Original Article
, Volume: 9( 6)Study of Cellulose Interaction with Various Liquids
- *Correspondence:
- Michael Ioelovich, Designer Energy Ltd, 2 Bergman Str, Rehovot, Israel, Tel: 972-8936661; Fax: 972-89366614; E-mail: bd895892@zahav.net.il
Received: July 28, 2016; Accepted: November 28, 2016; Published: December 01, 2016
Citation: Ioelovich M. Study of Cellulose Interaction with Various Liquids. 2016;9(6): 109.
Abstract
Interaction of dry cellulose samples with hydrocarbons, alcohols, ketones, organic acids, water, amines and some other organic liquids has been studied at 298 K using the methods of calorimetry and sorption. Heat of interaction and maximum equilibrium sorption value were determined. The study showed that interaction of various cellulose samples with non-polar (hydrocarbons) and low-polar liquids (higher alcohols, ketones, organic acids, etc.) was accompanied by a small exothermic heat effect, which was below 20 J/g. Unlike non- and low-polar liquids, interaction of cellulose with highly polar liquids (water dimethylformamide, dimethyl sulfoxide, etc.) caused a high exothermic heat effect exceeding 30 J/g DM. It has been found that maximum equilibrium sorption value (So) is a linear function of heat of interaction (ΔH); thus, the specific heat of interaction, ho=ΔH/So, was almost constant regardless of nature of the liquid and structural features of cellulose. Using the constant ho value, the sorption of diverse liquids by cellulose can be predicted.
Keywords
Cellulose; Structure; Liquids; Sorption; Heat of interaction; Specific heat methods
Introduction
During isolation, purification, modification and application, cellulose undergoes the action of various liquids. As a result of interaction, cellulose sorbs a liquid that is accompanied by diverse changes in structure and properties of this biopolymer depending on nature of the liquid. Negligible sorption of non-and low polar liquids (e.g. hydrocarbons, higher alcohols) has a small effect on mechanical properties [1] and reactivity [2] of cellulose. On the other hand, increased sorption of water and other highly polar liquids by cellulose reduces mechanical properties [2,3], lowers glass temperature and increases reactivity of the biopolymer [4].
As is known, sorption process of various liquids by cellulose samples is accompanied by an exothermic heat effect of interaction, which depends on the structure of cellulose and nature of the liquid [5-9]. It is also found that for the same sample the exothermic heat effect of interaction depends on nature of the liquid, and increases in the following order of the liquids: hydrocarbons < higher alcohols < lower alcohols < water < polar organic solvents [9].
Since the heat effect of interaction is caused by sorption, these two physicochemical characteristics should be correlated with each other. Unfortunately, a relationship between sorption value and heat of interaction has been investigated in a very limited extent, mainly for cellulose-water system [9,10]. Therefore, the purpose of this research was to study the dependence of maximum equilibrium sorption on heat of interaction using liquids of various nature and cellulose samples with different structural features. Another purpose was to use the obtained relationship to predict the sorption value and its impact on various properties of cellulose and cellulose materials.
Experimental Materials
The following cellulose samples were investigated (Table 1):
| Sample | α-Cellulose, % | DP | X | Y | AN2, m2/g |
|---|---|---|---|---|---|
| COC | 98 | 2700 | 0.70 | 0.30 | 1.8 |
| MEC | 99 | 2500 | 0.55 | 0.45 | 0.7 |
| MCC | 87 | 180 | 0.76 | 0.24 | 1.4 |
| RCF | - | 250 | 0.38 | 0.62 | 0.5 |
Table 1: Characteristics of cellulose samples.
• Purified and bleached cotton cellulose (COC) of Hercules Co
• Mercerized cotton cellulose (MEC)
• Microcrystalline cotton cellulose (MCC)
• Rayon fibers of regenerated cellulose (RCF) of Rayonier Inc.
Distilled water (W) and various chemical pure organic liquids were used, such as hexane (HE) absolute alcohols-methanol (ME), ethanol (ET), n-propanol (PR) and n-butanol (BU), acetic acid (AA), acetone (AC), dimethylformamide (DMF), dimethylsulfoxide (DMS), monoethanolamin (MEA) and butyl amine (BA).
Methods
The content of α-Cellulose was found according to TAPPI test method T-203 by treatment of the samples with 17.5% NaOH at room temperature for 1h. The average degree of polymerization (DP) was measured by viscosity method using diluted cellulose solutions in Cadoxen [11]. Specific surface area (AN2) of the samples was measured at -196°C by adsorption of nitrogen using Sorptometer QBET-NV1A of Porous Material Inc. The degree of crystallinity (X) and the content of non-crystalline domains (Y) were determined by physicochemical method using heat of cellulose interaction with water [10]:
 (1)
(1)
 (2)
(2)
Where ΔHw,o=167.5 J/g is theoretical heat of interaction of amorphous cellulose with water.
