Original Article
, Volume: 15( 1)Remediation of Fluoride Laden Water by Complexation with Triethylamine Modified Waste Polythene Material
- *Correspondence:
- Mwangi IW Department of Chemistry, Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya
Tel: 020 871090112, Ext. 57455; E-mail: isaacwaweru2000@yahoo.co.uk
Received Date: December 16, 2016 Accepted Date: January 21, 2017 Published Date: February 05, 2017
Citation: Singh G, Verma SS. Remediation of Fluoride Laden Water by Complexation with Triethylamine Modified Waste Polythene Material. Mater Sci Ind J. 2017;15(01):113.
Abstract
This paper reports on the development of an affordable, environmentally friendly, and sustainable method for mitigating effects of fluorides in water. This contaminant has leached from the soil and rocks in the earth’s crust that contributed to pollution of both surface and underground waters with negative effects on the safety of consumers. Dangers associated with consumption of fluorides include dental and skeletal fluorosis in man plus animals as well as impaired brain development. This implies that fluoride ions have a capability of limiting the intellectual ability of consumers to make them dependent throughout their entire life. The fluoride ion in drinking is a non-biodegradable pollutant that requires removal. Previously applied removal methods had several challenges such as expensive, ineffective, and not regeneratable. This study investigated the use of solid polythene material derived from the disposed waste thus meeting the criteria for low cost, value addition and sustainable technology. The polythene material was modified with a tertiary ammonium compound to enable its complexation with the fluoride ions in water. The modification was confirmed by FTIR analysis. Complexation parameters such as concentration, temperature, and pH were investigated. The complexation mechanism was found to fit the Langmuir model isotherm having an adsorption capacity of 18.545 mg g-1 and was of Pseudo first kinetics. This indicates that the complexation followed a physio sorption process. The results obtained demonstrated that the heterogeneous material is effective and has a potential application for treatment of fluoride laden water. It is envisioned that the material be packed in cartilages and applied at a point of use to improve the quality of fluoride laden waters and solve the problem of population in areas naturally fluoridated. Every drink taken from these sources is a potential poison that affects both the brain and bones. The use of this modified material to purify water will contribute towards the improvement of the peoples’ self-esteem and make them generous with their smile.
Keywords
Polythene papers; Epoxidation; Quaternization; Sorption; Fluoridation
Introduction
The structural chemical composition of water makes it have special properties as it has a lopsided electrical charge that attracts other atoms. Due to this hydrogen bonding and the polar nature of water, it becomes a universal solvent for polar substances which get easily dispersed uniformly within the water molecule. This dissolving power of water is very important for life. Thus, wherever water is, it harbors dissolved chemicals elements which at times are exploited as minerals and nutrients that support living things. This is property of water’s ability to split ionic compounds have contributed to 97% of the world’s water being salty [1]. Most of those salt ions occur naturally in the soil, sedimentary and ingenuous rocks in many other places of the earth’s crust. As they interact with the water, they are leached out of the land by rainwater and then introduced in ground water sources and other water bodies such as lakes and accumulate [2,3]. In Kenya, there are large deposits of fluorspar in Gilgil situated on the western slopes of the Aberdare range, which is the source of fluoride ions making the fluoride a serious pollutant in adjacent water bodies [4]. Water samples obtained from rift valley and analyzed for fluoride ranged from 2 μg ml-1 to 21 μg ml-1 in drinking water from rivers [5].
Fluorine is the most electronegative element in the halogen family and therefore chemically most energetic [6]. When it enters the body system, the ionic bond formed between fluorine and calcium is very strong due to high lattice energy [7]. This makes fluoride salts to be very fatal when ingested. They cause death in as little as 5 min from time of ingestion to not more than 24 h. The acute lethal dose is 105 mg per body [8]. Availability of clean water suitable for human consumption is therefore a concern and is a world crisis that cuts across rural and urban communities. This makes water scarcity a major challenge in Kenya [9].
Due to the reactivity of the fluoride ion, it easily reaches every organ resulting to many diverse toxic symptoms. According to the World Health Organization the maximum acceptable concentration of fluoride ions in drinking water lies below 1.5 ppm. Excess fluorides in drinking water cause dental and skeletal disorder [10]. This is because the fluoride ion is attracted by positively charged calcium ion in teeth or bones due to its strong electro negativity and later causes weakening of such bones and teeth resulting to a disease is known as fluorosis in human being. In some of the cases it may even interfere with carbohydrates, proteins, vitamins, and mineral metabolism and to DNA creation as well if intake excessively [11].
