Original Article
, Volume: 16( 3) DOI: 10.21767/0972-768X.1000279Selective N1-Alkylation of 1,3-Dibenzoylquinazoline-2,4(1H,3H)-Dione with Benzyl Chloride
- *Correspondence:
- Ozerov AA , Department of Pharmaceutical and Toxicological Chemistry, Volgograd State Medical University, Volgograd, Russia, Tel: +7-844-2943900, E-mail: prof_ozerov@yahoo.com
Received: June 19, 2018; Accepted: August 07, 2018; Published: August 09, 2018
Citation: Ozerov AA, Novikov MS. Selective N1-Alkylation of 1,3-Dibenzoylquinazoline-2,4(1H,3H)-Dione with Benzyl Chloride. Int J Chem Sci. 2018;16(3):279
Abstract
The reaction of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione with potassium carbonate in anhydrous DMF at room temperature leads to the formation of a potassium salt of 3-benzoylquinazoline-2,4(1H,3H)-dione, the alkylation of which in situ with benzyl chloride produces 1-benzyl-3-benzoylquinazoline-2,4(1H,3H)-dione. A one-pot method for the preparation of N1-monosubstituted derivatives of quinazoline-2,4(1H,3H)-dione was developed. The effect of an alkaline agent on the yield and ratio of the debenzoylation products was studied.
Keywords
One-pot synthesis; 1,3-Dibenzoylquinazoline-2,4(1H,3H)-dione; Debenzoylation; Selective alkylation; Benzyl chloride
Introduction
N-substituted derivatives of quinazoline-2,4(1H,3H)-dione demonstrate a broad range of pharmacological activity [1-3]. Of particular interest in this series are N-benzyl derivatives of quinazoline-2,4(1H,3H)-dione, which can suppress the reproduction of the HIV-1, respiratory syncytial virus, herpes simplex virus, and human cytomegalovirus in vitro [4-7]. The direct N1-alkylation of quinazoline-2,4(1H,3H)-dione with alkyl halides in a polar aprotic medium in the presence of bases, similarly to the case of uracil derivatives having substituents at position 6 of the pyrimidine system [8], proceeds with a low yield and is complicated by the formation of N1, N3-disubstituted derivatives. For this reason, the selective introduction of substituents to the nitrogen atom N1 is achieved by either using N3-benzoylated quinazoline-2,4(1H,3H)-dione or forming an already N1-substituted quinazoline system based on cyclization reactions [9]. Earlier, we developed a highly selective method for the synthesis of N1-substituted derivatives of 6-methyluracil, which consists in the direct alkylation of 1,3-dibenzoyl-6-methyluracil with alkyl halides or toluenesulfonates of primary alcohols in anhydrous DMF in the presence of potassium carbonate [10]. This article describes the use of this approach for the selective production of N1-substituted derivatives of quinazoline-2,4(1H,3H)-dione.
Experimental Procedure
Materials and measurements
All reagents were obtained at the highest grade available from Sigma and Acros Organics and used without further purification. NMR spectra were recorded with a Bruker Avance 600 spectrometer (600 MHz for 1H and 150 MHz for 13C) in DMSO-d6. Melting points were measured in glass capillaries with a Mel-Temp 3.0 device (Laboratory Devices Inc., USA). The element analysis was performed with a Vario EL Cube device. Yields refer to spectroscopically (1H and 13C NMR) homogeneous materials.
Synthesis of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1): Benzoyl chloride (25 mL, 0.215 mol) was added in one portion to a stirred suspension of quinazoline-2,4(1H,3H)-dione (3) (10.0 g, 0.062 mol) in anhydrous pyridine (25 mL, 0.309 mol) and acetonitrile (100 mL), and stirred at room temperature for 48 h. The reaction mass was evaporated in vacuo at a bath temperature not exceeding 60°C-65°C. The residue was cooled, partitioned between chloroform (200 mL) and water (200 mL); the organic phase was dried with sodium sulfate and filtered. The filtrate was evaporated in vacuo; the residue was triturated with diethyl ether (50 mL), maintained at 0-5°C for 24 h; the precipitate was filtered off, washed with diethyl ether, and air-dried to give 20.8 g (91%) of raw product with mp 145-150°C. Recrystallization from ethyl acetate (100 mL) gives 17.2 g (75%) of a white crystalline solid, mp 155.5°C-157°C (159-160°C [9]). Anal. Calcd for C22H14N2O4: C, 71.35; H, 3.81; N, 7.56. Found: C, 71.02; H, 3.89; N, 7.68. NMR 1H (600 MHz, DMSO-d6), δ ppm 7.11 (1H, d, J=8.4 Hz, H8), 7.44 (1H, t, J=7.6 Hz, H6), 7.58-7.64 (4H, m, aromatic H), 7.73-7.82 (3H, m, H7, aromatic H), 8.16 (1H, d, J=7.9 Hz, H5), 8.23-8.28 (4H, m, aromatic H). NMR 13C (150 MHz, DMSO-d6), δ ppm 118.39, 118.61, 127.93, 131.53, 132.88, 133.03, 134.20, 134.40, 134.49, 134.83, 139.15, 139.44, 139.56, 141.83, 151.13, 164.39, 172.00, 172.88.
