Review
, Volume: 8( 1)Recent Progress in Transition Metal Carbide Electrocatalysts for the Hydrogen Evolution Reaction
- *Correspondence:
- Feng-Xiang Yin, State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, P.R. China, Tel: 861064412054; Fax: 861064419619; E-mail: yinfx@mail.buct.edu.cn
Received: May 03, 2017; Accepted: May 29, 2017; Published: May 31, 2017
Citation: Zhao X, Yin FX, He X, et al. Recent Progress in Transition Metal Carbide Electrocatalysts for the Hydrogen Evolution Reaction. Res Rev Electrochem. 2017;S:105.
Abstract
The hydrogen evolution reaction (HER), performing as the cathode reaction of electrochemical water splitting, is an important green energy producing process. An increasing interest in transition metal carbides (TMCs) applied in HER has been aroused owing to their similarity in electronic structure to platinum (Pt). In this review, we mainly introduced the recent progress of the elements in group VIB (W, Mo, Cr) and their carbides applied in hydrogen evolution reaction. The structure designs, the catalytic activities and the pH adaptations were primarily investigated and compared. It was promising to obtain a Pt-like catalytic activity and an extraordinary stability in a wide pH range with the transition metal carbide. We also looked forward to the challenges in designing novel TMCs for electrochemical HER catalysis.
Keywords
Electrocatalysts; Carbides; Hydrogen Evolution Reaction (HER).
Introduction
With the development of modern industry, the consumptions of fossil fuels, such as coal, petroleum and natural gas, have increased rapidly in recent years, which resulted in the serious energy crisis and environmental pollution. Great efforts have been made to deal with the aforementioned issues recently. The development of the renewable and clean energy resources such as solar energy, wind energy and hydrogen energy is an efficient route to alleviate the energy crisis and environmental pollution. Hydrogen, as a renewable and clean energy resource, has been attracting considerable attentions in recent years because of its high combustion value and environmental friendliness. At present, the hydrogen is industrially produced through the reforming of natural gas [1-5]. However, this hydrogen production process is closely relative to a large amount of fossil fuel consumption.
Therefore, the clean and non-fossil fuel strategies are urgent to be developed. The electrochemical hydrogen evolution reaction (HER) has been regarded as a most promising process for the hydrogen production in recent years. The reaction also is the cathode reaction (2H++ 2e-→H2) of the electrochemical water splitting process [6,7]. This process only use water as the raw material and the productions are only H2 and O2 without any carbon emitting, which is extensively accessible worldwide. Moreover, the renewable and clean energy resources such as solar energy, wind energy and tidal can supply the electricity consumption for the HER [8].
Due to the complicated kinetics, the hydrogen evolution reaction process is not a one-step process [7,9]. The mechanism of HER has been investigated for a long history and the whole process is universally accepted as a two-step course. The first step is “the Volmer reaction”, in which a free proton distributed in aqueous solution combines with an electron to form an adsorbed hydrogen atom on the cathode. The adsorbed proton acts as an intermediate product of hydrogen evolution.
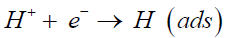 (1)
(1)
The second step is proton desorption step, known as “the Heyrovsky reaction”, in which the adsorbed proton combines with another free proton to form a gas-phase hydrogen molecule and breaks away from the catalyst surface,
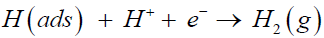 (2)
(2)
or the Tafel recombination reaction, in which the adsorbed hydrogen atom recombines with another same atom to form a gasphase hydrogen molecule and separates from the catalyst surface,
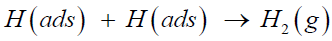 (3)
(3)
Generally, the electrochemical adsorption and desorption steps are competitive with each other, resulting in the sluggishness of the kinetics of HER. Each step mentioned above can be the rate controlling step because the performances of protons acting on the crystalline facets are different in various catalysts. When investigating the rate controlling step of the HER, the Tafel slope at the onset overpotential is commonly regarded as a judgment, which represents the slope in the plot of the applied overpotential vs. the log10 of the current density. The Tafel slopes are 116 mV dec-1, 40 mV dec-1, 30 mV dec-1, respectively, for the three steps mentioned above when each of them is the rate controlling step [1,10]. The sluggish kinetics unavoidably leads to high Tafel slope and onset potential, which indicate a large electrical energy cost. Thus, a highly efficient catalyst to reduce the potential cost is becoming a subject urgent to be developed.
An eligible catalyst always shows a low Tafel slope and indicates the kinetic process is mainly controlled by the reaction (3). It also displays a low overpotential at a specific current density (always chosen as 10 mA cm-2) on the cathode. At present, the catalysts for the HER can be separated into noble metal and non-noble metal catalysts. Noble metal catalysts, such as Pt, Pd and Ru, show excellent catalytic activities for the HER platinum. Among the noble metal catalysts, Pt-based catalyst has shown the lowest overpotential of ~40 mV at the current density of 10 mA cm-2 [11,12]. However, the expensive price, restricted reserve and low stability during the hydrogen evolution process hold back the development of this noble-metal catalyst [6]. Thus, due to the low cost and flexible structure, more and more attentions have been paid to the non-noble metal catalysts, among which non-noble metal oxides, carbides, sulfides and nitrides were continually synthesized to replace the platinum. However, the HER catalytic activities of non-noble metal catalysts still have a distance to catch up with the Pt. Great efforts have been made to improve the non-noble catalysts and there are still many undiscovered frontiers to explore.