Persons with stained teeth are hesitant to provide a gleaming smile and in many occasions, appear withdrawn with a low self-esteem. This is exacerbated by the fact that studies by Choi et al. have also shown a strong connection between exposure to fluoride in drinking water and decreased IQ scores in children [12]. This implies that this ion has a capability of limiting the intellectual ability of consumers. This is because it affects the development of the brain hence the consumers get low cognitive ability [13]. In the rift valley system of Kenya, it is possible to encounter such problems as it is known to have fluoride containing rocks which pollute the adjacent water bodies [4,14]. Continued consumption of such water means addition of toxins in the body from every drink taken.
The fluoride ions having negative charge, reacts with hydrogen atom of water molecule forms hydrogen bonding which is not easily degradable and will always be in the water available to cause poisoning. The only available option is removal. The removal methods had been previously achieved by methods such as precipitation and conventional membrane processes which are expensive [15]. These conventional techniques are also not effective when the concentrations are in trace levels ranging from 1 mg l-1 to 20 mg l-1 [16].
In the eastern rift valley, methods used for removal of fluoride ions in water are by the use of bone char. This has limitation of concern in that some religious groups the Jews and Muslim do not advocate the use of bones from unknown source to be associated with their food or drink [16,17]. A study by Bapurao reported that some dietary foods high in protein and vitamin C play a significant role in the effects fluorosis has on the body [18]. This is because such diets contain some specific functional groups capable of interacting with the fluoride ions. This offered an alternative site for complexation of fluorides alleviating its affinity to calcium within the bone material. Archaeologists in Sweden unearthed an ancient evidence of fish fermentation. It revealed that fish bones were preserved for more than 9000 years when the fish was fermented with some special herbs and seal brain [19]. Brain cells have endogenous quaternary ammonium compounds (QACs) broadly distributed in various tissues including the central nervous system, and many QACs have important biochemical functions in brain which could be responsible for the preservation [20,21]. Based on the reported findings, alternative removal options were investigated in this study by anchoring suitable functional groups (QACs) on a solid substrate to mitigate the negative effects of fluorine. This led to the development of cost-effective removal methods through complexation of the fluoride ion with a modified form of polystyrene. Polythene is a non-biodegradable solid material which is used for making cheap bags that are very popular and used extensively. However, disposal of these bags is a menace due to pollution of the environment thus, being a menace and affecting the scenic beauty [22]. The convenience and cost effectiveness associated with plastics has translated into the careless throw away culture in many societies. Furthermore, the increasing rate of urbanization has led to increased use of plastics hence increasing plastic bag waste generation [23]. Lack of proper methods of plastic bag waste management has become an increasing environmental and public health problem particularly in developing countries. Therefore, polythene is a challenge to solid waste management which this study intended to put into exploit.
The current study involves removal of fluoride ions by complexation with a heterogeneous modified plastic material. This was achieved by conversion of the polythene material that litters the environment and converted into a valuable polymer and forms complex with fluoride ions and thus extracts it from water. Factors that influence positive complexation were established and enhanced to enable the material applied on real water samples. The conversion process involved is a green method of removal for the solid pollutants from the environment through chemical epoxidation processes. It was first done through the solubilization of the polythene in dichloromethane then its epoxidation and the activation with an organic amine [24].
Materials and Methods
Research design
The focus of this study was to synthesis a sorbent material by modifying of waste polythene material. This was achieved by chemically anchoring a functional group on the material capable of interacting with fluoride ions. The final product was then used to extract the fluorides from the aqueous media. The protocol was to use non-toxic and environmentally friendly materials. This study was carried out in several parts. This comprised of the synthesis, characterization of the modified material, optimization of removal parameters and then its subsequent application for the removal of fluorides from both synthetic and environmental waters samples.
Chemicals and reagents
All the solutions were prepared in double distilled water and the reagents were of analytical grade. A fluoride standard stock solution of 1000 μg l-1 was prepared using sodium fluoride in a sodium acetate buffer solution. It was from this solution that subsequent working solutions were prepared from. A solution of 0.1 M nitric acid and 0.1 M sodium hydroxide solutions were also used to adjust the pH of the working solutions to the required value. The above chemicals as well as hydrogen peroxide, triethylamine, 1,2-dichloromethane and hydrochloric acid were all supplied by Kobian Kenya Ltd. which is Sigma Aldrich’s outlet in Kenya. The polythene waste materials were collected from within dumpsites in Nairobi.