Synthesis of 1-benzylquinazoline-2,4(1H,3H)-dione (5): Finely ground anhydrous potassium carbonate (2.5 g, 0.018 mol) and benzyl chloride (1.0 mL, 0,0087 mol) were added to a solution of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1) (2.5 g, 0,0068 mol) in anhydrous DMF (25 mL) and stirred at room temperature for 48 h. The mixture was filtered, the filtrate was evaporated in vacuo, and the residue was partitioned between chloroform (50 mL) and 1% solution of potassium hydroxide (50 mL). The organic layer was dried with sodium sulfate, filtered, and evaporated in vacuo. The residue was dissolved in boiling 95% ethyl alcohol (20 mL); then concentrated ammonium hydroxide (2 mL) was added; the solution was boiled for 5 min, maintained first at room temperature for 24 h, then at 0-5°C for 24 h. The precipitate was filtered off, washed with cold 95% ethyl alcohol, diethyl ether, air dried, and recrystallized from ethyl acetate (75 mL) to give 1.05 g (62%) of pale yellow needle crystals, mp 217°C-219°C. Anal. Calcd for C15H12N2O2: C, 71.42; H, 4.79; N, 11.10. Found: C, 71.22; H, 4.67; N, 10.40. NMR 1H (600 MHz, DMSO-d6), δ ppm 5.30 (2H, s, CH2), 7.19-7.33 (3H, m, H8, aromatic H), 7.25-7.33 (4H, m, H6, aromatic H), 7.60 (1H, t, J=7.8 Hz, H7), 8.01 (1H, d, J=7.7 Hz, H5), 11.76 (1H, s, NH). NMR 13C (150 MHz, DMSO-d6), δ ppm 48.50, 118.48, 119.27, 126.03, 129.80, 130.57, 130.96, 132.06, 138.53, 139.75, 144.21, 154.09, 165.19.
Synthesis of 1-benzyl-3-benzoylquinazoline-2,4(1H,3H)-dione (4) was synthesized similarly, though the residue after evaporation of the solution in chloroform was not subjected to ammonolysis but triturated with diethyl ether (20 mL) and maintained at 0-5°C for 24 h. The precipitate was filtered off, air dried, and recrystallized from acetone-hexane (1:1, 10 mL) to give 1.10 g (46%) of a white crystalline solid, mp 130°C-132°C (decomp). Anal. Calcd for C22H16N2O3: C, 74.15; H, 4.53; N, 7.86. Found: C, 73.89; H, 4.61; N, 8.17. NMR 1H (600 MHz, DMSO-d6), δ ppm 5.39 (2H, s, CH2), 7.25-7.43 (7H, m, H8, aromatic H), 7.59-7.63 (2H, m, aromatic H), 7.73-7.79 (2H, m, H6, H7), 8.10 (1H, d, J=7.2 Hz, H5), 8.14-8.17 (2H, m, aromatic H). NMR 13C (150 MHz, DMSO-d6), δ ppm 46.35, 115.82, 116.06, 123.89, 126.95, 127.82, 128.33, 129.20, 129.91, 131.00, 131.76, 135.92, 136.25, 136.54, 140.99, 149.88, 161.30, 169.73.
Synthesis of 3-benzoylquinazoline-2,4(1H,3H)-dione (6): Finely ground anhydrous potassium carbonate (3.75 g, 0,0271 mol) was added to a solution of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1) (5.00 g, 0.0135 mol) in anhydrous DMF (100 mL) and stirred for 24 h at room temperature. The reaction mixture was filtered, and the filtrate was evaporated in vacuo at a bath temperature of 85°C-90°C. The residue was partitioned between chloroform (100 mL) and 5% potassium hydroxide solution (100 mL).
The organic phase was dried with sodium sulfate, filtered, and evaporated in vacuo. The residue was cooled, triturated with diethyl ether (10 mL), and cooled at 0-5°C for 24 h. The precipitate was filtered off, washed with diethyl ether, air dried, and recrystallized from ethyl acetate (10 mL) to give 0.67 g (13%) of unreacted 1,3-dibenzoylquinazoline-2,4 (1H,3H)-dione (1), mp 155°C-157°C.
