Original Article
, Volume: 13( 6)Red Light and Nitrogen Depletion Stimulate the Synthesis of Lipids and N-Alkadienes Susceptible to be Used as Biofuels in Botryococcus braunii UTEX 2441 (Race A)
- *Correspondence:
- Rosa Olivia Cañizares-Villanueva Laboratorio de Biotecnología de Microalgas, Departamento de Biotecnología y Bioingeniería, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Av. IPN 2508, San Pedro Zacatenco, C.P. 07360, Cd. de México
Tel: +52 (55) 5747 3800; E-mail: rcanizar@cinvestav.mx
Received Date: December 08, 2017 Accepted Date: December 12, 2017 Published Date: December 15, 2017
Citation:Acuapan-Hernandez J, Cañizares-Villanueva RO, Cristiani-Urbina E. Red Light and Nitrogen Depletion Stimulate the Synthesis of Lipids and N-Alkadienes Susceptible to be Used as Biofuels in Botryococcus braunii UTEX 2441 (Race A). Biotechnol Ind J. 2017;13(6):155
Abstract
Effects of four light types, blue, green, white and red, as well as the incubation time were evaluated on culture growth and intracellular lipid and hydrocarbon production in Botryococcus braunii UTEX 2441. It was found that the microalga uses more efficiently the low energy photons to stimulate its cellular growth, and during the period of nitrogen deficiency, red light strongly influenced the microalga to synthesize high amounts of lipids and hydrocarbons. Compared with white light (control), red light significantly increased the specific growth rate, biomass productivity, photosynthetic efficiency, nitrogen consumption rate and lipids and hydrocarbons accumulation. At the end of the experiments, the lipid and hydrocarbon concentrations were 27% and 25%, respectively, by dried weight; 96% of the hydrocarbons produced were a mixture of the n-alkadienes C29H56 and C31H60. Results provide information of the combined effect of wavelength and nitrogen depletion over the intracellular lipid and hydrocarbon content, and the amounts in which they are produced, and suggests that the wavelength and incubation time are variables that can be used to manage the metabolism of biotechnological products in this alga.
Keywords
Botryococcus braunii; Light type; Lipids; Hydrocarbons; n-Alkadienes; Nitrogen depletion
Introduction
Light wavelength stimulates microalgal growth and productivity [1-3] because light absorption depends on the cellular photosynthetic pigments that capture photons from specific areas of the electromagnetic spectrum; this absorption regulates cellular processes that affect the composition of photosynthetic products [4,5].
In autotrophic cultures, the quantity and quality of light are essential to improving the yields of both biomass and metabolites synthesized by these organisms. Light is the energy that promotes the photochemical reactions for CO2 fixation and its eventual conversion to products of industrial interest. Currently, technology based on light emitting diodes (LEDs) is very important and provides low cost light that can be used in autotrophic cultures at a laboratory scale. The use of LEDs for this purpose has attracted interest because they have low energy consumption and the possibility of emitting in a narrower range of the electromagnetic spectrum than common bulbs. This last feature is important for providing light that can be efficiently exploited by autotrophic organisms (photosynthetically active light) to potentiate photochemical reactions and CO2 fixation. LED technology allows high biomass yield, increases growth rates, and is considered an economical and efficient source of energy for growing microalgae for biofuel production [6].
There are three races of Botryococcus braunii, and they are classified according the type of hydrocarbon synthesized. Race A preferably synthesizes n-alkadiene and triene hydrocarbons with odd C23-C33 chains. Race B synthesizes triterpenoid hydrocarbons with C30-C37 chains known as botryococcenes and C34 methylated hydrocarbons known as squalene which are ideal for producing liquid biofuels [7], such as diesel, jet fuel and gasoline [8]. Race L synthesizes licopadiene, a single tetraterpenoid hydrocarbon [9].
Of the three B. braunii races, Botryococcus braunii UTEX 2441 (race A) has not been shown to synthesize high percentages of lipids and hydrocarbons. For this reason, the purpose of this study was to evaluate the effect of wavelength and incubation time on the Botryococcus braunii UTEX 2441 growth rate and lipid and hydrocarbon production.
