Review
, Volume: 14( 2)Spatial Regulation of PKC by Pharmacological Approaches in Cancer: Are We There Yet?
- *Correspondence:
- Arbind A, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, U.P., India, Tel: +91 542 2307149, E-mail: acharya@bhu.ac.in
Received: March 15, 2018; Accepted: March 27, 2018; Published: March 30, 2018
Citation: Rishi Kant S, Sanjay K, Munendra Singh T, et al. Spatial Regulation Of PKC by Pharmacological Approaches in Cancer: Are We There Yet? Biotechnol Ind J. 2018;14(2):162.
Abstract
PKC is a ubiquitously expressed serine/threonine protein kinase, sit at the junction of many signaling pathway and play key roles in mediating myriad cellular functions. Therefore, the precise control of PKC directed signal transduction is essential for cellular homeostasis. Deregulation of PKC-mediated signaling leads to the several pathophysiological conditions including cancer. The activity of PKC is regulated by multiple molecular mechanisms such as phosphorylation, co-factor recruitment, interaction with binding partner proteins and cellular localization. Moreover, number of natural and synthetic compounds available that are involves in controlling the PKC mediated signal transduction, but a drug that can specifically regulate the activity of particular isoform of the PKC is not known. Therefore, it is essential to gain an insight into how the PKC activated and presumed to work. This review will provide comprehensive idea about regulation of PKC at various activation steps, a short account of some of available efforts in cellular transformation, challenges, and possibilities to design novel PKC modulators.
Keywords
PKC regulation; Pharmacological approach; Cancer; PKC inhibitor; Drug design
Introduction
Protein Kinase C (PKC) represents a family of highly related phospholipid dependent serine/threonine protein kinase. The PKC family occupies tip of the kinome and participated into the various signal transduction pathways. It also regulates various cellular functions such as cellular growth, proliferation, and differentiation [1]. Therefore, deregulation of PKC activities leads to the several pathophysiological conditions including cancer, Alzheimer, ischemic heart disease, Parkinson’s disease, diabetes, bipolar disease, and psoriasis [2]. Therefore, targeting PKC activity became an interesting approach in clinical biology to reverse pathophysiological status of the hosts.
Nowadays, number of drugs available that are involves in controlling the activity of PKC mediated signal transduction, but a drug that can specifically regulate particular isoform of the PKC not known. In 1982, when it was found that phorbol ester could activate intact PKC, provided a hope for a PKC specific drug discovery. Unfortunately, initial academic and pharmaceutic industrial efforts were unsuccessful in formulating a single pharmacological drug that can specifically target PKC isoform [2]. Why we failed to target PKC? Why cell contain multiple PKC isoform? Are they redundant in cellular function? Several questions remain to answer and have become a puzzle from last three decades in PKC field. It is daunting task to identify challenges associated with PKC field, it warrants the need of more extensive and consistent research to explore the structural feature, activation and interaction with various adopter proteins for downstream processing of individual PKC isozyme. Therefore, in this review, we discussed the molecular events and currently available pharmacological compounds that regulate PKC activities in detail. Further, the review will also put some light on the fate of clinical trials, and development of target specific approach to regulate PKC activity.
The PKC Family
PKC has been discovered more than 30 years ago as a lipid sensitive serine/threonine protein kinase. It was identified as a receptor for diacylglycerol (DAG), a product of phospholipase-C activation, and well-known signal transducer of many receptors [3]. Subsequently, based on the structure of regulatory domain, 10 different isoforms of PKC can be divided into three subfamilies: (i) the conventional or classical PKC isoforms (cPKC: α, βI, βII and γ), (ii) the novel PKC isoforms (nPKC: δ, θ, ? and ?), and (iii) the atypical PKC isoforms (aPKC: ζ, λ/?). The optimal activity of classical PKC isoform depends on negatively charged phospholipids like phosphatidylserine, DAG and calcium. The novel PKC isoforms require DAG and negatively charge phospholipids, whereas optimal activity of atypical PKC isoforms requires only negatively charge phospholipids [4].
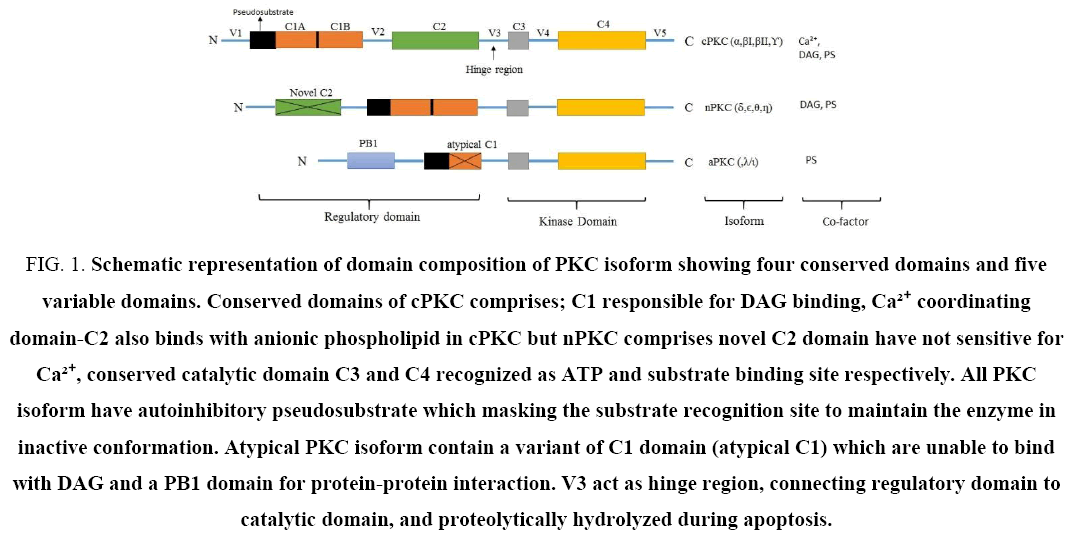
The cPKC is a single polypeptide chain comprises N-terminal regulatory domain and a highly conserved catalytic domain at C-terminal linked via flexible, proteolytically labile hinge region that is also known as V3 region. Primarily, cloning of cPKC isoforms demonstrated four conserved domains (C1-C4) and five variable domains (V1-V5). The regulatory N-terminal domain includes C1 and C2 domain as well as pseudosubstrate sequence [5]. The C1 domain contains two cysteine rich zinc fingers that comprise C1A and C1B tandem repeat which function as binding site for DAG and PMA. It is the C1B region which has binding ability in context of full length protein [6,7]. The C2 domain acts as a calcium dependent membrane targeting module. The regulatory domain primarily controls two functions: firstly, it engaged with lipid second messenger, and/or other interacting proteins to anchor the enzyme at various cellular locations such as plasma membrane, secondly it negatively regulates enzymatic activity. nPKC also contain tandem C1 domain for DAG binding and a variant of C2 domain, called novel C2 lack Ca2+ coordinating residue. Therefore, nPKC is not sensitive to Ca2+.aPKC contain an atypical C1 domain and PB1 (Phox and Bhem) domain. C1 domain of aPKC lack key ligand binding pocket, and therefore, aPKC is not sensitive to DAG and Ca2+. PB1 domain mediate interaction of aPKC to other PB1 domain containing proteins. Although, aPKC is not discussed further in this review, because this distant subfamily has not respond to same second messenger (DAG and Ca2+) as similar to another isoform. All isozyme has highly conserved catalytic domain consists N-terminal ATP binding loop C3, whereas at C-terminal; C4 act as protein kinase domain and have ability to recognize substrate leads to phosphorylation by PKC (Figure 1). In addition, pseudosubstrate sequence are found in all isoform at regulatory domain masks the substrate binding cavity at the C-terminus and maintaining the enzyme in inactive state [2,5,8].