In recent years, the transition metal carbides (TMCs) display extraordinary corruption resistance, high physical and chemical stability, low cost and potentially high catalytic activity [1,13-18]. The TMCs have already been widely applied in the electrochemical catalysis, such as the electrocatalysts, noble metal catalysts’ carriers and battery electrodes [19]. Hence, TMCs have drawn a lot of interest in the field of HER catalysis. In the relevant researches, it is discovered that the group VIB elements (chromium (Cr), molybdenum (Mo) and tungsten (W)), and their derivatives show excellent catalytic activities towards HER. The TMCs are often regarded as typical interstitial alloys with metal and carbon atoms. It has been demonstrated by density functional theory (DFT) that the transition metals’ electronic structures are modified by carbon during the formation of carbides [20]. The adjusted electronic structures of the VIB group transition metal atoms resemble that of the noble metal Pt, thus, resulting in the similar catalytic activity for HER as Pt. A qualified VIB group TMC catalyst should display a specific crystalline structure, such as the hexagonal Cr7C3, the orthorhombic Mo2C and the hexagonal WC or W2C. The bulk pure transition metal carbide cannot efficiently exert its potential as a HER catalyst because of the small specific surface area and the low electron conductivity. Thus, a good TMC catalyst also requires new synthesizing methods to shrink its particle size, and also combining with appropriate conducting substrates, such as activated carbon, carbon nanotube (CNT) or graphene, to improve its performance in conducting electrons. Besides, the common TMC synthesizing method is CH4/H2 thermal reduction, which presents a potential safety hazard. Simple and riskless synthesizing methods are encouraged to be developed. There is a large research prospect to apply TMCs in the HER catalysis.
In this review, we aim to make a general overview of the recent progress made in applying TMCs in the HER of the water splitting process. The whole review is constructed as shown in Figure 1, and the main literature review is separated into three parts: Chromium carbides, Molybdenum carbides and Tungsten carbides. Each part of the literatures review contains the representative arts and important advances in this field in recent years. The over-potential, Tafel slope and the experimental conditions are mainly investigated, and the novel methods to make the catalysts more efficient and stable are also described in the article. At last, the future developing trend is discussed to give a further train of thought in the application of TMCs as the electrocatalysts for HER.
Transition Metal Carbide Catalysts
During the formation of metal-carbon covalent bond, the interstitial atoms in the carbon atoms accept the electrons from metal atoms through charge transfer processes. The modified electronic structures of the metal atoms are believed in favor of decreasing the overpotential and the Tafel slope in the catalysis of HER. Group VIB metals include chromium, molybdenum and tungsten. The d-orbitals of these metal atoms are half-filled with electrons, revealing an outer electron configuration of nd5 (n+1) s1. When the five d-electrons hybridize with four outer electrons of carbon (2s22p2), the d-orbitals of the metals are broadened and similar to platinum (5d96s1) [20]. Thus, the carbides of Cr, Mo and W show the similar catalytic activities as Pt when applied in HER. The TMCs applied in HER have been reported frequently in recent studies. The development of this kind of materials is becoming more and more rapid with the advancement of material science. The synthesis methods are not only restricted in the single TMCs, but also extending to the composition with highly efficient catalytic materials, such as N-doped carbon [17], graphene [18] and nanoporous materials [19]. The specific progress of different transition metal carbides is introduced below.
