Viewpoint
, Volume: 11( 4)Review on Heterogeneous Electron Transfer in Graphene Electrode with Redox Couple
Betelihem Gesese*
Department of Chemistry, Mekdela Amba Universty, Addis Ababa, Ethiopia
*Corresponding author: B Gesese, Department of Chemistry, Mekdela Amba Universty, Addis Ababa, Ethiopia. E-Mail: betelihemgesese@gmail.com
Received: July 27, 2021; Accepted: August 10, 2021; Published: Augusr 17, 2021
Abstract
The development of Metal-Air Batteries MABs has been impeded by their poor rate capability and a lack of efficient and stable air catalysts, the former owing to the sluggish kinetics of the oxygen reduction reaction. Due to their high specific energy, low cost, and safety, Metal-Air Batteries (MABs), particularly rechargeable MABs, have reawakened attention as a viable energy storage/conversion solutionKeywords
Graphene; Cyclic voltammetry; Hetrognueoes; Electron transfer
Introduction
Graphene is a single carbon layer of the graphite structure, 2-dimensional, crystalline allotrope of carbon , sp2-hybridized carbon atoms, basic building block for graphitic materials of all other dimensionalities (0D fullerenes, 1D nanotubes, and 3D graphite) and describing its nature by analogy to a polycyclic aromatic hydrocarbon of quasi infinite size [1].
Materials and Methods
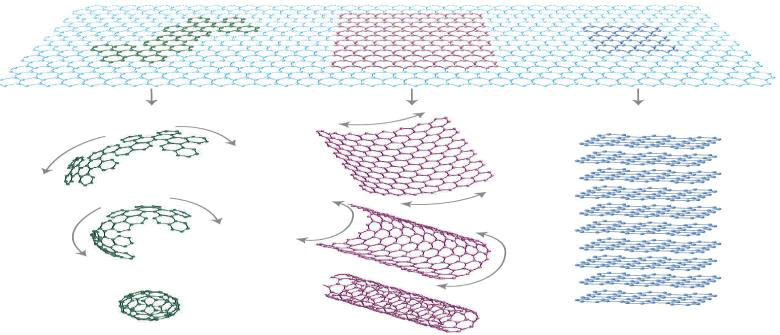
Studies on graphene electrochemistry have suggested the ability of graphene-based electrodes to carry a large amount of current at electron transfer rates superior to graphite and Carbon Nanotube (CNT) electrodes. The high conductivity, large surface area and high an area and high electron transfer rate of graphene are the underlying reasons for its extensive usage in modern electrochemistry related technologies. The overall aim of this review is to provide a critical overview of our understanding on the electrochemistry of graphene such as electron transfer of graphene in redox probe, doping of graphene and CV electrochemical response of graphene electrode with redox couple [2]. Electrochemistry provides a means to measure electron transfer kinetics, interactions between molecules and electrode surfaces (Figure 1).
FIG. 1. C60 fullerene molecules, carbon nanotubes, and graphite can all be thought of as being formed from graphene sheets, i.e. single layers of carbon atoms arranged in a honeycomb lattice.
Graphene have many applications in the world, such as electronics, energy storage and conversion (super capacitors, batteries, fuel cells, solar cells, and bioscience/biotechnologie because of its unique physicochemical properties (Table 1).
TABLE 1. Properties of grapheme.
| Property | Details |
|---|---|
| Electron mobility | 200,000 cm2 V-1 s-1 |
| Thermal conductivity | 5,000 W m-1 K-1 |
| Specific surface area | 2,630 m2 g-1 |
| Breaking strength | 42 N m-1 |
| Elastic modulus | 0.25 T Pa |
| Optical transparency | 0.977 |
| Electrical conductivity of graphene | 64 m S cm-1 |
The fundamental understanding of electron transfer at graphitic electrode materials indicates that they are electronically anisotropic in nature. This review can be considered electron transfer of graphene originates from two heterogeneous structures such as edge and base plane. In order to investigate the heterogeneous electron transfer properties of graphene electrode that performed cyclic voltammetric measurements in the presence of redox couples and this redox couple that describe electrochemical response. Cyclic voltammetric studies of redox couples in the presence of GNF function of graphene that have different effects for different solvents and doping of graphene functionalization. This review generalized compare and contrast electrochemistry of graphene structure and different electrode of graphene depends on electrochemical response for example conductance, rate of reaction, peak to peak separation, current, potential, electron transfer effect etc [3].
Results and Discussion
General objective
- To review heterogeneous electron transfer in graphene electrode with redox couple.