Figure 1: Schematic representation of domain composition of PKC isoform showing four conserved domains and five variable domains. Conserved domains of cPKC comprises; C1 responsible for DAG binding, Ca²? coordinating domain-C2 also binds with anionic phospholipid in cPKC but nPKC comprises novel C2 domain have not sensitive for Ca²?, conserved catalytic domain C3 and C4 recognized as ATP and substrate binding site respectively. All PKC isoform have autoinhibitory pseudosubstrate which masking the substrate recognition site to maintain the enzyme in inactive conformation. Atypical PKC isoform contain a variant of C1 domain (atypical C1) which are unable to bind with DAG and a PB1 domain for protein-protein interaction. V3 act as hinge region, connecting regulatory domain to catalytic domain, and proteolytically hydrolyzed during apoptosis.
Regulation of PKC Activation
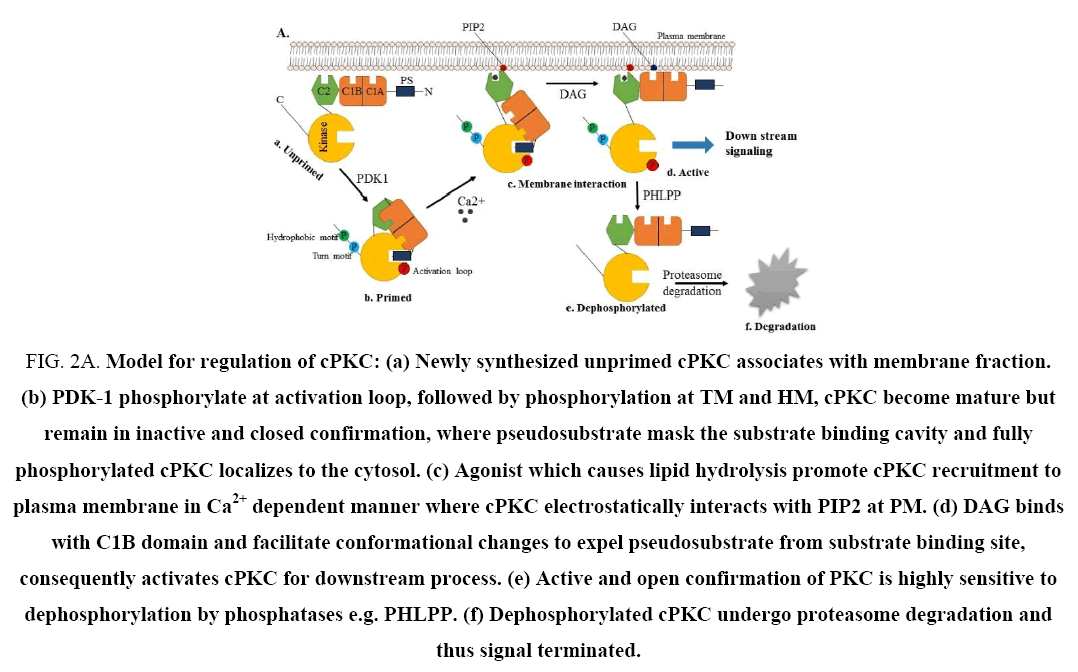
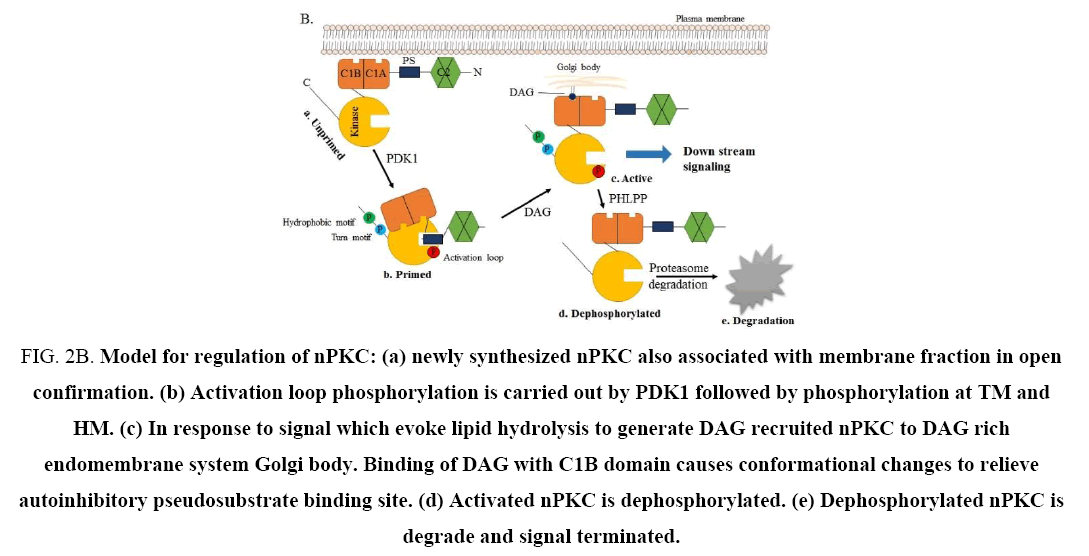
Newly synthesized PKC is processed by a tightly regulated coupled phosphorylation event in the conserved catalytic domain and kept in inactive form with the help of pseudosubstrate attached to the substrate binding cavity at C-terminus [9,10]. Canonically, PKCs activation are not depends on these phosphorylation events, but it requires translocation to specific cellular compartments to co-localize with substrate and activators, which is regulated by the binding ability of different isoform to second messenger and adopter proteins [11]. From this notion it might be possible that these activation step could be possible target to regulate PKC signal array. Thus, the signal transduction of PKC could be regulated by modulating at following step: first at phosphorylation level; which is necessary to achieve catalytically fully competent conformation and localization to the cytosol. Second at cofactor binding step; sole source for activation and translocation towards plasma membrane. Third step is during interaction between PKC and various cellular proteins or adopter proteins; responsible for specific cellular localization, and fourth at ATP binding site, which is essential to phosphorylate a substrate by PKC for downstream process (Figure 2A and 2B) [1,12]. Therefore, the aforesaid steps could be an emerging target to manage PKC signal transduction. It is necessary to develop a specific pharmacological tool to regulate these steps as well as PKC mediated signal transduction. However, few pharmacological agents and antisense oligonucleotide approaches are available to show modest selectivity for the PKC isoform and can be used to regulate the activity of this kinase. To achieve successful regulation of individual PKC, a consistent and extensive research involving PKC and currently used bonafied PKC pharmacological regulator is required.
Figure 2A: Model for regulation of cPKC: (a) Newly synthesized unprimed cPKC associates with membrane fraction. (b) PDK-1 phosphorylate at activation loop, followed by phosphorylation at TM and HM, cPKC become mature but remain in inactive and closed confirmation, where pseudosubstrate mask the substrate binding cavity and fully phosphorylated cPKC localizes to the cytosol. (c) Agonist which causes lipid hydrolysis promote cPKC recruitment to plasma membrane in Ca2+ dependent manner where cPKC electrostatically interacts with PIP2 at PM. (d) DAG binds with C1B domain and facilitate conformational changes to expel pseudosubstrate from substrate binding site, consequently activates cPKC for downstream process. (e) Active and open confirmation of PKC is highly sensitive to dephosphorylation by phosphatases e.g. PHLPP. (f) Dephosphorylated cPKC undergo proteasome degradation and thus signal terminated.
Figure 2B: Model for regulation of nPKC: (a) newly synthesized nPKC also associated with membrane fraction in open confirmation. (b) Activation loop phosphorylation is carried out by PDK1 followed by phosphorylation at TM and HM. (c) In response to signal which evoke lipid hydrolysis to generate DAG recruited nPKC to DAG rich endomembrane system Golgi body. Binding of DAG with C1B domain causes conformational changes to relieve autoinhibitory pseudosubstrate binding site. (d) Activated nPKC is dephosphorylated. (e) Dephosphorylated nPKC is degrade and signal terminated.
