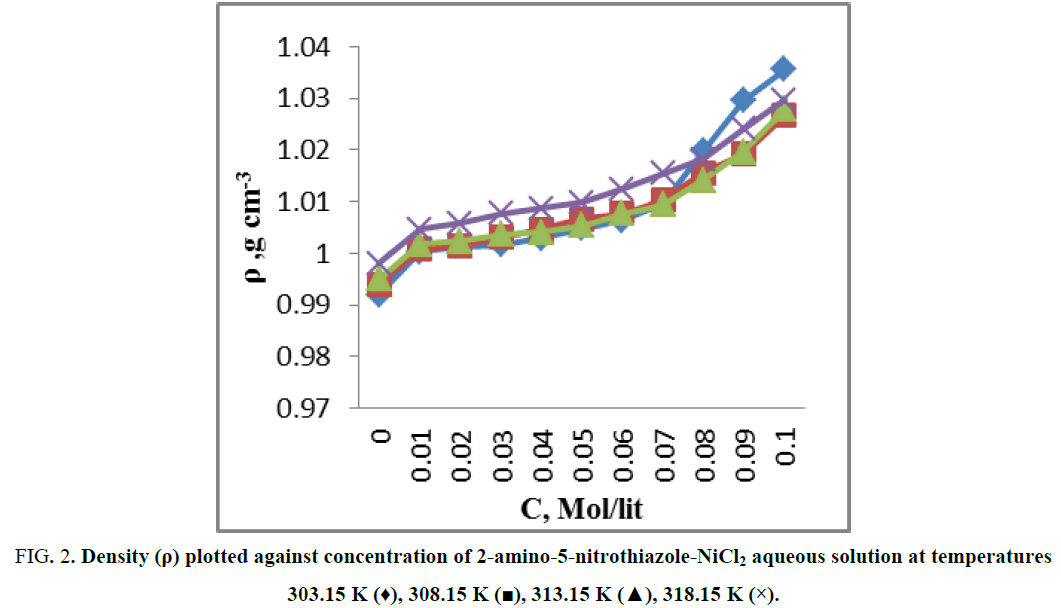
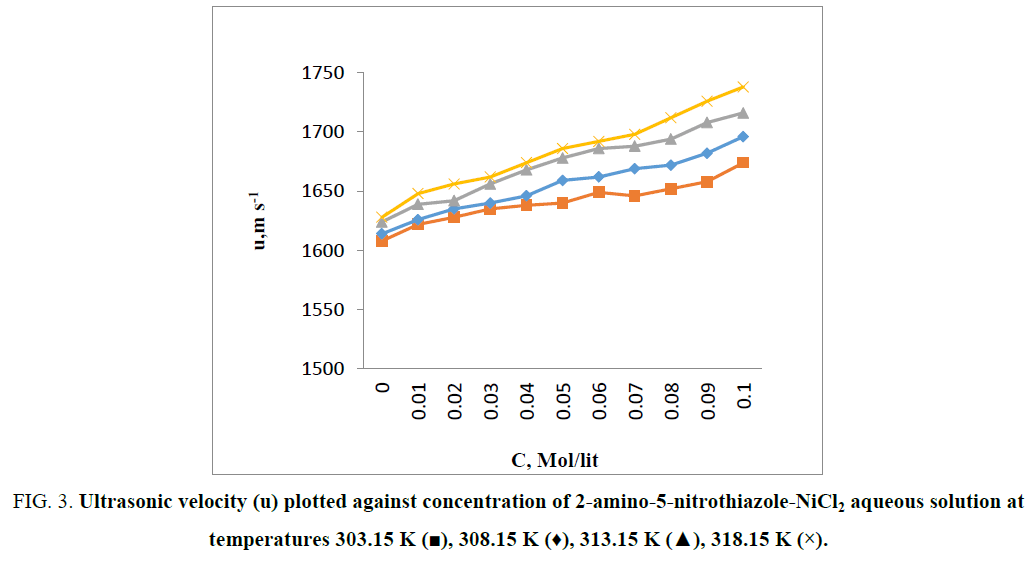
The measured values of ultrasonic velocities and densities for 2-amino-5-nitrothiazole-NiCl2 (Figure 1) aqueous solution at temperatures (303.15 K, 308.15 K, 313.15 K and 318.15 K) listed in Table 1, drawn in Figure 2 and Figure 3. From Figure 2 it is observed that the values of density increased as the concentration of 2-amino-5-nitrothiazole increases & same with temperature. From Figure 3 it is observed that the values of ultrasonic velocity increases with increase in concentration of 2-amino-5-nitrothiazole, this indicates the association of molecules in the experimental solution [6]. This may be due to dipole-dipole interactions and intermolecular hydrogen bonding between solute and solvent molecules. More number of monomeric water molecules is formed due to breaking down of hydrogen bonding between water molecules with increase in temperature. Monomeric water molecules get trapped in the vacant position present in the cage like structure of water molecules [7]. Due to addition of 2-amino-5-nitrothiazole to the aqueous NiCl2 the interaction between water and NiCl2 decreases as a result lowering of compressibility between molecules in the solutions. This result, polar molecules of 2-amino-5-nitrothiazole forms more compact structure with the water molecule through intermolecular hydrogen bonding [8]. The solution is predominantly composed of water-water interaction which forms the typical three dimensional cage-like structure of water and increase in association observed in the experimental solutions due to water structure enhancement brought by an increase in electrostriction/ means in presence of NiCl2 [7], due to which increase in ultrasonic velocity takes place.