Materials and Methods
Microalgae and inoculum preparation
Botryococcus braunii UTEX 2441 was acquired from the Microbial Collection at the University of Texas, USA. The inoculum was prepared by growing the cells in 1 L glass bottles flat surfaces and 850 mL of Bold’s basal medium (BBM) [10]. The incubation conditions were as follows: continuous illumination at an intensity of 60 μmol m-2s-1 supplied by white-light fluorescent lamps (OSRAM DULUX L Daylight, Germany), 22 ± 2°C and bubble mixing at 0.5 vvm using filtered atmospheric air. After incubation for 14 days, an 85 mL aliquot of the culture was collected during exponential growth to continue reseeding; this procedure was repeated four times to ensure inoculum viability. All the culture conditions were standardized previous to the experiments.
Effect of the wavelength on specific growth rate, nitrogen consumption and lipids and hydrocarbons production
From the fourth reseeding, an 85 mL aliquot was collected during exponential growth (197 mg L-1 dry biomass) for the inoculation of the treatments described below, which were also performed in glass bottles flat surfaces. Volume of each bottle and growth medium were the same as used to prepare the inoculum. Cells were autotrophically batch-cultured under the following conditions: 22 ± 2°C, bubble mixing at 0.5 vvm using filtered atmospheric air and continuous illumination with blue (λmax=460 nm), green (λmax=518 nm), red (λmax=634 nm) and white light (λmax=450-563 nm) using light-emitting diodes (LEDs) at 60 μmol m-2s-1. In this research, white light was used as control, which served to compared results found in monochromatic light of different wavelength. Irradiation intensity of each LED was determined using a Hansatech type QRT1 radiometer, and the wavelength was determined using a spectrometer (Spectrometer Photon Control Inc. model SPM002 with a 64 mm focal length and multimode optical fiber with a 1 mm diameter). All experiments were performed in triplicate with samples collected once a week to determine the growth and nitrogen consumption kinetics. Lipids and hydrocarbon produced were determined in each of the cultures exposed to the different wavelengths and two incubation times, as described below.
Determining incubation time effect on lipid and hydrocarbon synthesis
These experiments were concluded at two different incubation times due to the biomass requirement for the chemical analysis described below. The first experiment was concluded on day 55, when recorded the lipid and hydrocarbon content under favorable nutrient conditions. The second experiment was concluded on day 66 to allow for greater accumulation of both compounds in all cultures. The sample volume was 380 mL in both cases for each experimental unit.
Growth, specific growth rate and biomass productivity
Biomass concentration was determined using a 10 mL sample and the methodology from Hernández-Zamora et al. [11]. Specific growth rate of algal culture is a measure of the increase in biomass over time and it was determined by the following
equation: 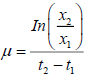
Where, μ is specific growth rate, X1 and X2, biomass content at time t1 (0 d) and t2 (55 d) respectively. Biomass productivity was determined in accordance to Okumura et al. [6].
Photosynthetic efficiency
Photosynthetic efficiency was calculated in accordance to Garibay-Hernández et al. [12] using the following equations:
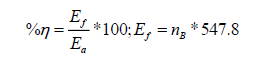
Where, 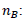 mol of biomass, 547.8: energetic content of the biomass
mol of biomass, 547.8: energetic content of the biomass 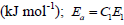
C1: total quantity of irradiated photons (mol), E1: energy per mole of photons (kJ mol-1).
Photosynthetic pigments quantification and nitrogen consumption
extract the photosynthetic pigments, it was used the procedure of Hernández-Zamora et al. [11], Chl-a, Chl-b and carotenois concentrations were determined with a UV-Vis spectrophotometer (Genesys 10 UV Thermo Electron Corporation) according to the equations of Wellburn [13]. Nitrogen consumption was determined using 5-mL aliquots that were vacuumfiltered using Millipore® membranes of 5.0-μm pore diameter; 3-mL of the filtrate were used and mixed with 0.6 mL of NaCl (30% w/w), 0.15 mL of Brucine-sulfamic acid reagent and 3 mL of sulfuric acid (1:1.25 in deionized water). The sample was kept in dry bath at 95°C for 20 min. After the sample was cooled at room temperature, the residual nitrogen concentration was calculated using standard methods [14].