Instrumentation
The fluoride content in the solutions was determined by potentiometric method using an ion selective electrode (JENWAY 3345 Ion Meter). The polymeric material was characterized using Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer 100 made in Waltham, MA, USA). The pH of the synthetic and real samples was monitored by a 3345-JENWAY (USA) pH meter. Refluxing the reaction mixture was carried out using a 1000 ml isomatle supplied by Gallenkamp UK.
Preparation of modified polythene material
Thirty grams of clean dry polythene bags were cut into small pieces and placed in a 500 ml three neck flask. To this, 300 ml of 1,2-dichloromethane were added and the resulting mixture refluxed for six hours on an isomantle. The resulting material was then allowed to cool resulting to fine crystals. The solvent was the filtered off and the solid material was weighed into a 250 ml three necked flask followed by addition of 10 ml of 1,2-dichloromethane, 10 g of sodium hydroxide pellets 50 ml of hydrogen peroxide and the mixture refluxed for 4 h. The resulting mixture was allowed to cool then filtrated by suction it for 13 h. The solid product was activated with 5 ml of triethylamine. The resulting viscous material was then thermally treated at 110°C for 3 h. A solid polythene material obtained was then grounded into a fine powder and then applied for complexation of the fluoride in solution in batch adsorption experiments.
Batch experiments
Complexation studies were carried out on a lab-line mechanical reciprocating shaker model (DKZ-1NO.1007827) using plastic screw cap bottles. Into these bottles, known weights (0.04 g) of the polymeric material were introduced and model solutions containing a known concentration of the fluoride ion solutions with their pH adjusted to values between pH 3 and pH 7. The sample solution was equilibrated at a constant shaking rate of 150 rpm and temperature of 25°C at a sufficient time. The resulting mixture was then filtered with Whatman NO 1 filter paper and the concentration of fluoride ions in the filtrates determined using a fluoride ion selective electrode on a 3345- JENWAY potentiometer.
Optimization of complexation parameters
Effects of various parameters on removal of fluoride ion from water were investigated by varying the parameter under investigation while maintaining the others constant. The pH of the medium was adjusted by using either sodium acetate solution or 0.1 M nitric acid drop wise. A solution of 0.1 M hydrochloric acid was used to investigate the effect of chloride ions on the adsorbent fluoride complexation.
Effect of pH
Batches of the powdered activated material approximately 0.1 g each were weighed and placed in 100 ml polythene screw cap bottles. Thereafter, 30 ml of the fluoride model solutions (20 mg l-1) prepared in an appropriate buffer solution (0.1 M sodium acetate/acetic acid) and their pH adjusted to values of between pH 2 and pH 7 were added. The mixtures were equilibrated for 1 h after which the fluoride ions in solution were analyzed potentiometrically.
Optimization of contact time
Aliquots of 30 ml model solutions (20 mg l-1) were dispensed into 100 ml polyethylene bottles containing approximately 0.1 g of powdered activated material. The initial pH of the model solutions was adjusted to the optimum pH value. The respective mixtures were allowed to equilibrate and then removed from the shaker at different time intervals. The solid material was filtered off through suction and the concentration of the fluoride ions in the filtrate determined.
Effect of sorbent dose on percentage fluoride removal
The effect of the amount of sorbent (dose) was carried out in 5 ml plastic SPE columns (Vac-master, Switzerland) packed with varying known weights (0.0 g to 0.1 g) of the solid material. The columns were previously washed successively with double distilled deionized water, preconditioned, and buffered to the desired pH before use. Buffered solutions (100 ml) containing 0.20 mg l-1 of metal ions were loaded onto the column at a flow rate of 3 ml min-1. The retained fluoride ions were stripped with 1 M hydrochloric acid solutions and the concentration of the eluent was analyzed for the levels of the fluoride ions.
Effect of fluoride ion concentration-Determination of complexation capacity
Complexation capacity was determined by varying the initial fluoride ion concentration in batches of 30 ml containing 0.3 g modified polythene material. The pH was adjusted to 4.0 and the mixture agitated for 1 h in a water bath shaker. After the equilibrium time was attained the mixture was filtered and the amount of fluoride ions determined using fluoride ion selective electrode. The data obtained was used in Langmuir and Freundlich isotherm models to obtain the complexation capacity.