Regulation at Phosphorylation Step
Phosphorylation of PKC
Maturation of PKC into catalytically competent and stable form of enzyme is mediated by a series of tightly regulated coupled phosphorylation events on the C-terminus [9,10]. Previous reports involving mass spectrometry and mutational analysis identified three conserved key phosphorylation sites known as the activation loop (AP), the turn motif (TM) and the hydrophobic motif (HF) [13,14]. Convincing evidence has providing clues that newly synthesized PKC undergoes phosphorylation just after synthesis at activation loop catalyzed by 3-phosphoinositide dependent protein kinase-1 (PDK1) [15]. Phosphorylation at activation loop is important for catalysis and stabilization of PKC [16]. This notion is further supported by the fact that the cell lacked PDK-1 have significantly low PKC levels [17]. Recent findings demonstrated that other than PDK1, several kinases are involved in phosphorylation of activation loop like human villirubin reductase (hBVR) phosphorylate activation loop of PKC-βII, while 5?-AMP-activated protein kinase (AMPK) involved in phosphorylation of PKC-ζ [18]. Structural analysis by molecular modelling and crystallization reveal that phosphorylation on TM mediate important interaction with other residues on the enzyme to stabilize the kinase domain of the enzyme [19-21]. Specially, phosphate on the TM interacts with a cluster of basic residues (Glycine loop) in a pocket to stabilize the conformation of active kinases. However, to setup the role of TM phosphorylation in context of enzyme stability is very contradictory because TM phosphorylation is essential for PKC-β activity, while unessential for PKC-α. [21,22].
Recently, it has been found that phosphorylation on TM of cPKC and PKCε is mediated by mammalian Target of Rapamycin (mTOR) signaling pathway. However, mTORC2 deficient cell shows vastly reduced expression levels of cPKC in murine embryonic fibroblast [23,24]. Though, it is difficult to know whether mTORC2 assists in TM phosphorylation, there are two possible mechanisms to hypothesized that mTORC2 positioning the newly synthesized PKC for phosphorylation by another kinase or it may directly phosphorylate. Hydrophobic motif (HM) phosphorylation was described as auto-phosphorylation event mediated by an intramolecular reaction [25]. Mutational analysis reported that HM phosphorylation have varying effect on the catalytic activity and function of enzyme [26,27]. HM phosphorylation is also directly controlled by mTORC2. The involvement of mTOR in HM phosphorylation of PKC-δ by using inhibitor rapamycine was first reported by Parker and co-workers [28]. Numerous study has demonstrated the involvement of HSP90 in the phosphorylation on HM. HSP90 with co-chaperon Cdc37 binds to a specific molecular clamp formed by conserved PXXP motif in the kinase domain with a conserved Tyr on the αE-helix in the kinase core. This molecular clamp is essential for HSP90 binding, an essential event for processing and stabilization of enzyme, and mutation in the molecular clamp result in PKC degradation [1].
Dephosphorylation of PKC
Metabolism of DAG results in the translocation of PKC back to the cytoplasm and termination of PKC signaling. PKC signaling is also terminated by dephosphorylation of cPKC at activation loop and HM in response to TNF-α [29]. Prolonged exposer of phorbol ester or sustained DAG stimulation results in downregulation of PKC activity via ubiquitin/proteasome system [30]. Further, a recent finding has identified the involvement of an additional okadiac acid-insensitive phosphatase known as PH domain and Leucine rich repeat Protein Phosphatases (PHLPPs) in dephosphorylation at HM upon prolonged phorbol ester treatment [31].
Inhibitors of PKC maturation
The initiation of PKC maturation begins with phosphorylation at AP catalyzed by PDK-1. PKC which does not undergoes phosphorylation are degraded by proteasome, thus the steady levels of PKC activity in cells depends on upstream PDK-1 activity. Many research have been conducted to regulate PDK-1 activity, however negative results were associated with active site inhibitors of PDK-1 [32]. Emerging evidence suggested that HSP90 and Cdc37 directly implicated in maturation of cPKC and nPKC by binding with molecular clamp. Therefore, inhibitors of HSP90, like geldanamycin, 17AAG and celastrol play significant role in inhibiting phosphorylation at HM as well as conventional and novel PKC maturation. Geldanamycin and 17-AAG involve in inhibition of ATP binding and hydrolysis which is necessary for HSP90?s activity, while celastrol inhibits both, HSP90?s ATP binding and interaction with co-chaperon Cdc37 which are essential for enzyme maturation following hydrophobic motif phosphorylation [33]. Further, phosphorylation on TM and HF have been depends on mTORC2 and do not occur in mTORC2 lacking cells [34,35]. Thus, mTORC2 specific inhibitor such as Torin1 can inhibit the phosphorylation at HM and TM to reduce the cellular levels of PKC.
Inhibitors of PKC downregulation
In the active confirmation PKC became catalytically-competent but highly sensitive to dephosphorylation that causes downregulation of their activities [1,36]. The downregulation of cPKC mediated by docking of peptidyl-prolyl isomerase Pin1 onto HM isomerizes the phospho-threonine-proline peptide bound from a cis to a trans confirmation of the TM. Therefore, pin1 inhibitor like PiB will be an emerging agent to inhibit cPKC downregulation and trap them in active condition [37].
Regulation at co-factor binding step
Canonically, the activity of mature but inactive cPKC depends on binding with second messenger DAG and Ca2+ while nPKC activity only depends on DAG [38]. Phosphorylated cPKC localized to the cytosol primarily and maintained in appropriate microenvironments by scaffold proteins [39]. The extracellular stimuli act on cell surface receptors leads to the activation of phospholipase-C and subsequently hydrolysis of phosphatidyl inositol-3 phosphate take place to generate DAG in membrane and IP3; a Ca2+ mobiliser. Ca2+ is a soluble ligand that binds with C2 domain of cPKC and increases its affinity for plasma membrane. Once anchored on the membrane, cPKC diffuses in the surface of lipid bilayer and mediate secondary C1A domain interaction with DAG assisted by phosphatidyl serine. The conformational changes produced from the binding of cPKC to DAG and anionic phospholipid is adequate to expel the autoinhibitory pseudosubstrate domain from substrate binding site, and as a result activation of the cPKC isoform takes place. nPKC have greater affinity for DAG than cPKC and activated in the absence of Ca2+. nPKCs does not have C2 domain, therefore it activated in the presence of Ca2+. Due to the presence of a tryptophan in place of tyrosine residue at position 22, The C1B domain of nPKC has 100-fold higher affinity for DAG as compare to the cPKC [40].
Another regulatory domain ligand
A wide range of structurally diverse natural or synthetic products is available which binds with high affinity to regulatory domain and activate or inactivate both cPKC and nPKC. As an activating agent of PKC, phorbol esters are much more efficient than DAG and have two-time greater affinity towards C2 domain than DAG. Nowadays, variety of structurally diverse phorbol esters are available with different substitutes on the 12th and 13th carbon and have shown different biological functions, e.g. 12-deoxyphorbol 13-monoesterates, long chain substituted 13-tetradecanoateact act as inflammatory and tumor promoting factor [41] while another derivative via short chain substitution are inflammatory but not tumor promoting [42]. Besides, structurally similar to phorbol esters, ingenol and daphnane esters are also involved in modulating the activity of PKC. Bacterial derived indole alkaloids such as lyngbyatoxin, telecocidines, and aplysiatoxin are polyacetate and actively participate in modulating the PKC activity [43]. Recently, researchers have developed short chain synthetic DAG-lactones [44] which are more lipophilic than DAG and canonically activate classical and novel isoform. A variety of diverse physiological Ca2+ agonists like histamine, a natural agent or pharmacological agonist thapsigarign or ionomycin are also present that can directly stimulate cPKC. Moreover, studies reveal that a large number of physiological stimuli causes elevation of DAG and consequently PKC activation. For example, histamine, bombesin, UTP, lysophosphatidic acid acts upon G-protein coupled receptor subsequently activation of PLC and generation of DAG and IP3 leads to cPKC and nPKC activation [45,46].
Furthermore, Bryostatins; a family of macrocyclic lactones isolated from the marine bryozoan Bugula neretina has shown to bind with C1 domain selectively. Structurally Bryostatins possess a large protruding ring, due to its unique structure when it is bound to C1 domain, form a cap that contribute to its specific biology [43,47]. In particular, short-term exposure of bryostatins 1 induces same effects as phorbol esters, but prolonged exposure antagonizes the effect of phorbol esters in various biological contexts, including cancer, due to membrane depletion of PKC [48].