The aqueous phase was acidified with glacial acetic acid and cooled at 0-5°C for 24 h. The precipitate was filtered off, washed with water, air dried, and extracted three times with boiling acetone (20 mL). The non-dissolved precipitate was air-dried, recrystallized from glacial acetic acid (5 mL) to give 0.56 g (26%) of quinazoline-2,4(1H,3H)-dione (3), mp>330°C.
The acetone extract was evaporated to dryness, the residue was recrystallized from acetone (20 mL) to give 1.90 g (53%) of 3-benzoylquinazoline-2,4(1H,3H)-dione (6) as white crystalline fibrous substance, mp 219°C-222°C (de?omp) (209°C-211°C [9]). Anal. Calcd for C15H10N2O3: C, 67.67; H, 3.79; N, 10.52. Found: C, 67.43; H, 3.66; N, 10.83. NMR 1H (600 MHz, DMSO-d6), δ ppm 7.25-7.31 (2H, m, H8, aromatic H), 7.58 (2H, t, J=7.7 Hz, aromatic H), 7.73-7.80 (2H, m, H6, H7), 7.93 (1H, d, J=7.2 Hz, H5), 7.79 (2H, d, J=7.8 Hz, aromatic H), 11.86 (1H, s, NH). NMR 13C (150 MHz, DMSO-d6), δ ppm 117.21, 119.27, 126.50, 130.61, 132.83, 133.87, 134.84, 138.82, 139.32, 143.78, 152.21, 165.19, 173.26.
Results and Discussion
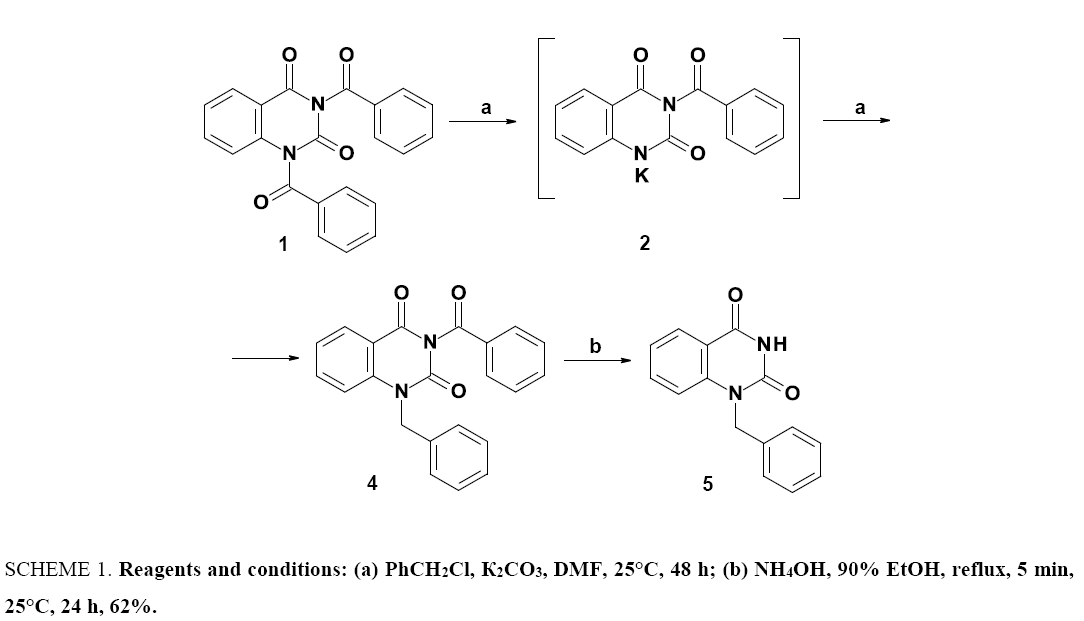
The reaction of the 1,3-dibenzoyl derivative of quinazoline-2,4(1H,3H)-dione (1) with potassium carbonate in anhydrous DMF at room temperature is accompanied by the evolution of carbon dioxide to form a highly soluble potassium salt of 3-benzoylquinazoline-2,4(1H,3H)-dione (2) and a slightly soluble potassium benzoate. In contrast to the dibenzoyl derivative of 6-methyluracil [10], N1-debenzoylation of the 1,3-dibenzoyl derivative of quinazoline-2,4(1H,3H)-dione (1) under the indicated conditions proceeds less selectively and, in addition to the desired 3-benzoylquinazoline-2,4(1H,3H)-dione (6) (in the form of potassium salt (2), partly gives the product of complete debenzoylation, quinazoline-2,4(1H,3H)-dione (3).
In the presence of an alkylating agent, in particular benzyl chloride, in the reaction mixture, the potassium salt of 3-benzoylquinazoline-2,4(1H,3H)-dione (2) formed in situ produces the corresponding N1-alkylation product (4), which is further easily released upon heating in a water-alcohol solution of ammonium hydroxide (SCHEME 1).