Tungsten carbides
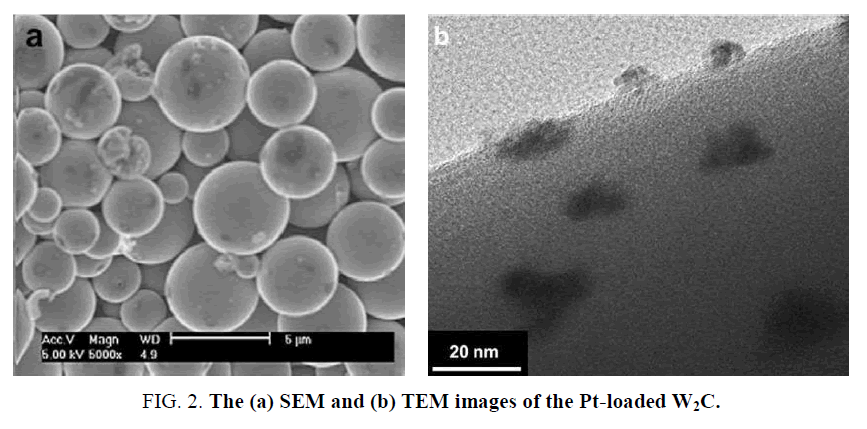
Due to W and Pt are both the sixth period elements, W carbides have been researched very frequently in HER catalysis aiming to replace Pt since 1930s [21]. The common forms of the W carbides include WC and W2C, both showing a hexagonal crystallization. The W carbides not only have a similar band structure as Pt, but also display a good poison resistance to H2S and CO [22]. Ham et al. [23] obtained high surface area W2C via annealing ammonium metatungstate and resorcinol-formaldehyde, and applied it into HER catalysis. The lowest overpotential at the current density of 10 mA cm-2 reached 200 mV in 1 M H2SO4 and the Tafel slope was 118 mV dec-1, which indicated the reaction followed a Volmer-Heyrovsky mechanism. They also loaded Pt onto the W2C substrate and compared with other commercial catalysts. As shown in Figure 2, the prepared spherical W2C substrate own a diameter of 5 μm and the Pt could be clearly observed on the W2C surface. As shown in Table 1, the prepared W2C displayed an advantage over the commercial WC. The sample 7.5 wt% Pt loaded on W2C shown a comparable catalytic activity to the Pt foil and the commercial 20 wt% Pt/C catalyst, which indicated the W2C had a brilliant promoting effect to the Pt catalyst. Chen et al. [24] recently obtained WCx/C by a combustion-carbothermal reduction method and applied it into HER. The overpotential for driving a current density of 10 mA cm-2 in 0.5 M H2SO4 was 264 mV and the Tafel slope was 85 mV dec-1. Garcia-Esparza et al. [25] synthesized WC via carbonization with mesoporous graphitic C3N4. When it was applied in HER, the overpotential at 10 mA cm-2 reached 125 mV, and the Tafel slope was 84 mV dec-1.
| Properties | WC (Commercial) | W2C microsphere | Pt Foil | 7.5 wt% Pt/W2C | 20 wt% Pt/C E-Tek |
|---|---|---|---|---|---|
| Capacitance (F/g) | 2.67 × 10-3 | 118 | - | 147 | -531 |
| Onset potential (mV) | -100 | 0 | -4 | -5 | -4 |
| Tafel slope (mV/dec) | -73 | -118 | -108 | -103 | -124 |
| io (×10-1 mA/cm2) | 0.18 | 2.81 | 18.8 | 20.8 | 15.4 |
| Specific activity at-0.1 V (mA/cm2) | 0.09 | 1.6 | 14.4 | 15.8 | 11.9 |
| Specific activity a-0.3 V (mA/cm2) | 9.3 | 23 | 109 | 101 | 55 |
| Mass activity at-0.1 V (mA/mgPt) | - | - | 0.12 | 74.5 | 32.4 |
| Mass activity at-0.3 V (mA/mgPt) | - | - | 0.91 | 476 | 150 |
Table 1. Electrocatalytic properties of tungsten-based catalysts towards hydrogen evolution reaction.
Tungsten carbides are not only applied as the electrocatalysts, but also the supports and promoters in order to reduce the whole cost when employing noble metal catalysts [12,26]. Ma et al. [27] synthesized a WC-supported Pt composite material (Pt/WC) by an impregnation method. It has been demonstrated the existence of WC could be a qualified Pt support. The catalytic activity can be comparable to pure Pt catalyst in 0.5 M H2SO4 and reduce the cost efficiently. Esposito et al. [28] took advantage of density functional theory (DFT) to calculate the hydrogen binding energy (HBE) of Pt/WC. It was concluded that the chemical property of Pt loaded on the substrate WC was very similar to bulk Pt in 0.5 M H2SO4, and showed Pt-like catalytic activity in HER. Pt was loaded on WC hollow microspheres by Tang et al. [29]. The WC hollow microspheres provide special 3-dimentional structure with large specific surface area, efficiently increasing the amount of the Pt catalytic site. The overpotential at 10 mA cm-2 was 80 mV and the Tafel slope was 73 mV dec-1 in 0.5 M H2SO4.
Although tungsten carbides are Pt-like catalysts and adequate support for Pt in acidic electrolyte for HER, there are few literatures reporting their applications in basic electrolyte. It is believed that WC can be partially oxidized into WO3 in the aqueous solution. Although WO3 is also a good catalytic site for HER, its dissociation rate strongly depends on solution pH. WO3 dissociates at high pH, and keeps stable at low pH [30,31]. Consequently, the tungsten carbides show a weak stability in basic conditions, which is not helpful to extensively employment for all kinds of water splitting conditions. Thus, there are still challenges to ensure tungsten carbides suitable for a wide pH range.