Calculation of metal ion sorption on modified and unmodified maize tassels
The amount of fluoride ions removal by the synthesized material during the series of batch investigations was evaluated using Equation (1):
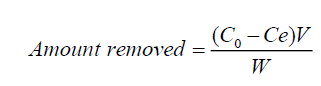 (1)
(1)
Where C0 and Ce are the initial and equilibrium concentration (mg l-1) of fluoride ions in solution respectively, V is the volume of solution (in liters) and W (g) is the mass of the solid material.
Regeneration of modified polythene material
The modified polythene material of 0.2 g, 0.5 g, 1.0 g, and 1.5 g was packed in a column and 50 ml of 20 μg ml-1 fluoride solution allowed to flow through it. The amount of fluoride solution at equilibrium was determined using fluoride ion selective electrode. Fluoridated modified polythene material was washed with 5 ml of 1.0 M hydrochloric acid and the content of fluoride ions attached were stripped the acid. The percentage recovery obtained using Equation (2).
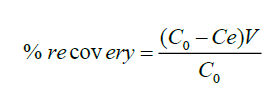 (2)
(2)
Analysis of environmental water sample
A similar treatment was done on Lake Baringo water samples whose fluoride ion content was found to be 41.24 mg l-1. To this samples, known concentrations of fluoride ions was spiked into a 50 ml of the water sample in 100 ml polythene bottles containing 0.3 g of modified polythene material. The pH of the mixtures was adjusted to 4.0 then equilibrated for 1 h in the thermostat shaker. The mixture was filtered and the amount of fluoride ions determined using fluoride ion selective electrode.
Results and Discussion
Characterization of modified polythene material
The original polythene, the modified, the fluoride complexed and the regenerated materials were characterized with FTIR (8400 Shimadzu model) and the resulting spectra are presented in Figure. 1-5. Figure. 1 shows the results of the unmodified polystyrene.
The results show strong bands 2850.6 cm-1 and 2920.0 cm-1. These may be attributed to stretching of C-H bonds [25]. Another band at 1463.9 was observed which may have been contributed by -C=C- stretching [25]. The material was then modified with strongly basic hydrogen peroxide and activated with an amino compound. The resulting material was analyzed with FTIR. The results obtained were presented in Figure. 2.
A band emerged at 1029.9 cm-1 and was attributed to the C-O deformation of the oxirane group, although some works done by [26]. Another was observed at 3400.3 cm-1. This could have been contributed by the C-H tension of the methylene group of the epoxy ring as the treatment was done in a dry environment [26]. FTIR results obtained were presented in Figure. 3 which was acquired in a transmittance mode.
The spectra in Figure. 2 show strong bands between 3319.3 cm-1. This was attributed to -NH groups [27]. The functional group is capable of interacting with the fluoride ion [7]. The material was reacted with a solution containing 20 mg l-1 of the test solution. The resultant was filtered, dried and the solid analyzed with FTIR. The results obtained are as presented in Figure. 4.
The band at 3319.3 cm-1 was observed to have shifted to 3456.2 cm-1 and its intensity was low. This could be attributed to the interaction of the amino functional group with the highly electronegative fluoride species forming a relatively strong bond. The electron density is concentrated around the fluorine, leaving the nitrogen relatively electron poor making it more positive [28]. This contributed to the shift in the wavelength of absorption [28]. When the material was treated with a solution of 0.1 M hydrochloric acid, the resulting solid obtained was analyzed and the results presented in Figure. 5.
It was observed that the band at 3456.2 cm-1 which had a low intensity had slightly shifted to 3442.7 cm-1 and the intensity increased in the regenerated material. This shows that the solid material had been stripped of the fluoride ion and replaced with the chloro group. This implies that the material could be reused for removal of fluoride ions in water.
Effect of pH on removal of fluoride ions by modified polythene material
Interaction of the fluoride ions with nitrogen containing moieties is pH dependent. This is because pH influences the charge of the nitrogen atom of the sorbent and the chemistry of the metal ion [29]. Thus, pH of the solution has a significant effect on the removal of fluoride ions from water since it determines the surface charge of the adsorbent. In this study the optimum uptake was determined by interacting model solutions buffered at various pH with the study material. The results were presented in Figure. 6.
It was observed that at higher pH values, the removal of fluoride ions from water was low. Decrease in fluoride removal at higher pH may be due to gradual increase in number of hydroxide ions on the adsorbent surface causing repulsion of fluoride ions [29]. At lower (2-4) pH values, removal of fluoride increased significantly but decreased at the physiologic pH of water. This could be due increasing the positive charge on the surface of the adsorbent leading to greater complexation of fluoride. A similar was observed by Sahira, et al. while they removed of fluoride from water using Zirconyl-Impregnated activated carbon prepared from lapsi seed stone [30].