Regulation at scaffold binding protein
Multiple PKC isozyme co-exist in the same tissues with overlapping substrate specificity and activators, perform distinct cellular functions. This phenomenon is based on their targeted subcellular localization after activation [49]. This specific subcellular localization is based on their interaction with scaffold protein, commonly known as Receptors for Activated C Kinase (RACKs), and hypothesized that it anchors specific PKC isozyme at unique subcellular locations; these were first identified two decades ago by Mochly- Rosen et al. For PKC, RACKs bind with regulatory domain as well as variable domain and activate them by reliving autoinhibition due to binding of pseudosubstrate. It also locks them in an active conformation by exposing substrate-recognition site. Thus, RACKs can mediate both localization and activation of specific PKC isoform. Moreover, a group of proteins have also been shown to binds with inactive PKC isoforms termed as Receptor for Inactive C Kinase (RICKs). Further, many cellular proteins have been identified by co-precipitation assay, by yeast two-hybrid technique and by sensitive proteomic approaches associated with PKC and regulate their activity [50]. Among these interacting proteins, some were associated with PKC prior to activation, while other including those proteins that interacted with activated PKC and rope them in active form. Dysregulation in PKC-C-KIP interaction likely to involves in pathophysiological states [51,52]. In the light of above study, it will be possible to control the interaction of Protein Kinase C interacting protein (PKC-C-KIP) provide a way for designing of novel pharmacological therapeutic targets to modulate PKC mediated isoform specific signaling array.
Inhibitors and activators of PKC translocation
Earlier, D. Mochly-Rosen and coworkers worked on PKC-RACK interaction and developed a series of peptide fragments for activation and inhibition of PKC translocation. Developments of these peptides were based on the interaction between PKCs and RACKs [50,53]. In case, peptide interfered during PKC-RICK interaction, it should act as an agonist for PKC isoforms; while in same manner when RACK binds to PKC just before the substrate binding, it acts as an inhibitor for translocation of specific PKC isoform. List of inhibitory peptides includes short oligopeptides (6-10 mer) with sequence homology to interacting partner and effectively inhibit the compatible protein-protein interaction. A brief summary of used isozyme selective peptides is discussed in Table 1. These derived peptides are more advantageous due to their flexibility and naturally selected as a suitable candidate for the interaction with binding partners [54,55].
| Peptide | PKC isoform selectivity | Effect on translocation | Reference |
|---|---|---|---|
| βC2-1 [Human (PKCβ: 209-216)] | cPKC | Inhibition | [57] |
| βC2-2 [Human (PKCβ: 186-198)] | cPKC | Inhibition | [56] |
| βC2-4 [Human (PKCβ: 218-226)] | cPKC | Inhibition | [56] |
| βIV5-3 [Rat(PKCβI:646-651)] | PKCβI | Inhibition | [56,57] |
| βIIV5-3 [Rat(PKCβII:445-450)] | PKCβII | Inhibition | [57] |
| εv1-2 [Rat (PKCε:14-21)] | PKCε | Inhibition | [58] |
| δv1 [Mouse (PKCδ: 8-17)] | PKCδ | Inhibition | [59] |
| γV5-3 [Human (PKCγ: 659-664)] | PKCγ | Inhibition | [60] |
| αV5-3 [Human (PKCα: 642-647)] | PKCα | Inhibition | [61] |
| ψεRACK [Rat (PKCε: 85-92)] | PKCε | Activation | [55,61] |
| PKC:Protein Kinase C | |||
Table 1: List of peptide inhibitors and activators for PKC isoform.
Unfortunately, these peptides do not inhibit PKC activity, because they are 6-8 residues long and unable to penetrate cell. There are several approaches have been used to introduce peptides into the cell like transient and stable transfection [54,55]. Major challenging task is to identify which C-KIP (Protein Kinase C interacting protein) have regulatory function, and to determine specificity by which it bind with isoform specific PKC and the mechanism by which PKC activity undergo regulation. Beside it, studies demonstrated that RACK proteins implicated in various cellular function are completely different from PKC. Therefore, it is required to study further in detail to develop complete understanding regarding the biological consequences of RACK before being used to modulate the activity of PKC in a cell.
Regulation by ATP Competitive Molecules
Inhibitors of kinase domain
Till date, designing of novel drugs as selective PKC inhibitor is based on inhibitor of ATP binding site. Although a rich list of inhibitory compounds is available which specifically inhibit either PKC or individual PKC isoform (Table 2). These effective ATP competitive compounds used to inhibit PKC kinase activity are natural compound or derived mostly from straurosporine [56-61].
| Inhibitors | PKC Isoform | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α | βI | βII | β | γ | δ | ε | η | |||
| Indolocarbazoles | ||||||||||
| UCN01 | 29 | - | 34 | - | 30 | 590 | 530 | - | ||
| Go6976 | 2.3 | - | 6.2 | - | - | - | - | - | ||
| Bisindolylmaleimides | ||||||||||
| Midostaurine | 22 | - | 30 | 31 | 24 | - | - | - | ||
| Go6983 | 7 | 7 | - | - | 6 | 10 | - | - | ||
| BIS1 | 20 | - | 17 | 16 | 20 | - | - | - | ||
| Ro320432 | 9 | - | 28 | 31 | 37 | - | 108 | - | ||
| Ro318220 | 5 | - | 24 | 14 | 27 | - | 24 | - | ||
| Enzastaurine | 39 | 6 | - | - | 83 | - | 110 | - | ||
| Rubboxystaurine | 360 | - | 4.7 | 5.9 | 400 | 250 | 600 | 52 | ||
Table 2: IC50 values (nM) for isoform specific PKC active site inhibitors.
Naturally derived kinase inhibitors
Rottlerin also known as mallotoxin a polyphenyl natural product isolated from Mallotus philippinesis. Although, rottlerin shows 5-10 time more selectivity towards PKC-δ (IC50=3-6 μM) than conventional PKC isoforms and 13-33 time than atypical and novel PKC isoforms [62], it has been dumped into list of discredited PKC inhibitors due to the fact that it does not inhibit phorbol ester stimulated PKC-δ activity in cells. Even more fatal, it shows continuous effect in cells isolated from PKC-δ knockout mice (absence of PKC-δ protein) [63,64].
Indolocarbazoles
This class include, Staurosporine, UCN01, Midostaurine, and Go6976. Staurosporine is the first known ATP competitive kinase inhibitor, isolated from Streptomyces staurosporeous and act as anti-proliferative agent [65]. Staurosporine strongly inhibit PKC-α, PKC-γ and PKC-η but fail to generate affinity towards another PKC isoform. Although it shows poor specificity for individual PKC isoform, nevertheless it served as parental compound from which many other novel pharmacological agents including UCN01, Midostaurine and Go6976 were developed to compete with ATP for PKC binding [66]. UCN01 or 7-hydroxy staurosporine, a potent inhibitor of PKC in nM concentration synthesized from addition of hydroxyl group at C-7 carbon of the lactam ring of staurosporine. UCN01 shows greater selectivity for inhibition of PKC (IC50= 4.1 nM). Another promising compound compete with ATP for PKC derived from staurosporine is Go6976 formed by deletion of the glycoside ring and further addition of methyl and cynoalkyl group to the nitrogen atom of staurosporine. Several studies have been performed to check the specificity of Go6976 and demonstrated that it inhibits conventional PKC isozyme more efficiently than novel or atypical PKC isoforms. It has been reported that at 500 nM Go6976 inhibited cPKC isoform by more than 80%, nPKC by 41-65%; and atypical isoform by 13% [67]. It has secured 7th position among a panel of 178 kinase inhibitors screened at 500 nM. The staurosporine derived Midostaurine (PKC412) or n-benzoylstaurosporine is a broad range kinase inhibitor. It interacts with ATP binding site of cPKC and strongly inhibited its action in nM concentration.