Extracting and quantifying the total lipids and hydrocarbons
Algal biomass was separated by centrifugation (3000 rpm for 10 min) at room temperature in a Thermo Scientific Heraeus Megafuge 8 centrifuge and then lyophilized and 100 mg was used; total lipid content was determined as described by Arias- Peñaranda et al. [15], and the total hydrocarbon content was measured using the technique of Eroglu et al. [16] with 100 mg of lyophilized biomass. The lipid and hydrocarbon concentrations were determined using gravimetry and are expressed as a percentage of the cellular dry-weight. The productivity of both compounds was determined in accordance to Converti et al. [17].
n-Alkadiene analysis, identification and quantification
n-Alkadienes were analyzed using a gas chromatograph with a flame ionization detector (GC-FID) (Perkin-Elmer. Mod. AutoSystem) and by injecting 2 μL aliquots of each sample that was previously suspended in HPLC-grade hexane. Before the analyses, a 2 μL aliquot of a standard reference was injected that contained a mixture of even-chain n-alkadienes (C21- C40) (Sigma-Aldrich # CAT. 04071), which allowed us to compare the retention times between the standard and sample. The analysis conditions were as follows: injector temperature: 260°C, detector temperature: 300°C, N2 as a carrier gas at 19.7 psia and a CPSIL capillary column that was 0.25 mm in diameter and 30 m in length. Method: Initial oven temperature: 220°C for 4 min, ramp at 5°C min-1 to 300°C, maintain for 10 min, ramp at 10°C min-1 to 330°C and maintain for 60 min.
n-Alkadienes were identified by injecting 2 μL aliquots from each sample into a gas chromatograph (Perkin Elmer. Mod. Clarus® 580) coupled to a mass spectrometer (Perkin Elmer. Mod. Clarus® SQ 8S). The analysis conditions were as follows: injector temperature: 250°C, split ratio=20:1; helium as the carrier gas; solvent delay: 3 min; transfer temperature: 220°C; source temperature: 180°C; and exploration: from 30 to 500 Da and 30 m × 320 μm column. Method: Initial oven temperature: 220°C for 4 min, ramp at 5°C min-1 to 300°C, maintain for 10 min, ramp at 10°C min-1 to 330°C and maintain for 40 min. n-alkadienes were quantified as described by Hirose et al. [18] using the following equations:
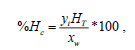
Where, HT: total hydrocarbons (mg), XW: biomass used in the extraction (mg)
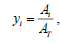
Where, Ai: n-alkadiene peak area, AT: chromatogram total area.
Statistical analysis
For results statistical analysis, it was used one-way analysis of variance (ANOVA) (p<0.05) and Tukey’s post hoc multiple comparison test using the software Sigma-Plot (ver. 11.0).
Results
Influence of light type on the specific growth rate, photosynthetic efficiency and biomass productivity
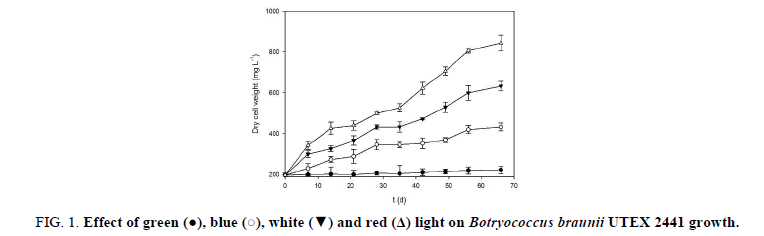
Figure 1 shows the change in dry weight for B. braunii UTEX 2441 cultures exposed to different wavelength light; the data show that the growth of B. braunii UTEX 2441 was stimulated by four tested light wavelengths in the following order: red>white>blue>green. After 66 days, the biomass concentrations were 844 mg L-1, 633 mg L-1, 433 mg L-1 and 223 mg L-1 for red, white, blue and green light color respectively. Under red light, microalgal growth increased beginning at day 7, and this improvement was maintained during the kinetics experiment. At the end of the experiment, biomass concentration increase relative to the initial concentration was 3.2, 2.2 and 1.2-fold for the red, white and blue light, respectively; green light did not significantly increase the biomass concentration (p>0.05). Compared with the control, the red light led to a 33% higher (p<0.05) biomass concentration after 66 days.