Molybdenum carbides
Molybdenum is a kind of transition metal with various valence states, among which +2, +4 and +6 states are commonly observed. Hence, Mo carbides exist in many forms, such as Mo2C, MoC and Mo3C2. Molybdenum carbides have been widely used in a variety of catalytic reactions owing to their stable chemical properties, such as industrial desulfurization, water-gas shift reaction, and hydrogenation [1]. The commercial Mo2C and its performance in HER catalysis have been studied by Vrubel et al. [32]. The overpotentials at 10 mA cm-2 were 210 mV and 190 mV at pH 0 and 14, respectively. Their work demonstrated that the commercial Mo2C owned a good stability in both acidic and basic electrolytes, which was a shortcoming in terms of Pt and WC.
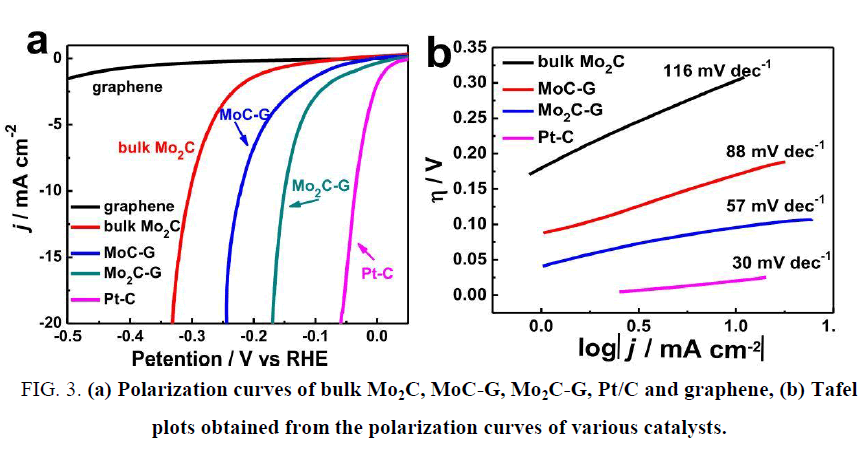
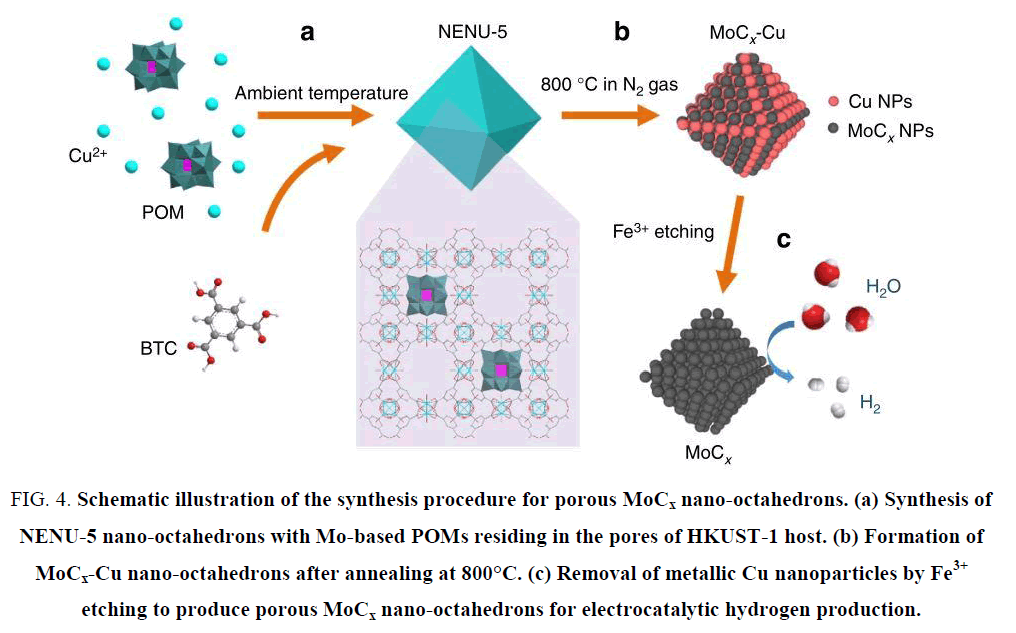
Although molybdenum carbides fit a wide pH range for HER catalysis, the catalytic activities cannot catch up with that of Pt or WC due to the difficulty to make their sizes small. The specific surface area (directly controlling the active sites distribution in catalyst) will be suppressed by large particle size. Many efforts have been made to shrink the molybdenum carbide particles, such as CH4-H2 mix reduction [33], chemical vapor deposition (CVD) [34], sol-gel method [35,36], microwave-assisted thermolytic method [37] and pyrolysis based of other nanoparticle substrates [38-42]. He et al. [39] synthesized MoC/Mo2C supported on graphene sheets and applied it into HER catalysis in acidic electrolyte as shown in Figure 3, the overpotential at 10 mA cm-2 reached 160 mV and the Tafel slope was 57 mV dec-1. It was believed that the extraordinary catalytic activity was attributed to the large number of active sites and the fast available electron transfer capabilities offered by the nanoscale graphene. Wu et al. [43] opened up a new thought by synthesizing molybdenum carbides from a MOF substrate, and obtained an admirable catalytic activity. This technique efficiently solved the problem that Mo2C owned small specific surface area. As shown in Figure 4, the HKUST-1 was directly used as the carbon source and the reduced metal Cu was washed out with FeCl3 solution, resulting in porous Mo carbide nanoparticles. The overpotentials at 10 mA cm-2 were 142 mV and 151 mV in pH 0 and 14 electrolytes, respectively, and the Tafel slopes were 53 mV dec-1 and 59 mV dec-1. This new train of thought brought about many relevant researches of synthesizing Mo carbides from MOFs [38,44,45]. Beside the bulk Mo carbides and their composite derivatives, many researches turned attentions to bimetallic carbides due to the polyvalency of molybdenum [46]. Zhao et al. [47] synthesized Co3Mo3C via carbonization with hexamethylenetetramine (HMT) and decorated with carbon nanotube. The catalytic performance in HER achieved a promotion than bulk Mo carbides. The overpotential at 10 mA cm-2 reached 125 mV at pH 7, and the Tafel slope was 250 mV dec-1.