Bisindolylmaleimide
Indolocarbazole structure has been used as parental compound to generate a second series of ATP competitive inhibitors termed as bisindolylmalemides. A range of inhibitory compounds available in this series show greater selectivity for individual PKC isoform. Among them Go6983 is a pan PKC inhibitor which inhibit all PKC isoform in vitro, however, it inhibits cPKC and nPKC isoform more selectively than aPKC [68]. Bisindolylmaleimide 1 also known as Go6850 or BIS1 is an ATP-competitive potent PKC inhibitor, possess average level of kinase promiscuity. Study involving X-ray crystallography reveals that dimethyl amino group of BIS1 make hydrogen bound to catalytic Asp 470 in ATP binding domain of PKCβII [32,69]. Further, extension in bisindolymalemides group were performed by Roche and collogues who synthesized various potent ATP competitive PKC inhibitor and marketed it. Among them, Ro320432 is a selective cell permeable, ATP competitive isoform specific PKC inhibitor. It selectively inhibits individual cPKC isoform. Ro318220 has been originally characterized as PKC inhibitor, however MAPKAP-K1β, MSK1, GSK3β and S6K1 are also target of this compound [70]. It is widely used as a cell permeable ATP competitive cPKC inhibitor. Enzastaurin and ruboxistaurin are the most promising PKC-β selective inhibitors with 10 to 100-fold greater affinity from other isoform at IC50 value 4.7-6nM. Ruboxistaurin, is a macrocyclic bisindolylmaleimide compound an orally active Protein Kinase C-β inhibitor. Ruboxystaurin was used for the treatment of diabetic peripheral retinopathy but unfortunately it failed to produce significant positive result and discontinued during Phase III trials [71,72].
Regulation by antisense oligonucleotide
In context to the inhibition of isoform specific PKC, another approach that implicated parallel to chemotherapeutic by researcher is the antisense oligonucleotide (ASO). ASO has been used to inhibit isoform specific expression of mRNA. In this series, aprinocarsen (ISIS3521); a 20mer phosphorothioate oligodeoxynuclotide, induces reduction of PKC-α protein level in concentration dependent manner [IC50=50-100nM] [73] in lung and bladder cell line [74]. Recently, several modifications were made in the parental aprinocarsen structure to derived 2-O-(methoxy)-ethyl termed ISIS9606; [75] a more selective, potent inhibitor of PKC-α expression. Furthermore, a class of nucleoside analogs were also discovered including ARC (4-amino-6-hydrazino-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]-pyrimidine-5-carboxamide) and Sangivamycin; shown anticancer activity by affecting DNA and RNA synthesis in proliferating cell. An additional feature associated with these nucleosides is its ability to inhibit positive transcription elongation factor, and therefore, ARC and sangivamycin are running in phase I trials against a range of malignancies [76,77].
Clinical applications of PKC regulators in oncology
The implication of PKC in cellular transformation, either by antagonizing or promoting different factors of biological function make it a potential target for anticancer therapy. Although various pharmacological regulators have been designed to regulate PKC activity are currently in clinical studies as chemotherapeutic agent. However, only few have been cross preliminary barrier and selected for drug efficacy trails (Table 3). Midostaurin is an orally available ATP competitive non-specific PKC inhibitor with broad range of anti-tumor activities. In phase-I study midostaurin was well tolerated in patients with low grade lymphoproliferative disorders e.g. Chronic B lymphocyte leukemia with some complications such as vomiting, diarrhea, nausea [78,79]. Unfortunately, in Phase-II study, midostaurin failed to generate statistically significant effect in malignant melanoma and has been discontinued from clinical trials. However, a recently published research article demonstrated that midostaurin showed efficacy in patients with FLT-3 mutated acute myeloid leukemia [80]. Further, researchers have also shown 60% response rate of midostaurin in the patients with advanced systemic mastocytosis, including highly fatal variant mast-cell leukemia to prove its chemotherapeutic potential [81]. Moreover, clinical trials are ongoing to assess the efficacy of midostaurin alone and/or combination with other chemotherapeutic drug for patient with aggressive systemic mastocytosis (ASM) or mast cell leukemia (Clinical Trials. gov Identifier: NCT01920204, NCT00233454), Adenocarcinoma of the Rectum (NCT01282502), Acute Myeloid Leukemia (Clinical Trials. gov Identifier: NCT01830361, NCT01883362), Adult Acute Myeloid Leukemia in Remission (Clinical Trials. gov Identifier: NCT02723435). UCN01 is a staurosporine analogue, selective inhibitor of cPKC and nPKC and possess antitumor activity against a variety of cancer cells. Further, UCN01 goes under phase I clinical trials in combination with other pharmacological agent such as cisplatin [82], topotecan [83] to reverse pathophysiological states of the patient. Recently a report has been published to report that UCN01 induces cell cycle arrest at G2/M phase through p53/p21waf1or CHK2/CDC25C pathways and also inhibit the invasion of human hepatoma cell line [84].
| Drugs | Indications | Phase | Status | Clinical trial no. | Outcome |
|---|---|---|---|---|---|
| Enzastaurin | Solid tumor, Lymphoma, Malignant | Phase 1 | Active | NCT01432951 | No response to various cancers, ongoing studies in Breast cancer and solid tumor lymphoma. |
| Enzastaurin and Erlotinib | NSCLC | Phase 1 | Completed | NCT00452413 | |
| Malignant tumor cell | Phase 2 | Completed | |||
| Enzastaurin | Neoplasm cancer | Phase 2 | NCT00309140 | ||
| Enzastaurin, Placebo and Fulvestrant | Breast cancer | Phase 2 | Active | NCT00451555 | |
| Enzastaurine and Warfarine | Solid tumor, Lymphoma, Malignant | Phase 1 | Completed | NCT01388335 | |
| Enzastaurine, Temozolomide, and radiation | Glioblastoma, | Phase 1 | Completed | NCT00402116 | |
| Gliosarcoma | Phase 2 | ||||
| Midostaurine | AML, ALL | Phase 1 | Completed | NCT00866281 | Ongoing studies in AML |
| Phase 2 | |||||
| Midostaurine | AML | Phase 2 | Active | NCT01830361 | |
| Midostaurine | AML with FLT3 mutation | …….. | Active | NCT02624570 | |
| NCT03114228 | |||||
| Midostaurine and placebo | AML | Phase 2 | Active | NCT03280030 | |
| Midostaurine and Decitabine | AML | Phase 2 | Active | NCT01846624 | |
| UCN01 | Lymphoma, T cell | Phase 2 | Terminated | NCT00082017 | No further clinical studies exit. |
| UCN01 and Topotecan hydrochloride | SCLC | Phase 2 | Completed | NCT00098956 | |
| UCN01 | Recurrent melanoma, Stage IV melanoma | Phase 2 | Terminated | NCT00072189 | |
| UCN01 | Breast cancer, Lymphoma, Neoplasm, Plastic neoplasm | Phase 1 | Completed | NCT00001444 | |
| UCN01 | Leukemia, Lymphoma, Unspecified adult solid tumor | Phase 1 | Completed | NCT00003289 | |
| UCN01 and Fluorouracil | Pancreatic cancer | Phase 2 | Completed | NCT00045747 | |
| Bryostatin-1 and Vincristine | HIV related Lymphoma | Phase 1 | Completed | NCT00022555 | Minimal or no effect to various cancers. Further development discontinued |
| Bryostatin-1 | Kidney cancer | Phase 2 | Complete | NCT00003968 | |
| Bryostatin-1 and Cisplatin | Stage III Gastric cancer, | Phase 2 | Complete | NCT00006389 | |
| Stage IV Gastric cancer | |||||
| Bryostatin-1 and Aldesleukin | Stage III Renal cell cancer, | Phase 2 | Complete | NCT00032188 | Limited benefit in various cancer |
| Stage IV Renal cell cancer | |||||
| Bryostatin-1 | Colorectal cancer | Phase 2 | Complete | NCT00003220 | |
| Bryostatin-1 | Breast cancer | Phase 2 | Complete | NCT00003205 | |
| LY900003 | Carcinoma, NSCLC | Phase 3 | Completed | NCT00034268 | |
| ISIS3521 | Lung cancer, Melanoma | Phase 2 | Completed | NCT00003989 | |
| ISIS3521, Carboplatin and Paclitaxel | Lung cancer | Phase 3 | Completed | NCT00017407 | |
| NSCLC: Non-Small Cell Lung Cancer; AML: Acute MyloidLeukemia; ALL: Acute Lymphoblastic Leukemia; SCLC: Small Cell Lung Cancer | |||||
Table 3: Summary of clinical trials of PKC regulator in various cancers.