Figure 2: Density (ρ) plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
Figure 3: Ultrasonic velocity (u) plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (?), 308.15 K (♦), 313.15 K (?), 318.15 K (×).
| C, m/lit | ρ ,kg m-3 | u, m s-1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 303.15 K | 308.15 K | 313.15 K | 318.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | |
| 0.00 | 0.992 | 0.994 | 0.995 | 0.998 | 1608.01 | 1614.02 | 1624.06 | 1628.01 |
| 0.01 | 1.0003 | 1.0007 | 1.0018 | 1.0046 | 1622.06 | 1626.04 | 1639.02 | 1648.02 |
| 0.02 | 1.0013 | 1.0015 | 1.0024 | 1.0058 | 1628.02 | 1635.08 | 1642.03 | 1656.09 |
| 0.03 | 1.0016 | 1.0031 | 1.0036 | 1.0076 | 1635.03 | 1640.02 | 1656.08 | 1662.01 |
| 0.04 | 1.0029 | 1.0046 | 1.0041 | 1.0086 | 1638.02 | 1646.06 | 1668.02 | 1674.02 |
| 0.05 | 1.0048 | 1.0067 | 1.0053 | 1.0098 | 1640.07 | 1659.06 | 1678.06 | 1686.02 |
| 0.06 | 1.0063 | 1.0076 | 1.0076 | 1.0124 | 1649.06 | 1662.01 | 1686.08 | 1692.03 |
| 0.07 | 1.0097 | 1.0103 | 1.0094 | 1.0154 | 1646.09 | 1669.03 | 1688.04 | 1698.08 |
| 0.08 | 1.0198 | 1.0156 | 1.0142 | 1.0182 | 1652.05 | 1672.04 | 1694.06 | 1712.05 |
| 0.09 | 1.0296 | 1.0192 | 1.0196 | 1.0241 | 1658.06 | 1682.08 | 1708.06 | 1726.02 |
| 0.01 | 1.0356 | 1.0267 | 1.0278 | 1.0297 | 1674.08 | 1696.04 | 1716.08 | 1738.04 |
| Uncertain ties in temperature, density and velocity are 0.1 K,1 m s-1 and 0.0005 k g m-3 | ||||||||
Table 1: Density (ρ) and ultrasonic velocity (u) values for 2-amino-5-nitrothiazole – NiCl2 aqueous solution at different concentrations and temperatures.