The heat of interaction of cellulose samples with various liquids was studied at 298K by means of the method of microcalorimetry [12]. Three of the same samples were dried at 380 K to constant weight and tested to calculate an average value (ΔH) and standard deviation that was in the range ± 0.02 J/g DM.
The vapor sorption of various liquids was carried out at 298 K on a vacuum Mac-Ben apparatus having helical spring quartz scales in the range of relative vapor pressure φ=p/po from 0.2 to 0.8. Prior to starting of the experiments the samples were dried at 380 K up to constant weight and additionally degassed in the sorption device. Three of the same dried samples were tested to calculate an average sorption value and standard deviation that was in the range ± 0.001 kg/kg DM. Sorption isotherms were described by linear equation [13]:
 (3)
(3)
where S is sorption value at φ; whereas So is maximum equilibrium sorption of a liquid by cellulose, which is found by extrapolation of S-values in the φ-range of 0.2-0.8 to the sorption value corresponding to φ=1 [13]. K is coefficient. Furthermore, the specific enthalpy of interaction (h) is calculated as follows:
 (4)
(4)
Results and Discussion
Interaction characteristics (ΔH and So) of cotton cellulose (COC) with liquids of various nature are shown in Table 2. As can be seen, the heat of interaction may vary in a wide range from 1 J to 140 J per 1g of dry cellulose material (DM), depending on nature of the liquid. Moreover, rise of the heat of interaction leads to a proportional increase in the sorption value.
| Liquid | ΔH, J/g DM | So |
|---|---|---|
| HE | 1 | 0.003 |
| AC | 8 | 0.024 |