Table 1 shows the values for specific growth rate (μ), biomass productivity (ν), photosynthetic efficiency (η) and photosynthetic pigment ratio (Chl-a:Chl-b:Car) for each light color tested. At the end of the kinetics experiment, the maximum values of μ, ν and η were obtained using red light compared with the control; these parameters increased by 25%, 51% and 52%, respectively (p<0.05); for the other wavelengths tested, the values were lower than those obtained with the control. The Chl-a:Chl-b:Car ratio was maintained at approximately 5.2:2.5:1; this ratio show that the Chl-a concentration was greater for all types of light tested.
Influence of light type on nitrogen consumption
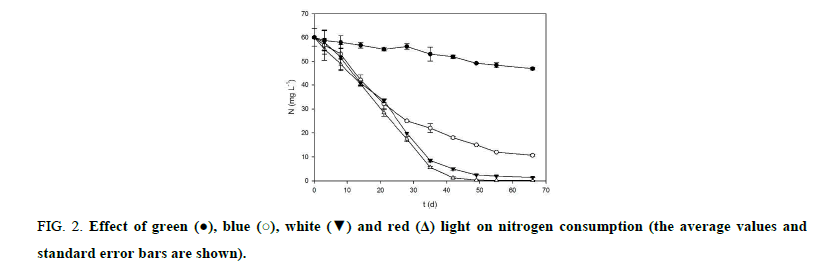
Figure 2 shows nitrogen consumption for B. braunii UTEX 2441 cultures grown at different wavelengths. Direct relationship exists between nitrogen consumption at different wavelengths and microalgal growth (Figure 1. Under red and white light, the specific rate of nitrogen consumption was greater due to the fact that the specific growth rate was higher in both light colors, the faster growth required more nitrogen to maintain the protein synthesis necessary for cell growth and photosynthetic metabolism; thus, the nitrogen depletion rate was higher than that obtained with the other light colors tested. During experiment, nitrogen concentration decreased faster in the cultures exposed to red and white light after 21 days, and by day 55, nitrogen was fully consumed in these. Before 55 days, nitrogen consumption rate was 1.2 mg L-1 d-1, 0.91 mg L-1 d-1, 0.68 mg L-1 d-1 and 0.17 mg L-1 d-1, under red, white, blue and green light, respectively. Under red light, nitrogen was depleted 32% more rapidly compared to control experiment (p<0.05).
Figure 2:Effect of green  blue
blue  white
white  and red
and red  light on nitrogen consumption (the average values and standard error bars are shown).
light on nitrogen consumption (the average values and standard error bars are shown).
Effect of the light type on intracellular lipid production
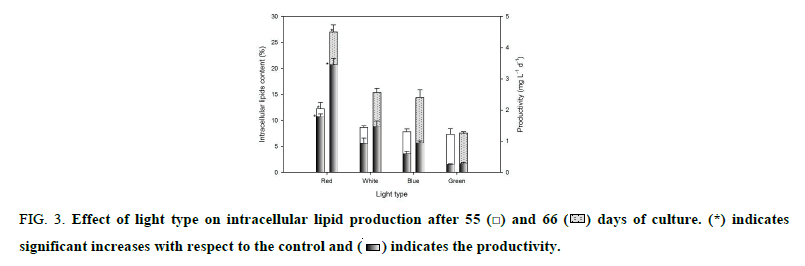
Figure 3 shows intracellular lipid and productivity after 55 and 66 days of incubation. At 55 days, intracellular lipid content was 12%, 9%, 8% and 7% under red, white, blue and green light, respectively, while productivity was 1.8 mg L-1 d-1, 0.9 mg L-1 d-1, 0.6 mg L-1 d-1 and 0.2 mg L-1 d-1, respectively. After 66 days, intracellular lipid content was 27%, 15%, 14% and 8%, and productivity was 3.5 mg L-1 d-1, 1.5 mg L-1 d-1, 1 mg L-1 d-1 and 0.3 mg L-1 d-1, respectively, for the aforementioned light types. After kinetics experiment, it was observed an 80% increase in intracellular lipid content and a productivity increase of 133% for red light compared with the control (p<0.05). With red light, the cell accumulated 59%, 72% y 90% more lipids than with white, blue and green light respectively.