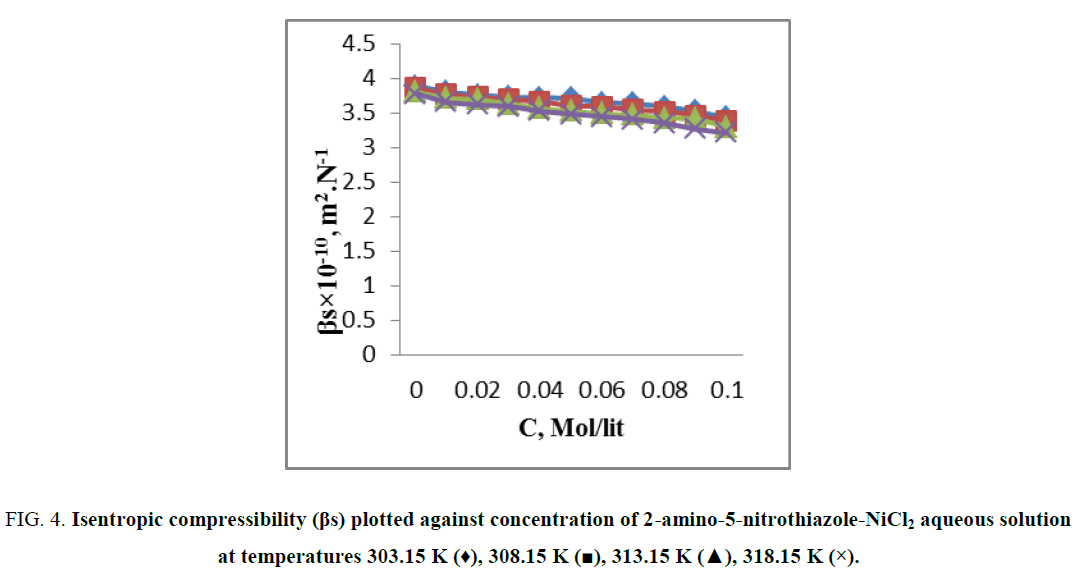
When ultrasonic waves incident on the solution, the molecules get perturbed. The reason is medium has some elasticity and due to this perturbed molecules regain their equilibrium positions [9]. When a solute is added to a solvent, its molecules attract certain solvent molecules toward them; this phenomenon is known as compression. Every solvent has a limit for compression and is known as limiting compressibility. The compressibility of the solution is lowers than that of solvent due to the increase in volumetric concentration of solution with decrease in compressibility of the solution. However the electrostrictive forces cause breakage in the structure of water with increase in solute concentration and compact packing occurs with the water molecules surrounding the solute due to which reducing of compressibility occurs. From Figure 4 it is observed that the isentropic compressibility for 2-amino-5-nitrothiazole- NiCl2 aqueous solution decreases with increase in the 2-amino-5-nitrothiazole concentration and temperature. This indicate the existence of strong solute- solvent interaction through dipole-dipole and acceptor – donor interactions of the –NH2 group of 2-amino-5-nitrothiazole with surrounding water molecules [9,10].