Figure 3: (a) Polarization curves of bulk Mo2C, MoC-G, Mo2C-G, Pt/C and graphene, (b) Tafel plots obtained from the polarization curves of various catalysts.
Figure 4: Schematic illustration of the synthesis procedure for porous MoCx nano-octahedrons. (a) Synthesis of NENU-5 nano-octahedrons with Mo-based POMs residing in the pores of HKUST-1 host. (b) Formation of MoCx-Cu nano-octahedrons after annealing at 800°C. (c) Removal of metallic Cu nanoparticles by Fe3+ etching to produce porous MoCx nano-octahedrons for electrocatalytic hydrogen production.
Chromium carbides
Chromium is the first element in group VIB, owning polyvalency and an active chemical property. The metal Cr can be easily oxidized when exposing to air or water during the hydrogen evolution process, and forms a compact Cr2O3 layer covering the surface of Cr. However, chromium can be highly efficient towards HER catalysis when it forms Cr carbides, such as CrC, Cr3C2, Cr7C3 and Cr23C6, among which the hexagonal Cr7C3 displays an extraordinary catalytic activity. Tsirlina et al. [48] revealed that Cr7C3 could achieve an overpotential of 180 mV at 10 mA cm-2, and the Tafel slope was 60 mV dec-1.
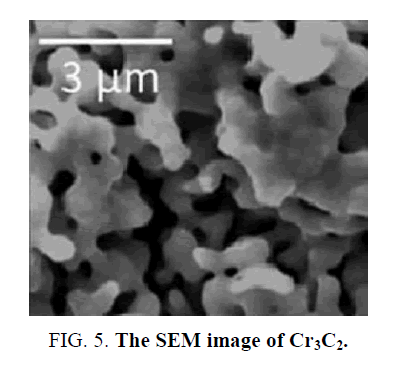
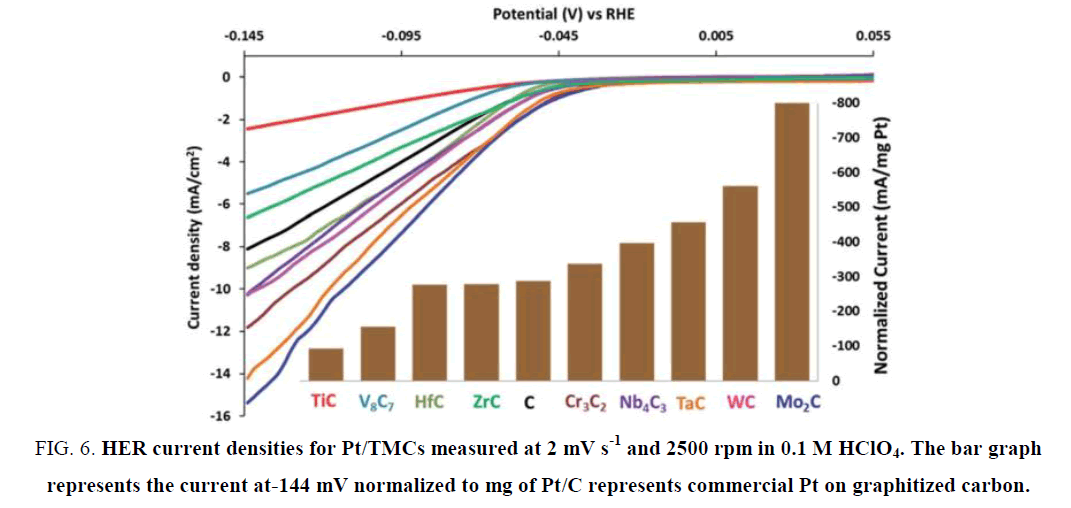
Regmi et al. [49] synthesized Cr3C2 by introducing a salt mixture on the carbon nanotubes sub-states to help crystallization. As shown in Figure 5, the synthesized Cr3C2 displayed a tubular network structure with the diameter of the tubes larger than common carbon nanotubes. Pt was loaded onto the Cr3C2 and other TMCs to form composite catalysts, and the overpotential of Pt/Cr3C2 at 10 mA cm-2 in pH 1 was 120 mV. According to Figure 6, Pt/Cr3C2 displayed a remarkable increase in catalytic activity when compared with Pt/C, which demonstrated the Cr carbide was an appropriate Pt promoter when catalyzing HER.