Figure 3: Effect of light type on intracellular lipid production after 55  and 66
and 66  days of culture. (*) indicates significant increases with respect to the control and
days of culture. (*) indicates significant increases with respect to the control and  indicates the productivity.
indicates the productivity.
Influence of light type on total hydrocarbon and n-alkadiene synthesis
Figure 4 shows weight percentage of the total intracellular hydrocarbons produced by B. braunii UTEX 2441 after incubation for 55 and 66 days. At day 55, for red, white, blue and green light, total hydrocarbon concentrations were 11%, 6%, 5% and 2% and productivities were 1.5 mg L-1 d-1, 0.5 mg L-1 d-1, 0.2 mg L-1 d-1 and 0.07 mg L-1 d-1, respectively. At day 66, hydrocarbon concentrations were 25%, 10%, 8% and 3%, and the productivities were 3.1 mg L-1 d-1, 0.8 mg L-1 d-1, 0.3 mg L- 1 d-1and 0.09 mg L-1 d-1, respectively. After 66 days, compared to the control (p<0.05), the red light to a 150% increase in the total hydrocarbon concentration and to a 288% increase in the productivity.
Figure 4: Influence of light type on hydrocarbon production at 55  and 66
and 66  days of culture. (*) indicates significant increases with respect to the control and
days of culture. (*) indicates significant increases with respect to the control and  indicates the productivity.
indicates the productivity.
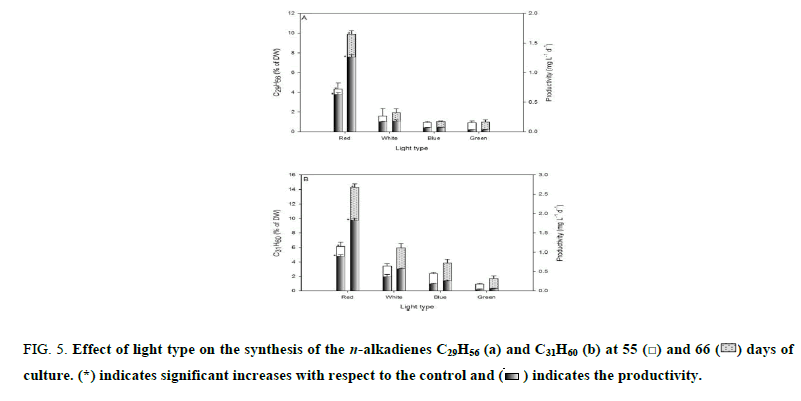
Figure 5 shows the n-alkadienes synthetized by B. braunii UTEX 2441 after incubation for 55 and 66 days. Of the total synthesized hydrocarbons, 96% were a mixture of the n-alkadienes C29H56 and C31H60. For red, white, blue and green light, at day 55, the weight percent of C29H56 was 4.3%, 1.6%, 0.9% and 0.9% with a productivity of 0.6, 0.1, 0.06 and 0.03 mg L-1 d-1, respectively (Figure 5a). At the same time point, the weight percent of C31H60 was 6.1%, 3.4%, 2.4% and 0.9% with a productivity of 0.9 mg L-1 d-1, 0.4 mg L-1 d-1, 0.2 mg L-1 d-1 and 0.04 mg L-1 d-1, respectively (Figure 5b). At day 66, when the aforementioned light types were used, the C29H56 weight percentages were 9.9%, 1.9%, 1.1% and 1% with a productivity of 1.3 mg L-1 d-1, 0.2 mg L-1 d-1, 0.07 mg L-1 d-1 and 0.04 mg L-1 d-1 (Figure 5a). At the same time point, the C31H60 weight percentages were 14.3%, 6%, 3.8% and 1.7% with a productivity of 1.8 mg L-1 d-1, 0.6 mg L-1 d-1, 0.3 mg L-1 d-1 and 0.07 mg L-1 d-1 (Figure 5b), respectively. Compared with the control (p<0.05), at the end of the red-light experiment, the weight percentages of C29H56 and C31H60 increased 4.2 and 1.4-fold, and the productivity increased 5.5 and 2-fold, respectively. In the present research, 96% of the total hydrocarbons synthesized in culture exposed to red light were a mixture of the n-alkadienes C29H56 and C31H60, which is 34% higher than that reported by other authors [18].