Figure 4: Isentropic compressibility (βs) plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
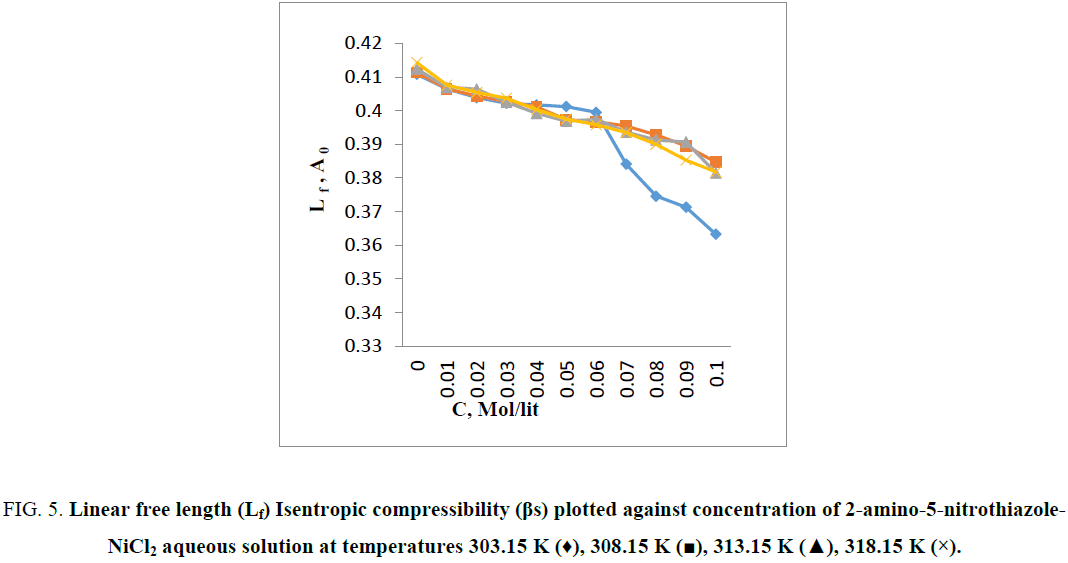
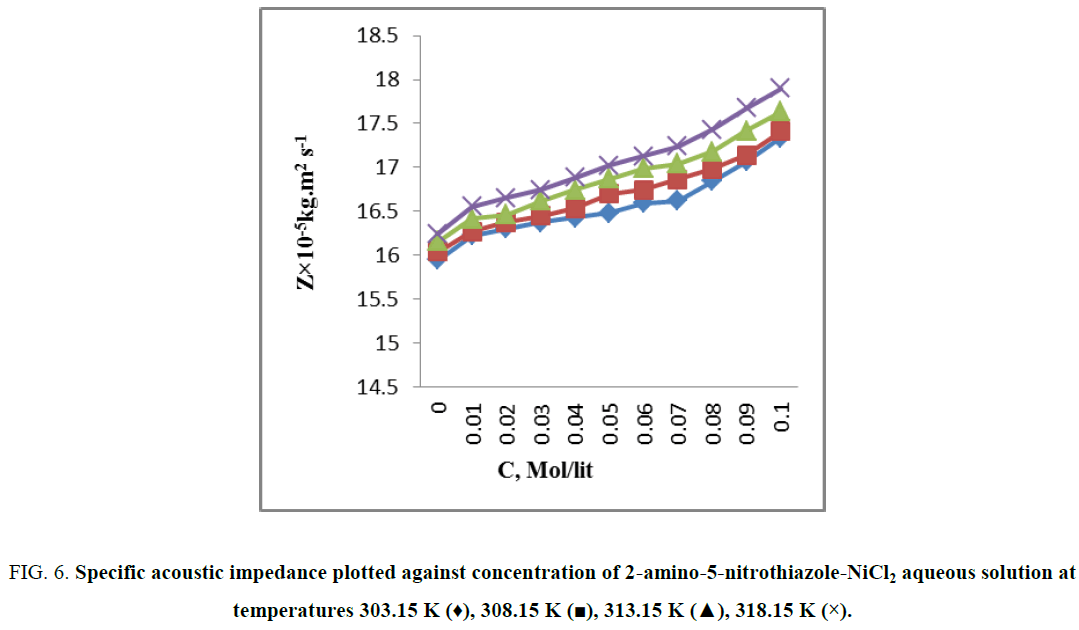
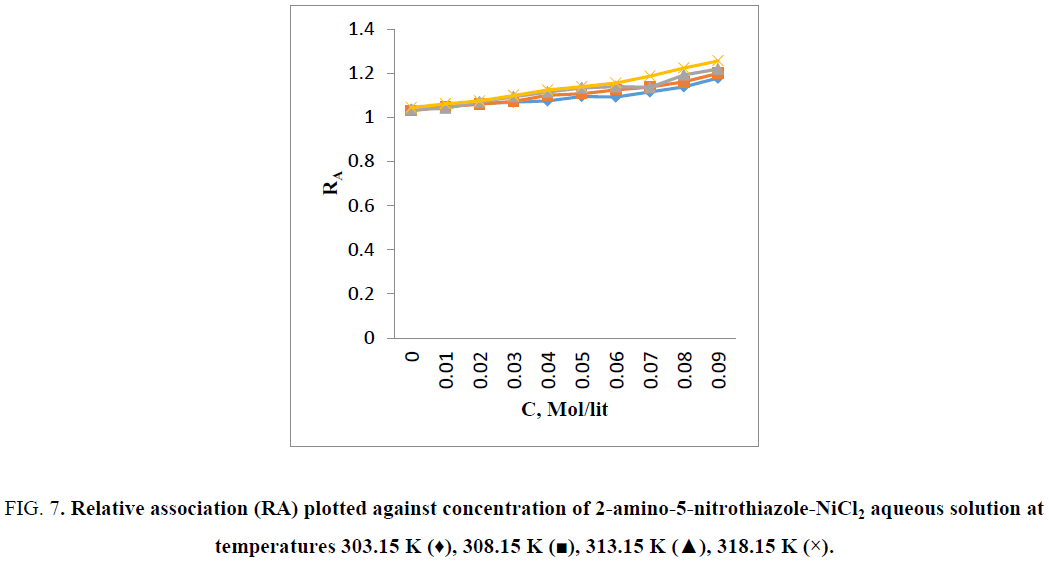
From Figure 5 it is observed that the intermolecular free length decreases which indicates closer packing [7]. Variation of ultrasonic velocity in a solution depends on the intermolecular free length on mixing. On the basis of a model for sound propagation given by Erying and Kincaid [11], ultrasonic velocity increases with decrease of free length (Lf) and vice versa. Intermolecular free length is a predominant factor for determining the variation of ultrasonic velocity in liquids and their solutions [12]. Intermolecular free length (LF) is the distance between the surfaces of the neighboring molecules and indicates a significant interaction between solute–solvent as well as metal-ligand [13] suggesting a structure promoting tendency of 2-amino-5-nitrothiazole-NiCl2 in aqueous solution [14]. When the ultrasonic wave travels through a solution, some part of it travels through the medium and remaining part of ultrasonic wave gets reflected by the solute [15] it means solutes restricts free flow of sound wave. The character that decreases this restriction or backward movement of sound waves is known as acoustic impedance (Z). The specific acoustic impedance is dependent on both concentration and temperature of the solution. As the internal pressure and cohesive energy [16] increases with solute concentration, strong intermolecular hydrogen bonding occurs between 2-amino-5-nitrothiazole and water molecule. Hence, an increase in specific acoustic impedance is caused by an increase in instantaneous pressure exert at any molecule (2-amino-5-nitrothiazole - NiCl2 in aqueous solution) with propagation of a sound wave (Figure 6). It is to be noted that the Ni–2-amino-5-nitrothiazole complex is formed by bonding between Ni (II) and the amino group of 2-amino-5-nitrothiazole [17,18]. Figure 7 shows that the relative association (RA) increases with increase in temperature and the increase of concentration of 2-amino-5-nitrothiazole. This is due to the solute-solvent interaction and metal -ligand interactions. It depends on either the breaking up of the solvent molecules on addition of solute molecules in solvent at certain temperature or the solvation of ions that are present [19,20].