SCHEME 1. Reagents and conditions: (a) PhCH2Cl, ?2??3, DMF, 25°C, 48 h; (b) NH4OH, 90% EtOH, reflux, 5 min, 25°C, 24 h, 62%.
The regioselectivity of the alkylation, in particular, the presence of the benzyl substituent on the nitrogen atom N1 of the quinazoline system in compound 5 is confirmed by correlation (NOESY) NMR spectroscopy.
3-Benzoylquinazoline-2,4(1H,3H)-dione (6) is a convenient synthon that allows obtaining various N1-substituted quinazoline derivatives. Publications provide a description of the synthesis of 3-benzoylquinazoline-2,4(1H,3H)-dione (6) by N1- debenzoylation of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1) with potassium carbonate in an aqueous dioxane medium. However, the yield of the desired compound did not exceed 23% [9]. In connection with this, we investigated the possibility of obtaining 3-benzoylquinazoline-2,4(1H,3H)-dione (6) under conditions corresponding to the developed alkylation method. It was found that the reaction of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1) with alkali metal carbonates at a molar ratio of 1:2 in anhydrous DMF at room temperature for 24 h was characterized by a various conversion of the starting compound and different ratios of products of N1-(6) and N1, N3-debenzoylation (3) depending on the nature of the alkaline reagent used (TABLE 1).
| Reagent | Yield (for the isolated compound), % | Molar ratio 6:3 |
||
|---|---|---|---|---|
| 1 | 3 | 6 | ||
| Li2CO3 | 45 | 30 | 16 | 0.53 |
| Na2CO3 | 25 | 15 | 52 | 3.47 |
| K2CO3 | 13 | 26 | 53 | 2.04 |
| Cs2CO3 | 0 | 44 | 49 | 1.11 |
TABLE 1. The effect of the nature of alkali metal carbonate on the yield and ratio of debenzoylation products.
It was found that the most effective debenzoylating reagents are sodium carbonate, which ensures the greatest selectivity of the reaction, and potassium carbonate, which provides a higher conversion of the starting compound with satisfactory selectivity.
Conclusion
Thus, the reaction of 1,3-dibenzoylquinazoline-2,4(1H,3H)-dione (1) with sodium or potassium carbonates in anhydrous DMF at room temperature yields a rather high amount of 3-benzoylquinazoline-2,4(1H,3H)-dione (6), which can be used in situ for the one-pot synthesis of N1-substituted quinazoline-2,4(1H,3H)-dione derivatives. The simple and convenient method for obtaining 3-benzoylquinazoline-2,4(1H,3H)-dione (6), developed by us, expands the synthetic capability of the series of quinazoline compounds.
References
- Khan I, Ibrar A, Ahmed W, et al. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur J Med Chem. 2015;90:124-69.
- Arora R, Kapoor A, Gill NS, et al. Quinazolinone: An overview. Int Res J Pharm. 2011;2:22-8.
- Rajput R, Mishra AP. A review: Quinazolin-4-ones as antifungal agents. Int J Pharm Pharm Sci. 2012;4:66–70.
- Novikov MS, Valuev-Elliston VT, Babkov DA, et al. N1, N3-disubstituted uracils as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem. 2013;21:1150-8.
- Matharu DS, Flaherty DP, Simpson DS, et al. Optimization of potent and selective quinazolinediones: Inhibitors of respiratory syncytial virus that block RNA-dependent RNA polymerase complex activity. J Med Chem. 2014;57:10314-28.
- Martinez A, Gil C, Castro A, et al. Benzothiadiazine dioxide human cytomegalovirus inhibitors: Synthesis and antiviral evaluation of main heterocycle modified derivatives. Antivir Chem Chemother. 2003;14:107-14.
- Glowacka IE, Andrei G, Schols D, et al. Design, synthesis, and the biological evaluation of a new series of acyclic 1,2,3-triazole nucleosides. Arch Pharm Chem Life Sci. 2017;350:e1700166.
- Wu F, Buhendwa MG, Weaver DF. Benzhydryl as an efficient selective nitrogen protecting group for uracils. J Org Chem. 2004;69:9307-9.
- Glowacka IE, Balzarini J, Wroblewski AE. The synthesis, antiviral, cytostatic and cytotoxic evaluation of a new series of acyclonucleotide analogues with a 1,2,3-triazole linker. Eur J Med Chem. 2013;70:703-22.
- Ozerov A, Novikov M, Khandazhinskaya A, et al. Selective N1-alkylation of 1,3-dibenzoyluracils: one-pot way to N1-monosubstituted uracil derivatives. Heterocycles. 2017;94:912-22.