Figure 5: Effect of light type on the synthesis of the n-alkadienes C29H56 (a) and C31H60 (b) at 55  and 66
and 66  days of culture. (*) indicates significant increases with respect to the control and
days of culture. (*) indicates significant increases with respect to the control and  indicates the productivity.
indicates the productivity.
Discussion
The different light colors used had an effect on the amount of biomass obtained from B. braunii UTEX 2441 when a constant photon flux density was maintained (Figure 1). Is known that autotrophy under red light is better for the process of photosynthesis, as green pigments preferentially absorb red light, whereas the light of other colors is partially absorbed, however, it seems that the different Botryococcus braunii races can exhibit different growth depending on the quality of the light they receive. Depending of the race, some may increase their growth in red light [Sakamoto] while others grow better with blue light [6]. In this work, monochromatic red light as the light that best stimulates the growth, biomass productivity and photosynthetic efficiency of B. braunii UTEX 2441 (Table 1). These differences could be attributed to the photosynthetic pigments content that they achieve to synthetize during the culture period, since the photosynthetic pigments (chlorophylls and carotenoids) are responsible for the absorption of light that is used to perform the process of photosynthesis [19], and when the quality of light changes during growth, the composition of the pigments is affected [20]. In our study, the chlorophyll a was the most abundant pigment in B. braunii UTEX 2441 (Table 1) and whose light spectrum ranges from 580 to 700 nm. Chlorophyll a is a light-harvesting pigment and is the core of the green algae reaction center pigments [21,22]. Chlorophyll a aids in efficient absorption of photons in this region of the spectrum to produce the chemical energy (NADH and ATP) used in CO2 capture during photosynthesis [23]. The above may cause the improved growth and biomass productivity in B. braunii UTEX 2441 under red light.
| Light type | µ (d-1) | ν (mg L-1 d-1) | η (%) | Chl-a:Chl-b:Car |
|---|---|---|---|---|
| Red | 0.025 ± 0.001a* | 10.9 ± 0.4a* | 9.6 ± 0.43a* | 5.3:2.4:1a |
| White | 0.020 ± 0.002b | 7.2 ± 1.5b | 6.3 ± 0.23b | 5.2:2.5:1a |
| Blue | 0.013 ± 0.002c | 4.0 ± 0.6c | 3.6 ± 0.16c | 5.4:2.5:1a |
| Green | 0.002 ± 0.001d | 0.4 ± 0.6d | 2.1 ± 0.18d | 4.9:2.6:1a |
Table 1. The effect of light type on the µ, ν, η values and the Chl-a:Chl-b:Car ratio. Different letters indicate significant differences between the analyzed parameters (Tukey’s test). (*) indicates significant increases with respect to the control.
The results presented in Figure 2 indicate that the cultures exposed to red and white light exhausted the nitrogen of the culture medium faster, this in response to a more accelerated growth of the microalga which demanded more of this nutrient to maintain its photosynthetic metabolism, whereas in cultures exposed to blue and green light, the same effect was not observed. In cultures exposed to red and white light there was a period of nitrogen depletion for 11 days (55 to 66 day), during this time the cells accumulated high amounts of lipids and hydrocarbons exceeding the values previously reported for this microalga [16,24]. This phenomenon causes a nutritional deficiency and possibly the activation of enzymes involved in lipid biosynthesis. In microalgae, the nitrogen depletion in culture medium stimulates the synthesis of lipids, due to nitrogen limitation decreased growth rate and protein synthesis, and increased lipids and fatty acid biosynthesis, promoting intracellular lipid accumulation [7,25,26,19]. Previous studies aimed at increasing the intracellular lipid content in B. braunii UTEX 2441 indicate that this microalga has low percentages of lipid accumulation (less than 20% dry weight) [16], it is at this point that the management of the variable nitrogen depletion associated with growth and wavelength can interact to improve these current levels of lipid accumulation in the microalga.
Figure 3 shows lipids content and productivity under different wavelengths in B. braunii UTEX 2441. During the 11 days of growth with nutrient deficiency, cultures exposed to red and white light accumulated an amount of intracellular lipids equal to the amount accumulated during the first 55 days; this result was determined by a higher percentage of extraction-, whereas in cultures exposed to blue and green light, the same effect was not observed. Although nitrogen was depleted approximately the same time in cultures exposed to red and white light, with red light, B. braunii 2441 accumulated more lipids than the control and all other colors tested (p<0.01). In culture exposed to red it was obtained lipid accumulation percentages higher than that reported previously [16,24]. The low accumulation of lipids in blue and green light was since the cultures did not completely deplete the nitrogen of the medium, this did not allow to generate the condition of depletion of nitrogen that could trigger the mechanism of lipid induction.