Specific objective
- To review electrochemistry of grapheme
- To review different graphene electrode with redox couple electrochemical response by using cyclic voltammetric study
- To review electrochemical response of edge and plane-electrodes with electrochemical probe
- To review doping of graphene
Electrochemistry of graphene
For the potential application of a certain kind of carbon material in electrochemistry, the basic electrochemical behaviors should be first studied to determine several important parameters of carbon electrodes such as: electrochemical potential window, electron transfer rate, redox potentials, density of state, peak to peak separation etc.
Redox couple can be classifieds in to two this are outer and inner redox couple or classifieds based on electrode surface such as surface sensitive, surface sensitive and oxide-sensitive and surface in sensitive. Cyclic voltammetry is the most extensively used technique for acquiring qualitative information about electrochemical reactions. It kinds the rapid identification of redox potentials distinctive to the electro active species under investigation, providing considerable information about the thermodynamics of a redox process, kinetics of heterogeneous electron-transfer reactions and analysis of coupled electrochemical reactions or adsorption processes [4].
This review can be study electrochemical response/activity of graphene toward different kinds of redox systems such as [Ru(NH3)6]3+/2+, [Fe(CN)6]3-/4-, and Fe3+/2+. As is known, [Ru(NH3)6]3+/2+ is a nearly ideal outer-sphere redox system that is insensitive to most surface defects or impurities on electrodes and can serve as a useful benchmark in comparing electron transfer of various carbon electrodes; [Fe(CN)6]3-/4- is “surface-sensitive” but not “oxide-sensitive”; [Ru(NH3)6]3+/2+ is surface in sensitive ; Fe3+/2+ is both “surface-sensitive” and “oxide-sensitive.
Compare different graphene electrode with redox couple electrochemical response by using cyclic voltammetric study
Hetrognueoes electron transfer of graphene can be determined redox mediator and biological molecules. Redox mediator by using cyclic voltammetry was indicated different electrochemical response/activities. Inner redox mediator most of the time more expressed electrochemical response for different structure of graphene. Electron transfer kinetics with inner sphere redox couple can be affected by Density of electron State (DOS) and micro surface structure. Edge plane defect on graphitic structure that accelerate rate of recreation and more electrochemical response than other graphene electrode. In cyclic voltammetry study different graphene electrode are different electrochemical response for example multilayer graphene poly (ethylene terephthalate) (PET) electrode (ML-G-PET), edge-plane pyrolytic graphite (EPPG), and basal-plane pyrolytic graphite (BPPG) electrodes are different rate of reaction, peak to peak separation ,DOS etc. In addition to inner sphere redox couple outer redox couple and biological molecule have affected electrochemical response in cyclic voltammetry. Generally the following figure can be compare and contrast electrochemical response of more than two graphene electrodes.
Electrochemistry at two structure of graphene was studies by using cyclic voltammetry in the presence of buffered solution and electrolyte ;but electrolyte is pure cyclic voltamogram both electrode rectangular in shape that indicated pure capacitive behavior. Edge electrode higher specific capacitance than base plane electrode because graphene edge electrode was formed by mechanical cutting leading to the formation of structural defect with dangling bond and this dandling bond unstable in reactive species or air.
Edge and base electrode have different properties in electrochemical redox probe. Edge and base plane electrode studies charge transfer in the presence of surface sensitive redox probe that follow different response for example different scan rate and current density. Electrochemical response can be controlled by diffusion electro active species. Like linear diffusion-based system and the peak current or wave current. Where t is time, D is the diffusion coefficient, L is length of edge-electrode W is width electrode, C is concentration, n is number of electron and F is Faraday constant.
The limitation of its diffusion law. For example, the flux in memory diffusion that based on the Fick’s first law is generated with a delay from the formation of concentration gradient. Furthermore, a fast electrochemical process can cause a delay of diffusion; the actual diffusion velocity is much slower than the theoretical value. The electro catalytic activities of the edge- and plane-electrodes have been studied by using three electrochemical probes, including ascorbic acid, beta-nicotinamide adenine dinucleotide (NADH) and oxygen so in this electrochemical probe that transfer information different electrochemical response.
Traditional consensus has been that electron transfer at graphitic materials is dominated by the edge plane, and and it has been shown that the intentional generation of oxygen-containing defects increases reactivity. The basal plane of the GNF is predominantly defect free and hence contains negligible oxygen content. In contrast, the edges of the flakes are decorated with carboxylic acid (COOH) functionalities.
The high density of edge COOH groups makes this an ideal material which to study the role of oxygen species on electrochemical response, as their influence is greatly amplified due to the small size of the flakes. Modification of the edge groups by transformation into amide groups also allows us to probe the influence of the edge group acidity and ability to hydrogen bond. COOH-terminated GNF (c-GNF) and amide-terminated GNF (a- GNF) modified electrodes interact with the common redox probes such as FcMeOH, ferricyanide and hydroquinone give different electrochemical response at different PH effect.