Effect of chloride ions
The effect of chloride ions on removal of fluoride ions by modified polythene material was investigated by varying the pH of the solution using 0.1 M hydrochloric acid while keeping all the other variables constant. This was meant to investigate the effect of chloride ions on the adsorbent fluoride complexation. The results obtained were presented in Figure. 7. It was observed that at pH values lower than 2.0, the chloride ions hindered the complexation of fluorides on the adsorbent. This could be due to competition between the fluoride and chloride ions for interaction sites. A similar observation was reported by Meenakshi and Maheshwari as investigated quaternary ammonium functional groups containing fluoride ions whose sorption capacity was affected negative by chloride ions [31]. When the concentration of the chloride is elevated, concentration becomes the driving force for the sites in the solid material [32]. From that observation, it was realized that the presence of a high chloride ion concentration in solution replaced the fluoride ions leading to recharge of the resin and starting the process again [32]. Other ions such as NO3- and SO42 had no effect on the interaction of fluoride and the study material. Such observation was also reported by Velazquez et al. in the study on the removal of fluoride ions by modified zeoiltic tuff. Therefore, our study exploited the use of hydrochloric acid to regenerate the synthesized material [33].
Effect of complex dosage
The effect of modified polythene material dosage was investigated by varying the modified polythene material masses from 0.01 g to 0.1 g, 30 ml of the model solution at an optimum pH of 4.0. The contact time was maintained at 30 minutes. The results obtained were presented as shown in Figure. 8.
Results show an increase in complexation capacity as the dosage was increased from 0.01 g to 0.1 g. This was due to the large number of binding sites resulting from increased adsorbent dosage and availability of more effective surface area of the adsorbent [34]. Similar findings were reported by [35].
Effect of initial fluoride concentration on complexation of fluoride ions
Studies of initial fluoride concentration was done by keeping all the other variables constant while changing the concentration of the solution (pH=4.0, dosage=0.04 g, temperature=25°C). The effect of initial fluoride concentration was varied from 10 ppm to 150 ppm as shown in Figure. 9:
The results show that an increase in initial fluoride ion concentration increased complexation of the ion. However, above 100 μg ml-1, there was no significant change in complexation of fluoride ions on modified polythene material and the curve remained relatively constant. This was probably due to saturation of binding sites of the modified polythene material inhibiting further complexation. Similar results were obtained by Aziz et al. on kinetic modeling and isotherm studies for copper (II) adsorption onto palm oil boiler mill fly ash (POFA) as a natural low-cost adsorbent [36].
Effect of contact time
The rate of fluoride uptake is related to the efficiency of the complexing sites to hold the ion, as well as the electronegativity of the halide, thus controlling the residence time of sorbate at the solid-solution interface [37]. This led to the investigation of the effect of contact time by varying the time of contact from 0 to 90 minutes while keeping all the other parameters constant. The results obtained were and presented in Figure. 10.
The general observation was that there was an initial rapid rate within the first 30 min followed by a subsequent slower rate upon saturation of the binding sites. However, the material could remove up to 95% of the metals in less than 30 min hence has a potential application for the remediation of polluted waters. From that observation, equilibration time was done for more than 30 min was used in all the other experiments.
Recovery of fluoride ions and Regeneration of modified polythene material
Effect of sorbate dose on the per cent recovery of fluoride ions was investigated by varying weights (0.2 g, 0.5 g, 1.0 g, and 2.0 g) of the modified resin material packed in a 6-ml polystyrene column. The model solutions buffered at optimum pH value of 4.0 then made passed through the column at 3 ml minute-1 flow rate. The retained fluoride ions were eluted from the sorbate with 1.0 M hydrochloric acid and the content of the fluoride in the solution analyzed. The results on percentage removal were presented in Figure. 11.
The general observation made for all the percentage removal decreased from 97% to 92% with increase in fluoride ion concentration using synthetic water samples. This is also attributed to the concentration being the driving force for the fluoride ions to occupy available adsorption sites [32]. When most of the complexation sites have been occupied, there will be no more removal of fluoride ions from solution. A similar experiment was carried out using water from lake Baringo. The results obtained were presented in Figure. 12 The results obtained were presented in Figure. 12.