Enzastaurin is an orally available ser/thr kinase inhibitor that targets ATP binding site and selectively inhibit PKC-β isoform. Enzastaurin was originally evaluated in human tumor xenograft-bearing mice [85]. Enzastaurin has also been studied clinically as antitumor agent in a number of oncology trials and established as well tolerable antitumor and anti-proliferative factor. In phase I study enzastaurin proves chemotherapeutic potential in patients with colorectal carcinoma, lung cancer and renal cancer [86]. Enzastaurin showed promising antitumor activity in Phase II study [87]. Further, Phase III study with enzastaurin showed that it was well tolerable and effective in patients with refractory diffuse large B cell lymphoma (DLBCL) [88]. In another Phase II study, enzastaurin did not add to the antitumor activity of pemetrexed and carboplatin in patients with stage IIIb or stage IV non-small cell lung cancer [89]. Moreover, clinical trials are ongoing to assess the efficacy of enzastaurin alone and/or combination with other chemotherapeutic drug for patient with breast cancer (Clinical Trials. gov Identifier: NCT00451555), and Advanced and/or Metastatic Solid Tumors or Lymphoma (Clinical Trials. gov Identifier: NCT01432951).
Aprinocarsen is an antisense oligonucleotide that reduces PKC-α protein levels in concentration dependent manner. In Phase I study aprinocarsen showed antitumor activity against non-Hodgkin lymphoma and ovarian carcinoma, however patient experienced several toxicities including nausea, vomiting, thrombocytopenia and fever [90,91]. Further, in combination with other chemotherapeutic agent like carboplatin and paclitaxelin in NSCLC it achieved 42% success rate and shown his chemotherapy potential. However, two continuous Phase III study in NSCLC with aprinocarsen and additional chemotherapy failed to improve pathophysiological state of the patient. Therefore, at present, clinical trial of aprinocarsen has been discontinued [92]. Bryostatin-1 is a prototype compound of bryostatins family derived from the Bulgula neritina [93]. It is a macrocyclic lactone which binds with regulatory domain and non-selectively activate both cPKC and nPKC followed by rapid downstream regulation. Due to its growth inhibitory activity against cancer xenograft model, its clinical trials were carried out during 1993, and founded promising result in phase-I study. During Phase-II trials in the patients with melanoma, multiple myeloma, colorectal cancer, ovarian cancer, gastric cancer and cervical cancer disappointing result was found [94]; however, significant result was appeared in patients with gastrophageal junction cancer [95]. Although, recently concluded phase-II study involving bryostatin-1 with vincristine showed modest effect in aggressive B cell non-Hodgkin lymphoma [96], overall response of bryostatin was disappointing and currently there is no any open trial exist.
Challenges in clinical implication of PKC regulators
A number of research have been carried out to develop novel drugs to target specific PKC or its different isoform, but unfortunately, almost all the preclinical trials have disappointed and failed to give significant results. Most of work have been made to designing drugs based on ATP binding site, but result is disappointing, because greater homology found between catalytic domain of other kinases in general, and PKC isoform in particular (approx 70% homology found within PKC family). Similarly, the PKC regulatory domain, DAG binding site in particular, within the PKC family is 60%-80% identical. Therefore, it has been challenging to generate a drug targeting catalytic and/or regulatory domain [97]. Recently, a brilliant work carried out by D. Mochely Rosen and colleagues they were designed a peptide inhibitor termed, separation-of-function inhibitor (SoF) [2]. These peptide inhibitors selectively inhibit phosphorylation of particular substrate without affecting other substrate of same kinase. Moreover, PKC activities is defined by translocation towards cellular compartments, and therefore it is necessary to emphasis on the investigation of such peptides or drugs which selectively inhibit translocation of PKC to particular cellular compartments without hampering localization to other part of cell. Further, drugs available in the market have shown concentration dependent selectivity, and this value is determined from in vitro studies. Therefore, determination of the dose of inhibitors in in vivo condition must be essential to achieve significant result. One of the major problem associated with PKC inhibitors is the route of drug delivery, and to exploit full function of drugs it could be most fruitful to delivered drugs intracellularly at their target.
Future perspectives of PKC modulators
The implication of PKC family in various pathophysiological states including cancer, undoubtably make it an interesting and challenging target for designing novel pharmaceutical therapeutic drug. Yet, both academic and pharmaceutical agencies have been failed to generate isoform specific PKC modulator, the current available PKC modulator are nonspecific in action due to some challenges as discussed earlier. Nevertheless, some of the available agents has already shown hope against isoform specific inhibition. For example, enzastaurin is a PKC-β selective inhibitor and currently used as therapeutic agent in cancer and diabetes [98]. However, it is under Phase II clinical trial with additional chemotherapeutic agents in various type of cancer. Rottlerin simplify the difficulties against PKC-δ and selectively act against this isoform [99]. The family of bryostatin also selectively inhibit function of cPKC and nPKC [93,100,101]. Aprinocarsen (ISIS3521) is an antisense oligonucleotide selectively inhibit PKC-α in concentration dependent manner [102]. Further, future of PKC inhibitor could be depending on development of peptide fragments that act as activator/inhibitor (RACK, RICK), which specifically inhibit localization of PKC to particular cellular compartments. Therefore, major objective of the upcoming years is to give emphasis on analysis of different PKC-interacting partners and analyzing motifs within each PKC interacting protein which is responsible for protein-protein interaction. Moreover, success with ASO to inhibit PKC-alpha isoform opened the door for designing of such ASO which will complimentary to targeted isozyme. Another novel strategy that might have potential to managed PKC mediated signaling is the use of separation of function inhibitors (SoF). Recently, it has been shown that SoF selectively inhibit phosphorylation of particular substrate without affecting another substrate of same PKC. Therefore, it will be an emerging strategy to regulate PKC mediated singling pathways. It has also been elucidated that PKC activity depends on posttranslational modification such as phosphorylation at tyrosine residue, oxidation of cysteine rich domain within C1 region, acetylation, nitrocylation and proteolytically cleavage at hinge region. Although, no research has been conducted based on post translational activation of PKC, it might be possible that modification of post translational event could regulate PKC activity. A most approachable and isoform selective inhibition of PKC will be mediated by ribozyme. Ribozyme hybridize and cleaved its complimentary mRNA to abort translational process, however to achieve further ribozyme mediated PKC isoform inhibition, extensive research is required. Further, PHLPP mediated dephosphorylation at HM is the first event in the down regulation of cPKC and nPKC, therefore, investigation of phosphatase inhibitors that can inhibit PHLPP or mutation of the HM will be a revolutionized step in cell signaling to regulate cPKC and nPKC activity.
Conclusion
Although, it is clear that PKC family implicated in both normal physiology and pathophysiological states, therefore this kinase family become an attractive target for drug discovery. Despite years of academic and pharmaceutical research, there is no any success achieved to generate a target specific single novel agent. As far our knowledge, the generation of enzymatically competent mature PKC molecule is associated with tightly regulated phosphorylation event, co-factor binding and intracellular localization within the cell. Although, healthy list of modulatory compounds are available, only few have shown specificity for either PKC alone or individual PKC isoform and undergo clinical trials as anticancer regime. Therefore, it might be useful to apply different strategy in drug designing to achieve success against PKC regulation, and it could be a small molecule kinase inhibitor, antisense oligonucleotide, peptide drugs to inhibit localization, protein substrate binding inhibitor, particular substrate phosphorylation inhibitor, modulator of post translational event pursued. Further, inhibitor against chaperonic protein like HSP90 and Cdc37 will be an emerging strategy to regulate cPKC as well as nPKC activity. However, fall out in designing a novel therapeutic drug, several pharmaceutical companies have turned their interest from PKC based therapeutic regimes, nevertheless academic affair with PKC is going strong in a single hope that discovery of only one novel drug can bring PKCs back to the top in the field of drug discovery.