There is little information on the effect of wavelength on lipid synthesis, however, the increase in lipid accumulation in red light may be due to a regulation of the enzymes involved in lipid synthesis mediated by the wavelength, mainly of the ribulose bisphosphate carboxylase/oxygenase (Rubisco) and carbonic anhydrase enzymes, which play a fundamental role in the regulation of the carbon cycle and lipid synthesis [3,21,27]. The red light and nitrogen depletion influence the photosynthetic apparatus of Botryococcus braunii UTEX 2441 capable of synthesizing lipid compounds, showing an overproduction of these [20].
In Botryococcus braunii UTEX 2441 the hydrocarbon synthesis is preferably stimulated in cultures exposed to red light during days of culturing in nutrient deficiency. This was higher than those reported previously [16,24]. In nitrogen starvation condition, microalgae such as B. braunii, activate mechanisms of some biosynthetic pathways that trigger the synthesis of secondary metabolites such as hydrocarbons, the activation of these pathways, increases the concentration of intracellular hydrocarbons and non-polar lipids [27,28].
Results of this study show that at all tested wavelengths, the most abundant n-alkadienes were C29H56 and C31H60 (Figure 5). The same effect observed for total hydrocarbons was present for n-alkadienes under the influence of red light. During days of culturing under nutrient deficiency, B. braunii increased n-alkadienes content in red light. This is possibly the result of a mechanism in this microalga responding to nutrient deficiency in the culture medium when growth is stimulated with monochromatic light, since monochromatic light on algae plays a fundamental role in the regulation of cellular processes [2,3], which influences the chemical composition of photosynthates [4,5], Eventually this variable could be used to increase the n-alcadiene production.
In microalgae, the light color can influence the biosynthesis of specific compounds [4,29]. Light color has also been shown to have effects at the transcriptional and post-transcriptional levels in cells. This regulates the expression of genes involved in chloroplast protein synthesis in bean plants [30]. Additionally, it has been shown that low intensity monochromatic light induces changes in electron flow in the transport chain or in the redox state of plastoquinone, which affects the expression of genes encoding chloroplast proteins [31]. The results of our study show that red light preferentially stimulates the synthesis of C29H56 and C31H60 n-alkadienes in B. braunii UTEX 2441. The n-alkadienes synthesized by B. braunii UTEX 2441 are subject of study for their characteristics to be used as sources of biofuels [24], from which a range of compounds such as gasoline and diesel [8] can be obtained with the potential to replace to fossil fuels.
Conclusion
Combined effects of wavelength as well as nitrogen depletion caused lipids and hydrocarbons to accumulate at higher levels than previously reported for B. braunii UTEX 2441. Different wavelengths influenced the composition of synthesized hydrocarbons, which suggest that wavelength as well as incubation time are variables that can be used to manage the metabolism of biotechnological products in algae.
Acknowledgments
The authors thank the Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional for the support to develop this investigation. Acuapan-Hernández J. received a post-graduate scholarship from Consejo Nacional de Ciencia y Tecnología (Grant no. 74757) for his doctoral studies.
References
- Abiusi F, Sampietro G, Marturano G, et al. Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M?M33 grown with LEDs of different colors. Biotechnol Bioeng. 2014;111(5): 956-64.
- Atta M, Idris A, Bukhari A, et al. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Technol. 2013;148:373-8.
- Vadiveloo A, Moheimani NR, Cosgrove JJ, et al. Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res. 2015;8:121-7.
- Marchetti J, Bougaran G, Jauffrais T, et al. Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp. (T-iso). J Appl Phycol. 2013;25(1):109-19.
- Michael C, del Ninno M, Gross M, et al. Use of wavelength-selective optical light filters for enhanced microalgal growth in different algal cultivation systems. Bioresour Technol. 2015;179:473-82.