| BU | 10 | 0.030 |
| AA | 12 | 0.036 |
| PR | 17 | 0.051 |
| ET | 20 | 0.060 |
| ME | 33 | 0.10 |
| W | 50 | 0.15 |
| DMF | 61 | 0.18 |
| DMS | 73 | 0.22 |
| MEA | 90 | 0.27 |
| BA | 140 | 0.42 |
Table 2: Interaction characteristics of cotton cellulose with various liquids.
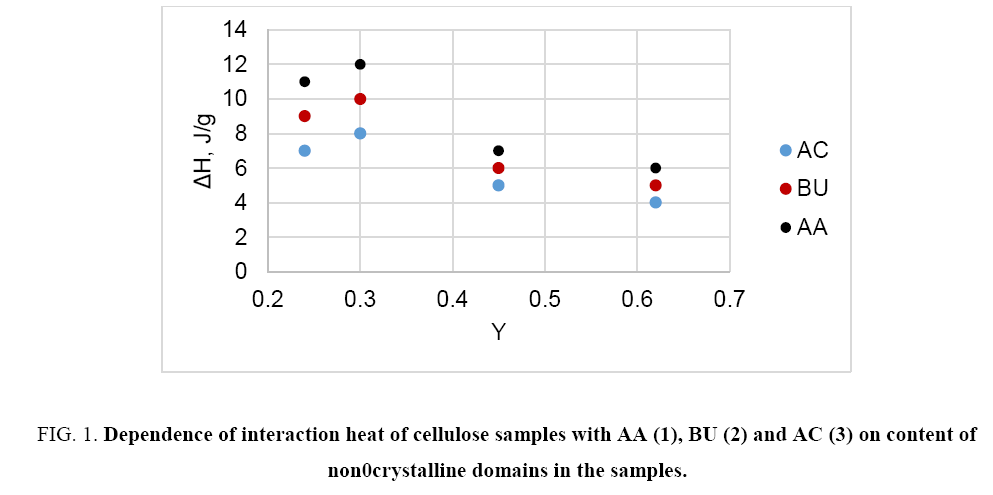
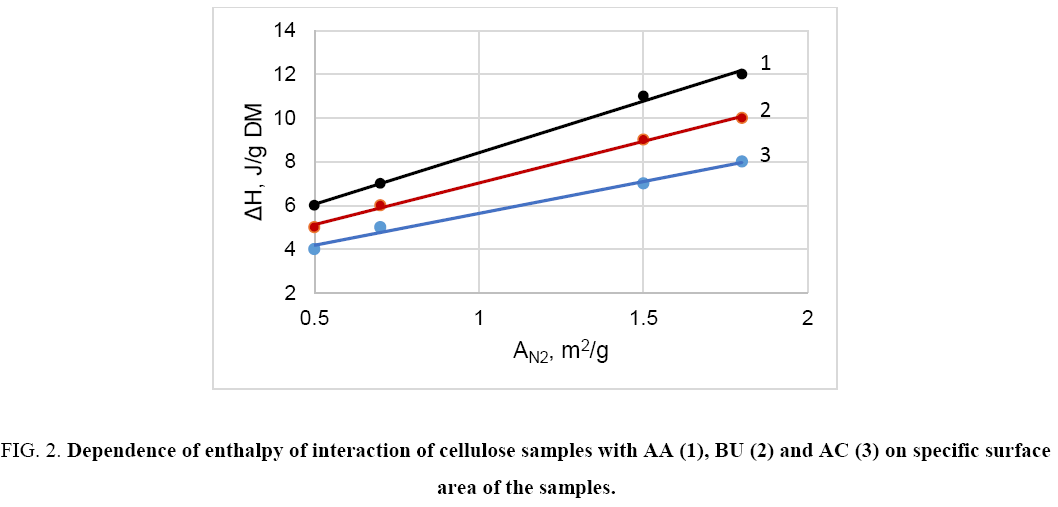
The study reveals that in the case of non- and low-polar liquids (e.g. hexane, acetone, acetic acid, higher alcohols, etc.) the heat of interaction of different cellulose samples with these liquids is relative small and does not correlate with crystallinity or content of non-crystalline domains (Y) of cellulose ( Figure 1). On the other hand, this interaction characteristic depends on specific surface area of the samples ( Figure 2). Thus, the interaction of non- or low-polar liquids occurs on accessible surface of cellulose only.
Figure 1: Dependence of interaction heat of cellulose samples with AA (1), BU (2) and AC (3) on content of non0crystalline domains in the samples.
Figure 2: Dependence of enthalpy of interaction of cellulose samples with AA (1), BU (2) and AC (3) on specific surface area of the samples.
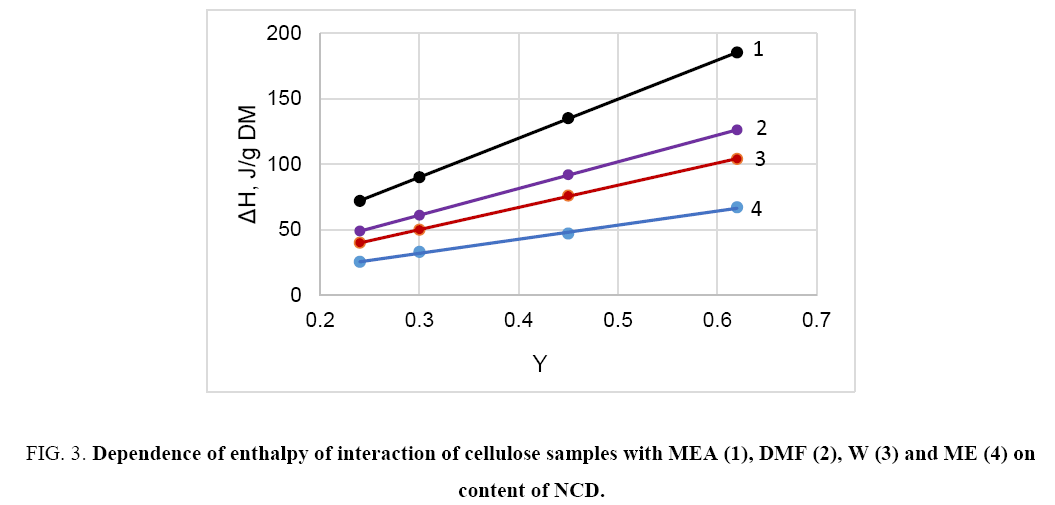
When the cellulose samples interact with highly polar liquids such as water, methanol, DNF, DMS, amines and some others, the obtained interaction heat is directly proportional to the content of non-crystalline domains (NCD); thereby the interaction of these liquids is carried out with polar groups of NCD ( Figure 3).
Figure 3: Dependence of enthalpy of interaction of cellulose samples with MEA (1), DMF (2), W (3) and ME (4) on content of NCD.