The removal efficiency decreased with increase in concentration from approximately 25% to 10%. Comparing the results obtained with those of synthetic water, it was observed that the percentage removal of fluoride ions in synthetic water was high at high that that of environmental water. This was probably due to availability of interfering ions like chloride in lake water.
Equilibrium studies
To determine the complexation of fluoride ions on modified polythene material, complexation isotherms models were used to provide the nature and physico-chemical interactions involved in the complexation [38]. In this study, Langmuir and Freundlich isotherm models were used to determine the maximum complexation capacity of modified polythene material [38,39]. The study was done by varying the initial concentration of fluoride solution from 10 ppm to 1000 ppm in a water bath shaker for 30 minutes and the data tabulated as shown in Table 1.
| Co (mg/L)) | Mass | Ce (mg/L) | Qe (mg/g) | Log Ce | log Qe |
|---|---|---|---|---|---|
| 10 | 0.0299 | 9.15 | 0.008423 | 0.961421 | -2.07454 |
| 30 | 0.1 | 21.3 | 0.08574 | 1.32838 | -1.06682 |
| 70 | 0.1 | 32.55 | 0.20349 | 1.512551 | -0.69146 |
| 100 | 0.1 | 48 | 0.2904 | 1.681241 | -0.537 |
| 150 | 1 | 942.75 | 0.26145 | 2.974397 | -0.58261 |
| 250 | 0.0306 | 262.5 | 0.213435 | 2.419129 | -0.67073 |
| 500 | 0.0299 | 590.25 | 0.413203 | 2.771036 | -0.38384 |
| 1000 | 0.0298 | 1200.9 | 0.822426 | 3.079507 | -0.0849 |
Table 1. Experimental adsorption data.
Langmuir isotherm model
Langmuir isotherm describes a monolayer complexation process on a homogeneous surface of an adsorbent. The model assumes uniform energies of complexation onto the surface and adsorbate do not transmigrate in the plane of the surface. Langmuir isotherm assumes that adsorbate molecules have equal affinity on the surface and that the adsorbed layer is one molecule in thickness [40]. The strength of the intermolecular attractive forces is believed to fall of rapidly with distance [ 41]. The linearized equation for Langmuir isotherm is as shown below:
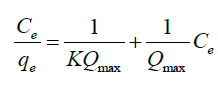 (3)
(3)
Where, Ce is the concentration at equilibrium (mg l-1), Qe is the amount of fluoride ions adsorbed (mg/g), Qmax is the complexation capacity and K is the apparent energy of sorption K and Qmax are calculated by plotting a graph of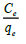 against Ce as shown in Figure. 13:
against Ce as shown in Figure. 13:
From the graph, it was observed that the complexation capacity of fluoride ions was 111.1 mg/g and the energy of complexation of fluoride ions was -0.046 L/mg. correlation coefficient R2 was 0.420.
Freundlich isotherm
Freundlich isotherm model describes a complexation process which takes place on a heterogeneous surface with unlimited binding sites [42]. It predicts that the fluoride concentration on the adsorbent will increase so long as there is an increase in fluoride concentration in the liquid. The linearized Freundlich equation used in this study was
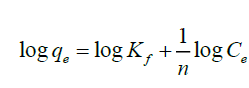 (4)
(4)
Where, Kf is Freundlich sorption capacity, 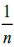 is the complexation intensity. When
is the complexation intensity. When 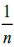 is less than 1, it is an indication of favorable conditions of complexation through Freundlich adsorption model [43]. Kf and
is less than 1, it is an indication of favorable conditions of complexation through Freundlich adsorption model [43]. Kf and 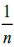 were determined by plotting a graph of log qe against log Ce as shown in Figure. 14.
were determined by plotting a graph of log qe against log Ce as shown in Figure. 14.
From the graph Kf value obtained was which was higher than that obtained by Auphedeous et al. in reduction of fluorine in water using clay mixed with hydroxyapatite [44]. The correlation coefficient R2 was 0.302. It was found out that Langmuir isotherm model had the highest R2 value hence the best fit. Therefore, complexation of fluoride ions on modified polythene material favored a monolayer complexation process.
Sorption mechanism
The kinetic data was fitted into Pseudo first order and Pseudo second order rate kinetics to determine the mechanism of fluoride ions uptake by the modified polythene material. The results are presented in Figure. 15 and 16 respectively.