Figure 5: Linear free length (Lf) Isentropic compressibility (βs) plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
Figure 6: Specific acoustic impedance plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
Figure 7: Relative association (RA) plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
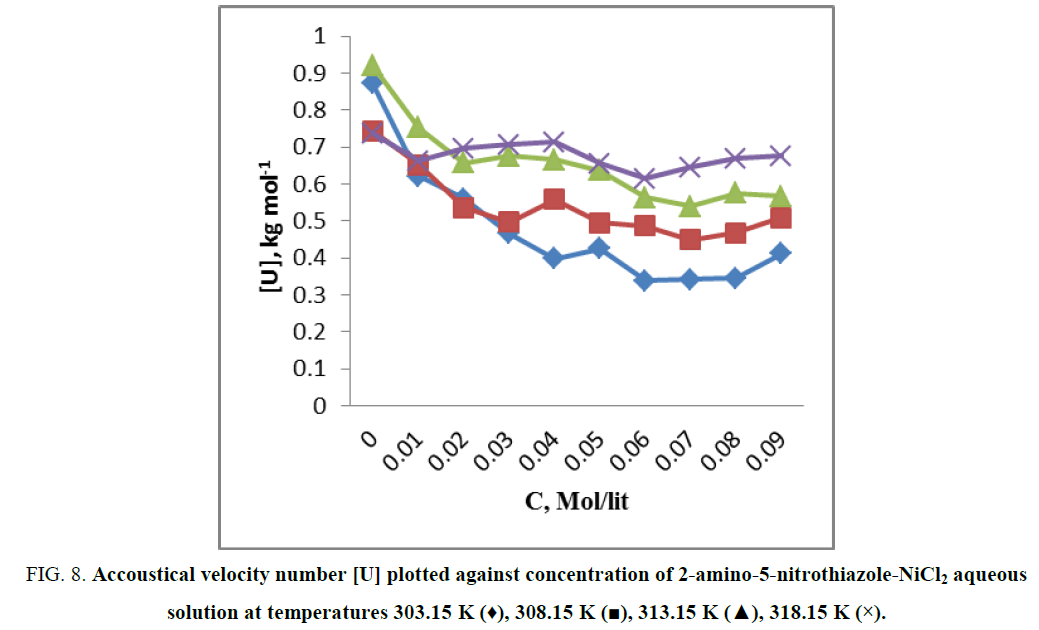
In general sound velocity number increases with increase in concentration of solute [21] and increase in temperature, however in present investigation there is first decrease in sound velocity number with increase in concentration but after some interval of increase in concentration, U remain constant (Figure 8). Again sound velocity number increase as temperature goes on increases (Table 2).
Figure 8: Accoustical velocity number [U] plotted against concentration of 2-amino-5-nitrothiazole-NiCl2 aqueous solution at temperatures 303.15 K (♦), 308.15 K (?), 313.15 K (?), 318.15 K (×).
| C, mol/lit | βs×10-10, m2.N-1 | RA | Z×10-5kg. m2 s-1 | L f, A 0 | [U], kg mol-1 |
|---|---|---|---|---|---|
| At 303.15 K | |||||
| 0.00 | 3.89 | - | 15.951 | 0.4108 | - |
| 0.01 | 3.81 | 1.035081 | 16.225 | 0.4065 | 0.8737 |
| 0.02 | 3.76 | 1.047478 | 16.301 | 0.4039 | 0.6221 |
| 0.03 | 3.73 | 1.061595 | 16.376 | 0.4022 | 0.5601 |
| 0.04 | 3.72 | 1.06882 | 16.427 | 0.4017 | 0.4665 |
| 0.05 | 3.71 | 1.074872 | 16.479 | 0.4012 | 0.3987 |
| 0.06 | 3.65 | 1.094112 | 16.594 | 0.3795 | 0.4254 |
| 0.07 | 3.64 | 1.092057 | 16.621 | 0.9741 | 0.3383 |
| 0.08 | 3.59 | 1.114944 | 16.847 | 0.3946 | 0.3423 |
| 0.09 | 3.53 | 1.137913 | 17.071 | 0.3913 | 0.3458 |
| 0.10 | 3.44 | 1.178058 | 17.336 | 0.8633 | 0.4108 |
| At 308.15 K | |||||
| 0.00 | 3.86 | - | 16.043 | 0.4114 | - |
| 0.01 | 3.77 | 1.029384 | 16.271 | 0.4065 | 0.7447 |
| 0.02 | 3.73 | 1.047518 | 16.375 | 0.4044 | 0.6524 |
| 0.03 | 3.70 | 1.058806 | 16.451 | 0.4027 | 0.5369 |
| 0.04 | 3.67 | 1.072103 | 16.536 | 0.4011 | 0.4962 |
| 0.05 | 3.60 | 1.100044 | 16.701 | 0.3973 | 0.5581 |
| 0.06 | 3.59 | 1.106814 | 16.746 | 0.3967 | 0.4955 |
| 0.07 | 3.55 | 1.123938 | 16.862 | 0.9454 | 0.4868 |
| 0.08 | 3.52 | 1.136244 | 16.981 | 0.3928 | 0.4493 |
| 0.09 | 3.46 | 1.160689 | 17.143 | 0.3895 | 0.4685 |
| 0.10 | 3.38 | 1.198541 | 17.413 | 0.3847 | 0.5081 |
| At 313.15 K | |||||
| 0.00 | 3.81 | - | 16.159 | 0.4124 | - |
| 0.01 | 3.71 | 1.034915 | 16.419 | 0.4069 | 0.9211 |