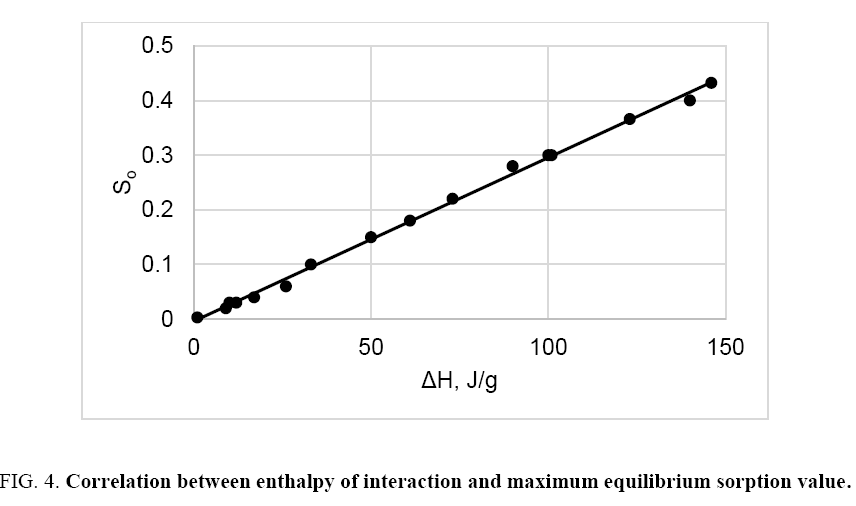
Generalized graph of maximum equilibrium sorption value as a function of heat of interaction for all investigated samples and liquids is shown in Figure 4. As can be seen, there is a linear correlation between ΔH and So; therefore, the average specific heat of interaction of different cellulose samples with liquids of various nature is constant, ho=334 J ± 3 J per g of liquids. Close value of the specific heat of interaction of cellulose with water, DMS and ethanol is found also in the papers [8,10]. Using the found ho value and determined heat of interaction, the value of maximum equilibrium sorption can be calculated:
This makes it possible to predict the sorption value and evaluate the So-dependent properties of the cellulose-liquid system, e.g. accessibility, reactivity, shift of glass-transition temperature, etc.
Conclusion
Interaction of different cellulose samples with hydrocarbons, alcohols, ketones, organic acids, water and some other organic liquids has been studied at 298 K using the methods of sorption and calorimetry. Heat of interaction (ΔH) and maximum equilibrium sorption value (So) were determined. The study showed that interaction of various cellulose samples with non- and low-polar liquids was accompanied by a small exothermic heat effect, due to sorption of these liquids on the accessible surface of the samples only. Unlike non- and low-polar liquids, interaction of cellulose with highly polar liquids caused a high exothermic heat effect, which was directly proportional to the content of non-crystalline domains in cellulose samples. The linear correlation So=f (ΔH) evidences that the equilibrium sorption of various liquids by cellulose is determined by enthalpy of interaction. Since the average specific heat of interaction of different cellulose samples with liquids of various nature was constant (ho=334 J ± 3 J per g of liquids), this, makes it possible to predict the maximum equilibrium sorption value using the enthalpy of interaction of cellulose with the liquid, as well as to evaluate the So-dependent properties of the cellulose-liquid system.
References
- Robertson AA. Interactions of liquids with cellulose. TAPPI. 1970;53:1331-9.
- Klenkova NI. Structure and reactivity of cellulose. Science L. Leningrad, 1976.
- KaiminI, IoelovichM, IvulonokZ. Effect of liquids on physico-mechanical properties of cellophane. Wood Chem.1984;2:78-83.
- Ioelovich M, KaiminI. Study of cellulose temperature transitions in liquid systems.J Polym SciPart B. 1979;21:621-5.
- Papkov SP, Fainberg EZ. Interaction of cellulose and cellulose materials with water, Khimiya, Moscow, 1976.232 p.
- ZvetkovV, IoelovichM, KaiminI, et al. Interaction enthalpy of cellulose with water. Wood Chemistry.1980;5:22-6.
- IoelovichM,AN, PrusovaSM, et al.Interaction of high-purity cellulose with binary systems: Water/DMSO and water/ethanol. J Chem and Chem Technol.2016;59:41-6.
- IoelovichM. Heat effects of interaction between cellulose and various polar liquids. SITA.2011;13:35-44 .
- IoelovichM. Physicochemical methods for determination of cellulose crystallinity. Chem Xpress.2016;9:245-51.
- IoelovichM. LeykinA. Nano-cellulose and its application. SITA, 2004;6:17-24.
- Ioelovich M. Study of thermodynamic properties of various allomorphs of cellulose. Chem Xpress. 2016;9:259-65.
- Ioelovich M, Leykin A. Study of sorption properties of cellulose and its derivatives. Bioresources. 2011;6:178-95.