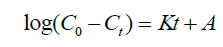 (5)
(5)
Where Co is the initial concentration, Ct is the concentration at time t, K is the equilibrium rate constant and A is the intercept. A plot of ln Co-Ct against
Pseudo second order kinetic
Pseudo second order rate expression is used to describe chemisorption involving valence forces through the sharing or exchange of electrons between adsorbent and adsorbate as covalent forces and ion exchange [45]. The rate limiting step in this model is a surface adsorption that involves physiochemical interactions between the two phases [46]. Equation (6) shows a second order sorption kinetics.
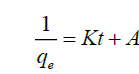 (6)
(6)
Where Qe is the amount of fluoride ions complexed at a unit mass (mg g-1), tis the time and A is the intercept. From the graphs, it was observed that the data fitted well in Pseudo first order kinetic with a correlation coefficient R2 of 0.324 which was higher than that obtained in Pseudo second order kinetic a rate constant of 0.034 confirming that complexation of fluoride ions by modified polythene material is a physiosorption process.
Conclusions
This study successfully modified polythene papers through epoxidation and then activated with triethylamine. The resulting solid material was applied on the removal of fluoride ions in water. It was found out that the adsorption capacity of 18.545 mg g-1 and prescribed to the Langmuir model isotherm. The material removed up to 95% of the fluoride ions within the first ten minutes of contact time and was regeneratable using a dilute solution (1 M) of hydrochloric acids 10 min. This implies that, waste modified polythene material has a potential application for the remediation of fluoride polluted waters.
References
- Smith A (2009) conference proceedings on chemistry international on global dialogue on nanotechnology and the Poor 31 Issue No (4) Meridian Institute. Opportunities and Risks 2006.
- Renault F, Sancey B, Badot PM, et al. Chitosan for coagulation/flocculation processes-an eco-friendly approach. Eur Polymer J. 2009;45(5):1337-48.
- Singh KK, Hasan SH, Talat M, et al. Removal of Cr (VI) from aqueous solutions using wheat bran. Chem Eng J. 2009;151(1):113-21.
- Amini M, Mueller K, Abbaspour KC, et al. Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ sci Tech. 2008;42(10):3662-8.
- Kahama RW, Kariuki DN, Kariuki HN, et al. Fluorosis in children and sources of fluoride around Lake Elementaita region of Kenya. Fluoride. 1997;30(1):19-25.
- Barrère F, van Blitterswijk CA, de Groot K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomed. 2006;1(3):317.
- Cotton F, Wilkinson J (1980) Advanced Inorganic Chemistry. 4th Ed. John Willey and sons NY., pp 563 & 810.
- Simons JH (1965) Fluorine Chemistry, 1st Ed. Academic press Inc. Lon pp14 & 377-81.
- Marshall S. The water crisis in Kenya: Causes, effects and solutions. Global Majority E-Journal. 2011;2(1):31-45.
- Sorg TJ. Treatment technology to meet the interim primary drinking water regulations for inorganics. J Am Water Works Assoc. 1978:105-12.
- Zhou P, Zou J, Tian F, Shang Z. Fluorine bonding-how does it work in protein-ligand interactions? J chem inf model. 2009;49(10):2344-55.
- Choi AL, Sun G, Zhang Y, Grandjean P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. 120(10):1362-68.
- Lu Y, Sun ZR, Wu LN, et al. Effect of high-fluoride water on intelligence in children. Fluoride. 2000;33(2):74-8.
- Williamson MM. Endemic dental fluorosis in Kenya. A preliminary report. East Afr med J. 1953;30:217-33.
- Cha D, Song J, Sarr D. Treatment technologies. Water Environ Resource. 1997;69:676-89.
- Fawell JK, Bailey K. Fluoride in drinking-water. World Health Organization; 2006.
- Anasuya A, Bapurao S, Paranjape PK. Fluoride and silicon intake in normal and endemic fluorotic areas. J Trace Elements Med Biol. 1996;10(3):149-55.
- Boethius A. Something rotten in Scandinavia: The world's earliest evidence of fermentation. J Archaeol Sci. 2016;66:169-80.
- Jones LL, McDonald DA, Borum PR. Acylcarnitines: Role in brain. Prog lipid res. 2010;49(1):61-75.
- Sarter M, Parikh V. Choline transporters, cholinergic transmission, and cognition. Nat Rev Neurosci. 2005;6(1):48-56.
- Aurah CM. Assessment of extent to which plastic bag waste management methods used in Nairobi city promote sustainability. Am J Environ Protec. 2013;1(4):96-101.