GNF in solution form that affected redox probe and electrochemical response can be inducted for these effects. In case of Ru(CN)63+/4+ that indicates small increasing peak to peak separation and small decreasing peak height in other words in case of [Fe(CN)6]3-/4- peak height drastically reduced, votamogram has sigmoidal shape that indicated electrode blocking. This is the same response as we obtained when COOH-terminated GNF were immobilized directly on the electrode surface.
Having acid functionality [Fe(CN)6]3-/4- CV experiments carried out low ionic strength solution with GNF immobilized electrode surface and either H2O or D2O used as a solvent. For CV experiment H2O used as CV significant inhibition first cycle and repeated cycle increasing peak height reversible of redox reaction and in case of D2O first cycle show inhibition electron transfer kinetics and repeated cycle increasing peak height, decreasing peak separation and reaction will be more revisable reaction. GNF in H2O used as a solvent that explained constant protonation and deprotonating carboxylic acid group and D2O used as a solvent inhibition redox, good proton exchange and electron closing species inhibition electron transfer.
Doping of grapheme
Doping can modify the electronic structure of graphene, and can also bring interesting physicochemical properties to the graphitic system. Doping of graphene can be classified in to two such as chemical and electronic doping. Chemical doping involves interactions of graphene with other chemical species or occurs due to charge transfer between graphene and surface adsorbates. Whether the charge transfer will take place is determined by the relative position of density of states (DOS) of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of the dopant and the Fermi level of graphene.
Adsorbates or substrates used to controlled type and concentration of carriers in graphene during transfer charge to graphene. P-type and n-type doping simply forming the difference between the highest occupied molecular level (HOMO) and the lowest unoccupied molecular level (LUMO) of the adsorbate and graphene. Charge is transferred from the dopant to the graphene layer, if the HOMO of the dopant is above the Fermi level of graphene resulting in n-type doping and for dopants with LUMO below the Fermi level of graphene, charge transfer occurs from the graphene layer to the dopant amounting to p-type doping. Electronic dopants are ability to dope graphene either n- or p-type has been demonstrated for various atomic and molecular adsorbates for example electropositive elements that easily donate their outer shell electrons are expected to be n-type dopants. Given the relatively low work function of graphene (−4.6 eV), n-type doping is perceived to be more challenging than p-type. P-type electronic doping is disparity between the work function of graphene and the electron affinity of the adsorbate [5]. The electrochemical doping of graphene occurs when certain surface adsorbates participate in electrochemical redox reactions in which graphene plays the role of an electrode. The total Gibbs free energy change is given by ΔG + W for p-doping and ΔG − W for n-doping, where ΔG is the free energy for the molecular reaction and W is the work function of graphen.
Conclusion
In summary, can be conclude Hetrognueoes electron transfer of different graphene electrode with redox couple. Redox couple is a redoxing species and it corresponding oxidized form .There are two types of redox couple such as inner and outer redox couple .Inner redox couple is sensitive to surface of graphene electrode but outer sphere redox couple is in sensitive to surface of graphene electrode .Electron transfer kinetics in graphene electrode that defect less base plane graphite and slower electron transfer toward the surface sensitive redox probe compared to that of base plane graphene electrode which is known to possess a certain density of edge plane defect site. In varies functionalization derivative of doped and undoped graphene oxide surface to probe the role of defects in determining the net electron transfer kinetics. Graphene basal plane and edge carbon atoms play a different role in electrochemistry at the graphene flak eelectrodes because of the used electrochemical species. Edge plane graphene electrode compared to BPPG, ML-GPET and quasi-graphene in CV study faster electrochemical properties in terms of HET kinetics.
References
- Pumera M. Electrochemistry of Graphene: New Horizons for Sensing and Energy Storage. Chem Rec. 2009;211?223.
- McCreery RL. Advanced carbon electrode materials for molecular electrochemistry. Chem Rev. 2008;108;2646?2687.
- Pumera M, Ambrosi A, Bonanni A, et al. Graphene for electrochemical sensing and biosensing. Trends Anal. Chem. 2010;29: 954?965.
- Banerjee S, Shim J, Rivera J, et al. Electrochemistry at the edge of a single graphene layer in a nanopore. ACS Nano. 2012;7834?843
- Le Drogoff B, El Khakani MA, Silva PRM, et al. Effect of the Microelectrode geometry on the diffusion behavior and the electroanalytical performance of Hg-electroplated iridium microelectrode arrays intended for thedetection of heavy metal traces. Electro Analysis. 2001;13:1491?1496.