Acknowledgement
We are grateful to the CSIR-UGC, New Delhi for the financial support to RKS for his research work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Newton AC. Protein Kinase C: Poised to signal. Am J PhysiolEndocrinolMetab. 2010;298:E395-E402.
- Mochly-Rosen D,Das K, Grimes KV. Protein Kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11(12):937-57.
- Rossum van RL, Patterson DB, Nikolaidis N, et al. Phospholipase C-gamma: Diverse roles in receptor-mediated calcium signaling. Trends Biochem Sci. 2005;30:688-97.
- Hofmann J. The potential for isoenzyme-selective modulation of Protein Kinase C. FASEB J 1997;11:649-669.
- Steinberg SF. Structural basis of Protein Kinase C isoform function. Physiological Reviews. 2008;88:1341-1378.
- Kazanietz MG, Wang S, Milne GWA, et al. Residues in the second cysteine-rich region of Protein Kinase C δ relevant to phorbol ester binding as revealed by site-directed mutagenesis. J Biol Chem.1995;270:21852-9.
- Pu Y, Garfield SH, Kedei N, et al. Characterization of the differential roles of the twin C1a and C1b domains of Protein Kinase C-delta. J Biol Chem. 2009;284:1302-12.
- Newton AC.Protein Kinase C: Structural and spatial regulation by phosphorylation, cofactors and macromolecular interactions.Chem Rev. 2001;101:2353-64.
- Newton AC. Regulation of the ABC kinases by phosphorylation: Protein Kinase C as a paradigm. Biochem J.2003;370:361-71.
- Parker PJ, Parkinson SJ. AGC protein kinase phosphorylation and Protein Kinase C. BiochemSoc Trans.2001;29:860-3.
- Dorn GW, Mochly-Rosen D. Intracellular transport mechanisms of signal transducers. Annu Rev Physiol. 2002;64:407-429.
- Antal CE, Newton AC. Tuning the signaling output of Protein Kinase C. A Biochemical Society Focused Meeting Held at Science Gallery, Trinity College Dublin, Ireland; 2014.
- Orr JW, Newton AC. Requirement for negative charge on "activation loop" of Protein Kinase C. J Biol Chem. 1994;269(44):27715-18.
- Tsutakawa SE, Medzihradszky KF, Flint AJ, et al. Determination of in vivo phosphorylation sites in Protein Kinase C. J Biol Chem. 1995;270(45):26807-12.
- Le Good JA, Ziegler WH, Parekh DB, et al. Protein Kinase Cisotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281(5385):2042-5.
- Liu Y, Belkina NV, Graham C, et al. Independence of Protein Kinase C-δ activity from activation loop phosphorylation. J Biol Chem. 2006;281(17):12102-11.
- Balendran A, Hare GR, Kieloch A, et al.Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of Protein Kinase C (PKC) isoforms. FEBS Lett. 2000;484:217-23.
- Michael F, Dermot K, Long A. Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cellular Signalling. 2011;23:753-62.
- Messerschmidt A, Macieira S, Velarde M, et al. Crystal structure of the catalytic domain of human atypical Protein Kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol. 2005;352(4):918-31.
- Grodsky N, Li Y, Bouzida D, et al. Structure of the catalytic domain of human Protein Kinase C beta II complexed with a bisindolylmaleimide inhibitor. Biochemistry. 2006;45(47):13970-81.
- Hauge C, Antal TL, Hirschberg D, et al. Mechanism for activation of the growth factor-activated AGC kinases by turn motif phosphorylation. EMBO J. 2007;26(9):2251-61.
- Zhang J, Wang L, Schwartz J, et al. Phosphorylation of Thr642 is an early event in the processing of newly synthesized Protein Kinase C beta 1 and is essential for its activation. J Biol Chem. 1994;269(30):19578-84.
- Ikenoue T, Inoki K, Yang Q, et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 2008;27(14):1919-31.
- Facchinetti V, Ouyang W, Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and Protein Kinase C. EMBO J. 2008;27(14):1932-43.
- Behn-Krappa A, Newton AC. The hydrophobic phosphorylation motif of conventional Protein Kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728-37.
- Gysin S, Imber R. Replacement of Ser657 of Protein Kinase C-α by Alanine Leads to Premature Down Regulation after Phorbol-Ester-Induced Translocation to the Membrane. Eur J Biochem. 1996;240(3):747-50.
- Edwards AS, Newton AC. Phosphorylation at conserved carboxyl-terminal hydrophobic motif regulates the catalytic and regulatory domains of Protein Kinase C. J Biol Chem. 1997;272(29):18382-90.
- Ziegler WH, Parekh DB, Le JA, et al. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr Biol.1999;9:522-9.
- Lee JY, Hannun YA, Obeid LM. Functional dichotomy of Protein Kinase C (PKC) in tumor necrosis factor-alpha (TNF-alpha) signal transduction in L929 cells. Translocation and inactivation of PKC by TNF-alpha. J Biol Chem. 2000;275(38):29290-8.
- Lee HW, Smith L, Pettit GR, et al. Ubiquitination of Protein Kinase C-α and degradation by the proteasome. J Biol Chem. 1996;271(35):20973-6.
- Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of Protein Kinase C. J Biol Chem. 2008;283(10):6300-11.
- Hoshi N, Langeberg LK, Gould CM, et al. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell. 2010;37:541-50.
- Gould CM, Kannan N, Taylor SS, et al. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of Protein Kinase C through a conserved PXXP motif in the C-terminal tail. J Biol Chem. 2009;284:4921-35.
- Ikenoue T, Inoki K, Yang Q, et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 2008;27:1919-31.
- Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell. 2006;11:859-71.
- Gould CM, Newton AC. The life and death of Protein Kinase C. Curr Drug Targets. 2008;9:614-25.
- Abrahamsen H, O’Neill AK, Kannan N, et al. Peptidyl-prolyl isomerase Pin1 controls down-regulation of conventional Protein Kinase Cisozymes. J Biol Chem. 2012;287:13262-78.
- Scott AM, Antal CE, Newton AC. Electrostatic and hydrophobic interactions differentially tune membrane binding kinetics of the C2 domain of Protein Kinase Cα. J Biol Chem. 2013;288:16905-15.
- Schechtman D, Mochly-Rosen D. Adaptor proteins in Protein Kinase C-mediated signal transduction. Oncogene. 2001;20:6339-47.
- Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel Protein Kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826-30.
- Zayed S, Sorg B, Hecker E. Structure activity relations of polyfunctionalditerpenes of the tigliane type, VI1. Planta Med. 1984;50:65-9.
- Furstenberger G, Hecker E. Mode of action of carcinogenic plant components. Planta Med. 1972;22:241-66.
- Blumberg PM, Kedei N, Lewin NE, et al. Phorbol esters and diacylglycerol: The PKC activators. Protein Kinase C in Cancer Signaling and Therapy Springer. New York. 2010;25-53.
- Marquez VE, Nacro K, Benzaria S, et al. The transition from a pharmacophore-guided approach to a receptor-guided approach in the design of potent Protein Kinase C ligands. PharmacolTher. 1999;82:251-61.
- Kajimoto T, Sawamura S, Tohyama Y, et al. Protein Kinase Cδ-specific activity reporter reveals agonist-evoked nuclear activity controlled by Src family of kinases. J Biol Chem. 2010;285:41896-910.
- Gallegos LL, Kunkel MT, Newton AC. Targeting Protein Kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem. 2006;281:30947-56.
- Keck GE, Poudel YB, Welch DS, et al. Substitution on the A-ring confers to bryopyran analogues the unique biological activity characteristic of bryostatins and distinct from that of the phorbol esters. Org Lett. 2009;11:593-6.
- Hennings H, Blumberg PM, Pettit GR, et al.Bryostatin 1, an activator of Protein Kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987;8:1343-6.
- Mochly-Rosen D, Gordon AS. Anchoring proteins for Protein Kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35-42.
- Alastair PW, Giordano P, Ingeborg H, et al. PKC-interacting proteins: From function to pharmacology. TRENDS in Pharmacological Sciences. 2004;25:528-35.