- Peters K. Community-based waste management for environmental management and income generation in low-income areas: A case study of Nairobi, Kenya. Published by City Farmer, Canada's Office of Urban Agriculture. 1998 Mar.
- Adam W, Roschmann KJ, Saha-Möller CR, et al. Cis-Stilbene and (1α, 2β, 3α)-(2-Ethenyl-3-methoxycyclopropyl) benzene as mechanistic probes in the MnIII (Salen)-catalyzed epoxidation: Influence of the oxygen source and the counterion on the diastereo selectivity of the competitive concerted and radical-type oxygen transfer. J Am Chem Soci. 2002;124(18):5068-73.
- Coates J. Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry. 2000.
- Dannenberg H, Harp Jr WR. Determination of cure and analysis of cured epoxy resins. Anal Chem. 1956;28(1):86-90.
- Stuart B. 1996. Modern infrared spectroscopy. New York: John Wiley & Sons Inc.
- Blanksby SJ, Ellison GB. Bond dissociation energies of organic molecules. Acc chem res. 2003;36(4):255-63.
- Ünlü N, Ersoz M. Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater. 2006;136(2):272-80.
- Tembhurkar AR, Dongre S. Studies on fluoride removal using adsorption process. J environ sci engi. 2006;48(3):151-6.
- Joshi S, Adhikari M, Pradhananga RR. Adsorption of fluoride ion onto zirconyl-impregnated activated carbon prepared from lapsi seed stone. J Nepal Chem Soc. 2013;30:13-23.
- Garg VK, Malik A. Groundwater quality in some villages of Haryana, India: focus on fluoride and fluorosis. J Hazard mater. 2004;106(1):85-97.
- Ilhan S, Nourbakhsh MN, Kiliçarslan S, et al. Removal of chromium, lead and copper ions from industrial waste waters by Staphylococcus saprophyticus. Turkish Electron J Biotechnol. 2004;2(2):50-7.
- Velazquez-Peña GC, Solache-Ríos M, Martínez-Miranda V. Competing effects of chloride, nitrate, and sulfate ions on the removal of fluoride by a modified zeolitic tuff. Water Air Soil Pollut. 2015;226(1):2236.
- Aravind J, Kanmani P, Devisri AJ, et al. Equilibrium and kinetic study on chromium (VI) removal from simulated waste water using gooseberry seeds as a novel biosorbent. Glob J Environ Sci Manag. 2015;1(3):233-44.
- Dutta M, Ray T, Basu JK. Batch adsorption of fluoride ions onto microwave assisted activated carbon derived from Acacia Auriculiformis scrap wood. Arch Appl Sci Res. 2012;4(1):536-50.
- Aziz AS, Manaf LA, Man HC, et al. Kinetic modeling and isotherm studies for copper (II) adsorption onto palm oil boiler mill fly ash (POFA) as a natural low-cost adsorbent. BioResources. 2013;9(1):336-56.
- Demirbas E, Kobya M, Senturk E, et al. Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water Sa. 2004;30(4):533-9.
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica, and platinum. J Am Chem soc. 1918;40(9):1361-403.
- Freundlich HM. Over the adsorption in solution. J Phys Chem. 1906;57(385):e470.
- Dada AO, Olalekan AP, Olatunya AM, et al. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem. 2012;3(1):38-45.
- Rajamohan N. Equilibrium studies on sorption of an anionic dye onto acid activated water hyacinth roots. Afr J Environ Sci Tech. 2009;3(11).
- Chou KS, Tsai JC, Lo CT. The adsorption of Congo red and vacuum pump oil by rice hull ash. Bioresource Technol. 2001;78(2):217-9.
- Dang-i AY, Boansi AO, Pedevoah MM. Reduction of fluorine in water using clay mixed with hydroxyapatite. Int J Appl. 2015;5(2).
- Smith A. (2009) Conference proceedings on chemistry international on global dialogue on nanotechnology and the Poor 31 Issue No (4) Meridian Insitute. Opportunities and Risks 2006.
- Sun Y, Zhang JP, Yang G, et al. Removal of pollutants with activated carbon produced from K2CO3 activation of lignin from reed black liquors. Chemical and biochemical engineering quarterly. 2006;20(4):429-35.
- Wang YY, Chai LY, Chang H, et al. Equilibrium of hydroxyl complex ions in Pb2+-H2O system. Trans Nonferrous Met Soc China. 2009;19(2):458-62.