- Okumura C, Saffreena N, Rahman MA, et al. Economic efficiency of different light wavelengths and intensities using LEDs for the cultivation of green microalga Botryococcus braunii (NIES?836) for biofuel production. Environ Prog Sustain Energy. 2015;34(1):269-75.
- Li Y, Horsman M, Wang B, et al. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol. 2008;81(4):629-36.
- Tran NH, Bartlett JR, Kannangara GSK, et al. Catalytic upgrading of biorefinery oil from micro-algae. Fuel. 2010;89(2):265-74.
- Metzger P, Largeau C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl Microbiol Biotechnol. 2005;66(5):486-96.
- Dayananda C, Sarada R, Usharani M, et al. Autotrophic cultivation of Botryococcus brauniifor the production of hydrocarbon and exopolysaccharides in various media. Biomass Bioenerg. 2007;31:87-93.
- Hernández-Zamora M, Perales-Vela HV, Flores-Ortíz CM, et al. Physiological and biochemical responses of Chlorella vulgaris to Congo red. Ecotoxicol EnvironSaf. 2014;108:72-7.
- Garibay-Hernández A, Vázquez-Duhalt R, Sánchez-Saavedra M, et al. Biodiesel a partir de microalgas. BioTecnología. 2009;13(3):38-61.
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144(3):307-13.
- APHA-AWWA-WPCF. Standard methods for the examination of water and wastewater. APHA, Washington, DC. 1992.
- Arias-Peñaranda MT, Cristiani-Urbina E, Montes-Horcasitas C, et al. Scenedesmus incrassatulus CLHE-Si01: A potential source of renewable lipid for high quality biodiesel production. Bioresour Technol. 2013;140:158-64.
- Eroglu E, Okada S, Melis A. Hydrocarbon productivities in different Botryococcus strains: Comparative methods in product quantification. J Appl Phycol. 2011;23(4):763-75.
- Converti A, Casazza AA, Ortiz EY, et al. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process: Process Intensification. 2009;48(6):1146-51.
- Hirose M, Mukaida F, Okada S, et al. 2013. Active hydrocarbon biosynthesis and accumulation in a green alga, Botryococcus braunii (Race A). Eukaryot Cell. 2013;12(8):1132-41.
- Ort DR, Kramer D. Photosynthesis. In: Encyclopedia of Life Sciences (ELS). John Wiley and Sons, Ltd. Chichester.
- Baba M, Kikuta F, Suzuki I, et al. Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresour Technol. 2012;109:266-70.
- Teo CL, Atta M, Bukhari A, et al. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol. 2014;162:38-44.
- Wang CY, Fu CC, Liu YC. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem Eng J. 2007;37(1):21-5.
- Kim TH, Lee Y, Han SH, et al. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour Technol. 2013;130:75-80.
- Li Y, Moore RB, Qin JG, et al. Extractable liquid, its energy and hydrocarbon content in the green alga Botryococcus braunii. Biomass Bioenerg. 2013;52:103-12.
- Ren HY, Liu BF, Ma C, et al. A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: Effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels. 2013;6:143.
- Ruangsomboon S. Effects of different media and nitrogen sources and levels on growth and lipid of green microalga Botryococcus braunii KMITL and its biodiesel properties based on fatty acid composition. Bioresour Technol. 2015;191:377-84.
- Cheng P, Wang J, Liu T. Effect of cobalt enrichment on growth and hydrocarbon accumulation of Botryococcus braunii with immobilized biofilm attached cultivation. Bioresour Technol. 2015;177:204-8.
- Cheng P, Ji B, Gao L, et al. The growth, lipid and hydrocarbon production of Botryococcus braunii with attached cultivation. Bioresour Technol. 2013;138:95-100.
- Kula M, Rys M, Mo?d?e? K, et al. Metabolic activity, the chemical composition of biomass and photosynthetic activity of Chlorella vulgaris under different light spectra in photobioreactors. Eng Life Sci. 2014;14(1):57-67.
- Glick RE, McCauley SW, Gruissem W, et al. Light quality regulates expression of chloroplast genes and assembly of photosynthetic membrane complexes. Proc Nat Acad Sci. 1986;83(12):4287-91.
- Fey V, Wagner R, Bräutigam K, et al. Photosynthetic redox control of nuclear gene expression. J Exp Bot. 2005;56(416):1491-8.