| 0.02 | 3.70 | 1.041250 | 16.459 | 0.4064 | 0.5532 |
| 0.03 | 3.63 | 1.069486 | 16.620 | 0.4025 | 0.6572 |
| 0.04 | 3.57 | 1.093330 | 16.748 | 0.3992 | 0.6766 |
| 0.05 | 3.53 | 1.114523 | 16.869 | 0.3969 | 0.6650 |
| 0.06 | 3.49 | 1.133166 | 16.988 | 0.3974 | 0.6364 |
| 0.07 | 3.47 | 1.139153 | 17.039 | 0.3936 | 0.5627 |
| 0.08 | 3.43 | 1.134959 | 17.181 | 0.3913 | 0.5387 |
| 0.09 | 3.42 | 1.192092 | 17.415 | 0.3907 | 0.5746 |
| 0.10 | 3.30 | 1.218686 | 17.637 | 0.3815 | 0.5666 |
| At 318.15 K | |||||
| 0.00 | 3.78 | - | 16.247 | 0.4143 | - |
| 0.01 | 3.66 | 1.044188 | 16.556 | 0.4076 | 0.7377 |
| 0.02 | 3.62 | 1.060869 | 16.656 | 0.4054 | 0.8624 |
| 0.03 | 3.59 | 1.074205 | 16.746 | 0.4037 | 0.6961 |
| 0.04 | 3.53 | 1.098751 | 16.884 | 0.4003 | 0.7065 |
| 0.05 | 3.48 | 1.123885 | 17.025 | 0.3975 | 0.7126 |
| 0.06 | 3.45 | 1.138871 | 17.130 | 0.3958 | 0.6554 |
| 0.07 | 3.41 | 1.154542 | 17.242 | 0.3935 | 0.6148 |
| 0.08 | 3.35 | 1.186535 | 17.432 | 0.3900 | 0.6452 |
| 0.09 | 3.27 | 1.222864 | 17.676 | 0.3853 | 0.6689 |
| 0.10 | 3.21 | 1.255418 | 17.896 | 0.3818 | 0.6758 |
Table 2: Values isentropic compressibility (βs), Relative association, Specific acoustic impedance, linear free length and sound velocity number for 2-amino-5-nitrothiazole – NiCl2 aqueous solution at different temperature and concentrations.
Conclusion
The ultrasonic method is a powerful tool for characterizing physicochemical properties and existence of molecular interaction in a metal – ligand solution. The result reveals that the density and ultrasonic velocity of 2-amino-5-nitrothiazole-NiCl2 aqueous solution increases with increase in concentration and temperature. It is also seen that the formation of a linear, between respective parameters indicated that the stronger solute- solvent interaction and metal –ligand interaction. These results clearly indicate scope for further studies on solute– solvent interaction and metal-ligand interaction and effects of temperature and concentration. These results also give the scope for investigating acoustical parameters of various substituted thiazoles as a ligand with aqueous metal solution.
References
- Thakare A, Naik B. Study of molecular interaction of 2-amino-5-nitrothiazole in NNDMF, acetonitrile and ethanol using acoustical parameters. Cogent chemistry. 2016;(2).