Figure 6. HER current densities for Pt/TMCs measured at 2 mV s-1 and 2500 rpm in 0.1 M HClO4. The bar graph represents the current at-144 mV normalized to mg of Pt/C represents commercial Pt on graphitized carbon.
Tomás-García et al. [50] obtained a relatively thick carburized layer of two main carbides Cr3C2 and Cr7C3 by reducing chromium oxide with CH4/H2 at 800°C. The overpotential at 10 mA cm-2 was about 130 mV, and the Tafel slope was 76 mV dec-1. In their work, W and Mo carbides were also synthesized as a comparison. It was found that the catalytic activities presented an order from high to low: W, Cr, Mo, which indicated the chromium carbides possessed a potential to perform better than molybdenum carbides in HER catalysis. Nevertheless, Cr is not a popular element to constitute a catalyst for HER because of its poisoning property to the environment. That is also the reason for the lack of relevant reports about Cr carbides applied in HER catalysis. If the problems of stability and recycling are settled, Cr carbides are believed to be promising catalysts for the electrochemical HER.
Conclusion and Future Outlook
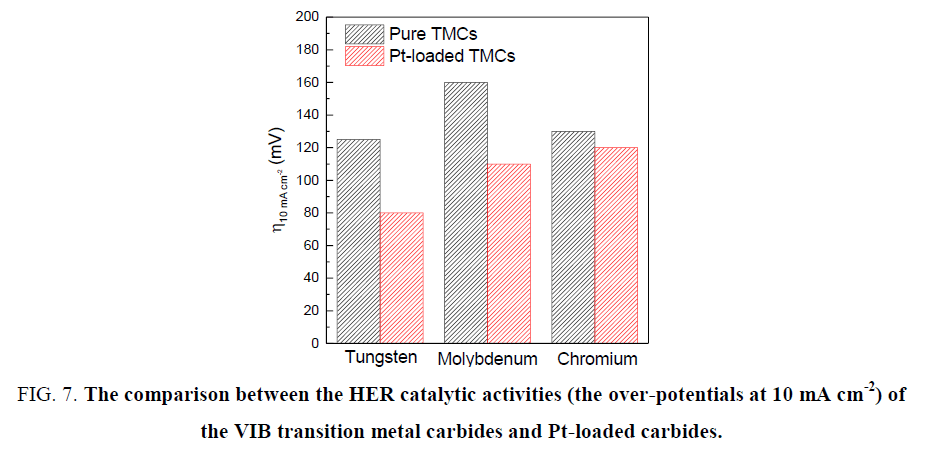
The performances of transition metals W, Mo and Cr carbides in catalyzing hydrogen evolution reaction still have a distance towards Pt-based catalyst. Great efforts have been made to shrink the distance in order to realize the replacement of Pt. The TMCs not only can be directly applied as the HER catalysts, but also can act as the Pt-promoters to reduce the material cost as concluded in Figure 7 [12,25,29,39,49,50], the catalytic activities of the pure TMCs follow the order from high to low: W, Cr, Mo. However, when loading Pt, the Mo carbide displays a better catalytic activity than Cr. In consequence, tungsten carbides display the best among these materials but they are lack of stability in the environment at high pH. Novel synthesis methods are waiting for exploration to make W carbides stable in a wide pH range. Molybdenum carbides show a wide suitable pH range when applied in HER catalysis, but the problem of large particle size remains to be solved. To deal with this problem, many feasible methods reported recently could lead Mo carbides to a vast potential for future development. Although chromium carbides are commendable HER catalysts, there is lack of relevant researches due to the poisoning property of Cr. More efforts should be made to explore highly efficient TMCs-based catalyst for HER.
Figure 7. The comparison between the HER catalytic activities (the over-potentials at 10 mA cm-2) of the VIB transition metal carbides and Pt-loaded carbides.
Owing to the special outer electron structures of the VIB group transition metal carbides, they show significant potentials to replace noble metal catalysts such as Pt on the catalysis in HER. However, because of the disadvantages of three TMCs mentioned above, the target catalysts should be designed to own wide pH adaptation, small particle size and low poisonousness, simultaneously. Thus, other synthesizing methods are encouraged to replace the common thermal reduction, such as sol-gel, CVD and electric arc methods. Besides, the catalyst’s structure analysis shouldn’t be restricted in X-ray diffraction, X-ray photoelectron spectroscopy or Raman spectra. More advanced characterizations are supposed to be carried out, such as in-situ analysis and synchrotron radiation. More in-depth theoretical researches are encouraged to be combined to simulate the catalytic mechanism, such as DFT and kinetic Monte Carlo (KMC) modeling. It should be noted that most of the relative works on TMCs carbides applied in HER focus on simplex pH conditions, such as only pH=0 or 14. It will be helpful to investigate the suitability for a wide pH range, which will play a key role in the development of the whole water splitting technology.