- Prevostel C, Alvaro V, Boisvilliers de F, et al. The natural Protein Kinase C alpha mutant is present in human thyroidneoplasms. Oncogene. 1995;11:669-74.
- Vallentin A, Lo TC, Joubert D, et al. A single point mutation in the V3 region affects Protein Kinase Calpha targeting and accumulation at cell-cell contacts. Mol Cell Biol. 2001;21:3351-63.
- Churchill EN, Qvit N, Mochly-Rosen D. Rationally designed peptide regulators of Protein Kinase C. Trends EndocrinolMetab. 2009;20:25-33.
- Hundle B, McMahon T, Dadgar J, et al. An inhibitory fragment derived from Protein Kinase C epsilon prevents enhancement of nerve growth factor responses by ethanol and phorbol esters. J Biol Chem. 1997;272:15028-35.
- Dorn GW, Souroujon MC, Liron T, et al. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon Protein Kinase C translocation. ProcNatlAcad Sci. 1999;96:12798-803.
- Ron D, Luo J,Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of β Protein Kinase Cin vivo. J Biol Chem. 1995;270:24180-7.
- Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II Protein Kinase C. J Biol Chem. 2001;276:29644-50.
- Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-Protein Kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945-51.
- Chen L, Hahn H, Wu G, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. ProcNatlAcadSci USA. 2001;98:11114-9.
- Sweitzer SM, Wong SM, Peters MC, et al. Protein Kinase C epsilon and gamma: Involvement in formalin-induced nociception in neonatal rats. J PharmacolExpTher. 2004;309:616-25.
- Kim J, Thorne SH, Sun L, et al. Sustained inhibition of PKC alpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011;30:323-33.
- Gschwendt M, Muller HJ, Kielbassa K, et al.Rottlerin, a novel protein kinase inhibitor. BiochemBiophys Res Commun. 1994;199:93-8.
- Leitges M, Elis W, Gimborn K, et al. Rottlerin-independent attenuation of pervanadate-induced tyrosine phosphorylation events by Protein Kinase C-δ in hemopoietic cells. Lab Invest. 2001;81:1087-95.
- Pula G, Schuh K, Nakayama K, et al. PKCδregulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035-44.
- Hoshi N, Langeberg LK, Gould CM, et al. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell. 2010;37:541-50.
- Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci.2000;21:181-7.
- Anastassiadis T, Deacon SW, Devarajan K, et al. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039-45.
- Gschwendt M, Dieterich S, Rennecke J, et al. Inhibition of Protein Kinase Cμby various inhibitors. Differentiation from Protein Kinase Cisoenzymes. FEBS Lett. 1996;392:77-80.
- Grodsky N, Li Y, Bouzida D, et al. Structure of the catalytic domain of human Protein Kinase Cβ II complexed with a bisindolylmaleimide inhibitor. Biochemistry. 2006;45:13970-81.
- Alessi DR. The Protein Kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997;402:121-3.
- Roffey J, Rosse C, Linch M Hibbert, et al.Protein Kinase C intervention: The state of play. CurrOpin Cell Biol. 2009;21:268-79.
- Bain J, Plater L, Elliott M, et al.The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297-315.
- Dean NM, McKay R, Condon TP, et al. Inhibition of Protein Kinase C-alpha expression in human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J Biol Chem. 1994;269:16416-24.
- Dean N, McKay R, Miraglia LJ,et al. Inhibition of growth of human tumor cell lines in nude mice by an antisense oligonucleotide inhibitor of Protein Kinase C-α expression. Cancer Res. 1996;56:3499-507.
- McKay RA, Miraglia LJ, Cummins LL, et al. Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human Protein Kinase C-α expression. J Biol Chem. 1999;274:1715-22.
- Stockwin LH, Yu SX, Stotler H, et al.ARC (NSC 188491) has identical activity to Sangivamycin (NSC 65346) including inhibition of both P-TEFβ and PKC. BMC Cancer.2009;9:63.
- Robins RK, Revankar GR. Purine analogs and related nucleosides and nucleotides as antitumor agents. Med Res Rev.1985;5:273-96.
- Virchis A, Ganeshaguru K, Hart S, et al.Novel treatment approach for low grade lymphoproliferative disorders using PKC412 (CGP41251), an inhibitor of Protein Kinase C. Hematol J.2002;3:131-6.
- Ganeshaguru K, Wickremasinghe RG, Jones DT, et al. Actions of the selective Protein Kinase C inhibitor PKC412 on B-chronic lymphocytic leukemia cells in vitro. Haematologica.2002;87:167-76.
- Gallogly MM, Lazarus HM. Midostaurin: An emerging treatment for acute myeloid leukemia patients. J Blood Med. 2016;19:73-83.
- Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety ofMidostaurinin advanced systemic mastocytosis. N Engl J Med. 2016;374:2530-41.
- Lara PN, Mack PC, Synold T, et al. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: A California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res. 2005;11:4444-50.
- Hotte SJ, Oza A, Winquist EW, et al.Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: A Princess Margaret Hospital Phase II Consortium Study. Ann Oncol.2006;17:334-40.
- Wu G, Lin N N, Xu L, et al. UCN-01 induces S and G2/M cell cycle arrest through the p53/p21(waf1) or CHK2/CDC25C pathways and can suppress invasion in human hepatoma cell lines. BMC Cancer. 2013;13:167.
- Keyes KA, Mann L, Sherman M, et al.LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer ChemotherPharmacol.2004;53:133-40.
- Carducci MA, Musib L, Kies MS, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral Protein Kinase C beta inhibitor, in patients with advanced cancer. J ClinOncol.2006;24:4092-9.
- Kreisl TN, Kotliarova S, Butman JA, et al.A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol.2010;12:181-9.
- Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a Protein Kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J ClinOncol.2007;25:1741-6.
- Socinski MA, Raju RN, Stinchcombe T, et al. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small cell lung cancer. J ThoracOncol. 2010;5:1963-9.
- Cripps MC, Figueredo AT, Oza AM, et al. Eisenhauer, Phase II randomized study of ISIS 3521 and ISIS 5132 in patients with locallyadvanced or metastatic colorectal cancer: A National Cancer Institute of Canada clinical trials group study. Clin Cancer Res. 2002;8:2188-92.
- Matassa AA, Carpenter L, Biden TJ, et al. PKC delta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem.2001;276:29719-28.
- Paz-Ares L, Douillard JY, Koralewski P, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a Protein Kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J ClinOncol.2006;24:1428-34.
- Schaufelberger DE, Koleck MP, Beutler JA, et al. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J Nat Prod.1991;54:1265-70.
- Marengo B, De Ciucis C, Ricciarelli R, et al. Protein Kinase C: An attractive target for cancer therapy.Cancers.2011;3:531-67.
- Ku GY, Ilson DH, Schwartz LH, et al. Phase II trial of sequential paclitaxel and 1 h infusion of bryostatin-1 in patients with advanced esophageal cancer. Cancer ChemotherPharmacol.2008;62:875-80.
- Lazarus HM, Cooper BW, Schluchter MD, et al. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am J Hematol.2009;84:484-7.
- Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nature Biotechnol. 2008;26:127-32.
- Glimelius B, Lahn M, Gawande S, et al. A window of opportunity phase II study of enzastaurin in chemonaive patients with asymptomatic metastatic colorectal cancer. Ann Oncol. 2010;21:1020-6.
- Lee KW, Kim SG, Kim HP, et al.Enzastaurin, a Protein Kinase C beta inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Res. 2008;68:1916-26.
- Zimmermann J, Caravatti G, Mett H, et al. Phenylamino-pyrimidine (PAP) derivatives: A new class of potent and selective inhibitors of Protein Kinase C (PKC). Archiv Der Pharmazie. 1996;329:371-6.
- Parker PJ, Parkinson VSJ Meyer, Müller M, et al. AGC protein kinase phosphorylation and Protein Kinase C. BiochemSoc Trans. 2001;29:860-3.
- Ali AS, Ali S, El-Rayes BF, et al. Exploitation of Protein Kinase C: An useful target for cancer therapy. Cancer Treat Rev. 2009;35:1-8.