- Thakare A, Dongapure A. Acoustical study of substituted thiazole in acetonitrile at different concentrations and temperature. GIMRJ, 2016;(3):242-51.
- Thakare A, Dongapure A, Rathod V, et al. Acoustical study of substituted thiazole in NNDMF-water mixture at different temperature. IJRBAT. 2015;(6).
- Shah P. Synthesis and biological activity of novel imidazole. Current Trends in Biotechnology and Chem Res. 2012;(2):45-51.
- Syal V, Patial B, Chouhan S. Ultrasonic velocity, viscocity and density studies in binary mixture of dimethylformaide and ethyl methyl ketone at different temperatures. Indian J Pure and Applied Phys. 1999;(37):366-70.
- Kharat S. Density, viscosity and ultrasonic velocity studies of aqueous solutions of sodium acetate at different temperatures. J Mol Liq. 2008;(140):10-4.
- Santosh M, Bhat D. Solute–solvent interactions in aqueous glycylglycine–CuCl2 solutions: Acoustical and Molecular Dynamics Perspective. J Solution Chem. 2011;(40):1657-71.
- Rao NP, Verrall RE. Ultrasonic velocity, excess adiabatic compressibility, apparent molar volume, and apparent molar compressibility properties of binary liquid mixtures containing 2-butoxyethanol. Can J Chem. 1987;(65):810-6.
- Godhani R, Dobariya B, Sanghani A, et al. Thermodynamic properties of binary mixtures of 1,3,4-oxadiazole derivative with chloroform, NN-dimethylformamide 303.15-313.15 K and atmospheric pressure. Arabian J Chem. 2012;10.1016/arabjc/2012.10.002.
- Nikam P, Ansari H, Hasan M. Acoustical properties of fructose and maltose solutions in water and in aqueous 0.5 M NH4Cl. J Mol Liq. 2000;(84):169-78.
- Kincaid J, Eyring H. The liquid state. J Chem Phys. 1938;(6):587-620.
- Landge M, Badade S, Kendre B. Density, ultrasonic velocity and viscosity measurements Glucose-Alcohol-Water mixtures at various temperatures. Int J Res Chem Environ. 2013;(3):348-52.
- Yadav P, Kumar M, Yadav R. Study of molecular interaction in binary liquid mixtures of ethyl acetoacetate with chloroform and dimethylsulphoxide using excess acoustic parameters and spectroscopic methods. Phys and Chem of Liqs. 2014;(52):331-41.
- Raman PV , Kolandaivel P, Perumal K. Ultrasonic and computational study of intermolecular association through hydrogen bonding in aqueous solutions of D-mannitol. J Mol Liq. 2007;(135)46-52.
- Kharkale S, Bhaskar C, Agarwal P, et al. A study of substituded dihydroformazan in predicting the acoustical and its allied properties in different solvents at 288.15K. Int J of Emerging Techs in Computational and App Scis. 2013;(5):327-9.
- Palani R, Jayachitra K. Ultrasonic study of ternary electrolytic mixtures at 303, 308 and 313 K. Indian J Pure Appl Phys. 2008;(46):251-4.
- Planka TS, Rockenbauer A, Korecz L. ESR study of the copper (II)–glycylglycine equilibrium system in fluid aqueous solution. Computer analysis of overlapping multispecies spectra. Magn Reson. Chem.1999; (37):484-92.
- Kim MK, Martell AE. Copper (II) complexes of glycylglycine. Biochemistry. 1964;(3):1169-74.
- Ambomase S, Tripathy S, Tripathy M, et al. Study on water-polymer interaction in the presence of aceclofenac at 298.15K. E-Journal Chem. 2011;(8):63-70.
- Meshram B, Agrawal P, Chandak H, et al. A study of acoustical behaviour of paracetamol in 70% methanol at various temperatures. IJETCAS. 2013;(5):369-73.
- Chauhan S, Kumar K, Patial B. Study of acoustic parameters of proline in lecithin-ethanol mixture at varying temperature. Indian J Pure and Applied Phys. 2013;(51):531-41.