Acknowledgments
We gratefully acknowledge the Natural Science Foundation of China (21276018), the Natural Science Foundation of Jiangsu Province of China (BK20140268 and BK20161200), Fundamental Research Funds for the Central Universities (buctrc201526), Changzhou Sci and Tech Program (CJ20159006 and CJ20160007), and the Advanced Catalysis and Green Manufacturing Collaborative Innovation Centre of Changzhou University (ACGM2016-06-02, ACGM2016-06-03).
References
- Chen WF, Muckerman JT, Fujita E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem Commun. 2013;49(79):8896-909.
- McCrory CC, Jung S, Ferrer IM, et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc. 2015;137(13):4347-57.
- Jahan M, Liu Z, Loh KP. A graphene oxide and copper?centered metal organic framework composite as a tri?functional catalyst for HER, OER, and ORR. Adv Funct Mater. 2013;23(43):5363-72.
- Wang J, Cui W, Liu Q, et al. Recent progress in cobalt?based heterogeneous catalysts for electrochemical water splitting. Adv Mater. 2016;28(2):215-30.
- Yang Y, Liu J, Guo S, et al. A nickel nanoparticle/carbon quantum dot hybrid as an efficient electrocatalyst for hydrogen evolution under alkaline conditions. J Mater Chem A. 2015;3(36):18598-604.
- Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev. 2015;44(15):5148-80.
- Li X, Hao X, Abudula A, et al. Nanostructured catalysts for electrochemical water splitting: current state and prospects. J Mater Chem. A. 2016;4(31):11973-2000.
- Zhong Y, Xia X, Shi F, et al. Transition metal carbides and nitrides in energy storage and conversion. Adv Sci. 2016;3(5):1-28.
- Bagotsky VS. Fundamentals of electrochemistry. 2nd ed. John Wiley and Sons, Inc., New Jersey. 2006.
- Sheng W, Gasteiger HA, Shao-Horn Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J Electrochem Soc. 2010;157(11):B1529-36.
- Liu Y, Mustain WE. Evaluation of tungsten carbide as the electrocatalyst support for platinum hydrogen evolution/oxidation catalysts. Int J Hydrogen Energy. 2012;37(11):8929-38.
- Kelly TG, Lee KX, Chen JG. Pt-modified molybdenum carbide for the hydrogen evolution reaction: From model surfaces to powder electrocatalysts. J Power Sources. 2014;271:76-81.
- Xiao Y, Hwang JY, Sun YK. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J Mater Chem A. 2016;4(27):10379-93.
- Meyer S, Nikiforov AV, Petrushina IM, et al. Transition metal carbides (WC, Mo 2 C, TaC, NbC) as potential electrocatalysts for the hydrogen evolution reaction (HER) at medium temperatures. Int J Hydrogen Energy. 2015;40(7):2905-11.
- Yamanaka K, Mori M, Sato K, et al. Characterisation of nanoscale carbide precipitation in as-cast Co–Cr–W-based dental alloys. J Mater Chem B. 2016;4(10):1778-86.
- Harnisch F, Sievers G, Schröder U. Tungsten carbide as electrocatalyst for the hydrogen evolution reaction in pH neutral electrolyte solutions. Appl Catal B. 2009;89(3):455-8.
- Zhong Z, Liu N, Chen H, et al. Molybdenum carbide supported by N-doped carbon: Controlled synthesis and application in electrocatalytic hydrogen evolution reaction. Mater Lett. 2016;176:101-5.
- Ojha K, Saha S, Kolev H, et al. Composites of graphene-Mo 2 C rods: Highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim Acta. 2016;193:268-74.
- Borchardt L, Hoffmann C, Oschatz M, et al. Preparation and application of cellular and nanoporous carbides. Chem Soc Rev. 2012;41(15):5053-67.
- Kitchin JR, Nørskov JK, Barteau MA, et al. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catal Today. 2005;105(1):66-73.
- Levy RB, Boudart M. Platinum-like behavior of tungsten carbide in surface catalysis. Science. 1973;181(4099):547-49.
- Stellwagen DR, Bitter JH. Structure-performance relations of molybdenum-and tungsten carbide catalysts for deoxygenation. Curr Green Chem. 2015;17(1):582-93.
- Ham DJ, Ganesan R, Lee JS. Tungsten carbide microsphere as an electrode for cathodic hydrogen evolution from water. Int J Hydrogen Energy. 2008;33(23):6865-72.
- Chen Z, Qin M, Chen P, et al. Tungsten carbide/carbon composite synthesized by combustion-carbothermal reduction method as electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy. 2016;41(30):13005-13.
- Garcia?Esparza AT, Cha D, Ou Y, et al. Tungsten carbide nanoparticles as efficient cocatalysts for photocatalytic overall water splitting. ChemSusChem. 2013;6(1):168-81.
- Kelly TG, Hunt ST, Esposito DV, et al. Monolayer palladium supported on molybdenum and tungsten carbide substrates as low-cost hydrogen evolution reaction (HER) electrocatalysts. Int J Hydrogen Energy. 2013;38(14):5638-44.
- Ma C, Sheng J, Brandon N, et al. Preparation of tungsten carbide-supported nano Platinum catalyst and its electrocatalytic activity for hydrogen evolution. Int J Hydrogen Energy. 2007;32(14):2824-9.
- Esposito DV, Hunt ST, Stottlemyer AL, et al. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates. Angewandte Chemie International Edition. 2010;49(51):10055-8.
- Tang C, Wang D, Wu Z, et al. Tungsten carbide hollow microspheres as electrocatalyst and platinum support for hydrogen evolution reaction. Int J Hydrogen Energy. 2015;40(8):3229-37.
- Bozzini B, De Gaudenzi GP, Fanigliulo A, et al. Anodic behaviour of WC?Co type hardmetal. Mater Corros. 2003;54(5):295-303.
- Andersson KM, Bergström L. Oxidation and dissolution of tungsten carbide powder in water. Int J Refract Met Hard Mater. 2000;18(2):121-9.
- Vrubel H, Xile HU. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angewandte Chemie International Edition. 2012;51(51):12703-6.
- Nagai M, Zahidul AM, Matsuda K. Nano-structured nickel–molybdenum carbide catalyst for low-temperature water-gas shift reaction. Appl Catal A. 2006;313(2):137-45.
- Hanif A, Xiao T, York AP, et al. Study on the structure and formation mechanism of molybdenum carbides. Chem Mater. 2002;14(3):1009-15.
- Zhao L, Fang K, Jiang D, et al. Sol-gel derived Ni-Mo bimetallic carbide catalysts and their performance for CO hydrogenation. Catal Today. 2010 Dec 22;158(3):490-95.
- Danks AE, Hall SR, Schnepp Z. The evolution of ‘sol-gel’chemistry as a technique for materials synthesis. Mater Horiz. 2016;3(2):91-112.
- Pang M, Li C, Ding L, et al. Microwave-assisted preparation of Mo2C/CNTs nano-composites as efficient electrocatalyst supports for oxygen reduction reaction. Ind Eng Chem Res. 2010;49(9):4169-74.
- Qamar M, Adam A, Merzougui B, et al. Metal-organic framework-guided growth of Mo2C embedded in mesoporous carbon as a high-performance and stable electrocatalyst for the hydrogen evolution reaction. J Mater Chem A. 2016;4(41):16225-32.
- He C, Tao J. Synthesis of nanostructured clean surface molybdenum carbides on graphene sheets as efficient and stable hydrogen evolution reaction catalysts. Chem Commun. 2015;51(39):8323-5.
- Chen WF, Wang CH, Sasaki K, et al. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ Sci. 2013;6(3):943-51.
- Shi Z, Wang Y, Lin H, et al. Porous nano-MoC @ graphite shell derived from a MOFs-directed strategy: An efficient electrocatalyst for the hydrogen evolution reaction. J Mater Chem A. 2016;4(16):6006-13.
- Mahmood A, Guo W, Tabassum H, et al. Metal?organic framework?based nanomaterials for electrocatalysis. Adv Energy Mater. 2016;6(17).
- Wu HB, Xia BY, Yu L, et al. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat Commun. 2015;6: 6512-20.
- Fan L, Liu PF, Yan X, et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat Commun. 2016;7: 10667-4.
- Xu X, Nosheen F, Wang X. Ni-decorated molybdenum carbide hollow structure derived from carbon-coated metal-organic framework for electrocatalytic hydrogen evolution reaction. Chem Mater. 2016;28(17):6313-20.
- Liu Y, Li GD, Yuan L, et al. Carbon-protected bimetallic carbide nanoparticles for a highly efficient alkaline hydrogen evolution reaction. Nanoscale. 2015;7(7):3130-6.
- Zhao Y, Tang Q, He B, et al. Carbide decorated carbon nanotube electrocatalyst for high-efficiency hydrogen evolution from seawater. RSC Advances. 2016;6(96):93267-74.
- Tsirlina GA, Petrii OA. Hydrogen evolution on smooth stoichiometric tungsten and chromium carbides. Electrochim Acta. 1987;32(4):649-57.
- Regmi YN, Waetzig GR, Duffee KD, et al. Carbides of group IVA, VA and VIA transition metals as alternative HER and ORR catalysts and support materials. J Mater Chem A. 2015;3(18):10085-91.
- Tomás-García AL, Jensen JO, Bjerrum NJ, et al. Hydrogen evolution activity and electrochemical stability of selected transition metals in concentrated phosphoric acid. Electrochim Acta. 2014;137:639-46.