Original Article
, Volume: 15( 4)Rapid and Sensitive RP-HPLC Method for Determination of Potential Genotoxic Impurity in Dasatinib Drug Substance
- *Correspondence:
- Sunil V Lanke, Oncogen Pharma (Malaysia) Sdn Bhd, No.3, Jalan Jururancang U1/21, Hicom Glenmarie Industrial Park, 40150 Shah Alam, Selangor, Malaysia, Tel: (+)60179886374; E-mail: sunil.lanke@oncogenpharma.com
Received: August 04, 2017; Accepted: August 14, 2017; Published: August 21, 2017
Citation: Sunil VL, Shahnawaz SS, Shekhawat DS, et al. Rapid and Sensitive RP-HPLC Method for Determination of Potential Genotoxic Impurity in Dasatinib Drug Substance. Int J Chem Sci. 2017;15(4):177.
Abstract
A simple and sensitive reverse phase liquid chromatography (RP-HPLC) method has been developed and subsequently validated for the determination of potential genotoxic carboxamide [2-Amino-N-(2-chloro-6-methylphenyl) thizaole-5-carboxamide] impurity at trace level in Dasatinib drug substance. Separation was achieved with a Inertsil C8-3, (Make: GL Sciences); 150 × 4.6 mm I.D; particle size 5 μm column and buffer was prepared by dissolving 1.36 g of potassium dihydrogen phosphate into 1000 ml water; and pH adjusted to 6.0 with dilute potassium hydroxide solution. The flow rate was set 1 ml/min and the column temperature was 40°C. UV detection was performed at 299 nm and injection volume 20 μL with ambient sample temperature. The method is simple, rapid, and specific. The described method is linear, precise and accurate over a range of 1.5 μg/mL to 20.0 μg/mL. The method precision for the determination of genotoxic carboxamide impurity was below 2.0% RSD. The percentage recoveries of genotoxic carboxamide impurity ranged from 95% to 113%. It can be conveniently adopted for routine analysis of determination of genotoxic carboxamide impurity in Dasatinib drug substance.
Keywords
RP-HPLC; Carboxamide impurity; [2-Amino-N-(2-chloro-6-methyl phenyl) thizaole-5-carboxamide]; Dasatinib; Genotoxic impurity
Introduction
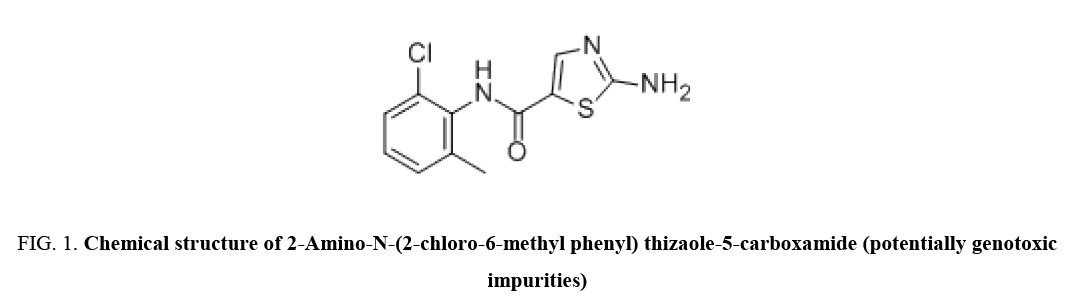
Dasatinib is an inhibitor of multiple tyrosine kinases. Dasatinib inhibited the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. The chemical name for Dasatinib is N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl] amino]-5-thiazole carboxamide. The molecular formula is C22H26CIN7O2S.H2O, which corresponds to a formula weight of 506.02 (monohydrate). The anhydrous free base has a molecular weight of 488.01. Dasatinib is a white to off-white powder. The drug substance is soluble in dimethyl sulphoxide and practically insoluble in water and slightly soluble in ethanol and methanol. Dasatinib is available in market with different strengths like 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg [1]. Literature survey reveals that a few LC-MS (Liquid chromatography-mass spectrometry) and UPLC Ultra performance liquid chromatography) method reported for determination of genotoxic impurities, but there is no method available on conventional high-performance liquid chromatograph (HPLC) for determination of genotoxic carboxamide impurity in Dasatinib drug substance [2-5]. Therefore, the present study aims to develop and validate a simple, sensitive, fast and economical RP-HPLC method for determination of genotoxic carboxamide impurity in Dasatinib drug substance (Figure 1).
Figure 1: Chemical structure of 2-Amino-N-(2-chloro-6-methyl phenyl) thizaole-5-carboxamide (potentially genotoxic impurities)
Description of brief synthetic process of Dasatinib
The Dasatinib has been synthesized by using key raw material 2-Amino-N-(2-chloro-6-methyl phenyl) thizaole-5-carboxamide (Carboxamide); it reacts with 4, 6-dichloro-2-methyl-pyrimidine followed by the addition of 2-piperazine-1-yl-ethanol forms Dasatinib and upon purification yields pure Dasatinib [6-8].
Genotoxicity and carcinogenicity study
The carboxamide [2-Amino-N-(2-chloro-6-methyl phenyl) thizaole-5-carboxamide] impurity has been considered as a genotoxic carcinogen as per structural alert. The alerts have been taken from the ViTAL toxicity alert is software program of V Life science a division of Novalead pharma pvt. ltd (Estimation of toxic hazard: A decision tree approach) version 2.4. The maximum daily dosage of Dasatinib is about 140 mg. The genotoxic carboxamide impurity limit 10 ppm, calculated as per the calculation provided in ICH guideline M7, considering 140 mg maximum daily dose of Dasatinib and based acceptable intake of 1.5 μg/day the threshold of toxicological concern (TTC) was protective for a lifetime of daily exposure [9].
Materials and Methods
Apparatus
The analysis was carried out on a waters liquid chromatography system equipped with photodiode array detector 2998, separations module Alliance 2695 and Empower-3 data handling system and a reverse phase HPLC column Inertsil C8-3, (Make: GL Sciences); 150 × 4.6 mm I.D; particle size 5 μm was used.
Chemicals and solvents
Millipore generated water used for HPLC, acetonitrile (HPLC grade), potassium dihydrogen phosphate (AR grade), sodium-1-octane sulphonic acid (HPLC/AR grade), methanol (HPLC grade) dimethylsulphoxide (DMSO) and potassium hydroxide of (AR grade) were obtained from E. Merck Ltd. Potential genotoxic carboxamide impurity is a starting material of Dasatinib and purchased from China. Dasatinib sample is synthesized at research center of Oncogen pharma (Malaysia) Sdn. Bhd [10-14].
Method development
Different chromatographic conditions were experimented to achieve better efficiency of the chromatographic system. Parameters such as mobile phase composition, wavelength of detection, column, column temperature, sample concentration and sample solubility, gradient program and diluents were optimized. Optimized system suitability parameters like tailing, theoretical plates and run time were the major task while developing the method, Finally, following chromatographic condition were found suitable for determination of genotoxic carboxamide impurity in Dasatinib drug substance [15,16].
Optimized chromatographic condition
Instrument: e2695 Waters LC system equipped with photodiode array detector 2998, and Empower-3 data handling system.
Chromatographic conditions:
Column : Inertsil C8-3,150 × 4.6, 5 μ
Flow Rate : 1.0 ml/min
Column Temperature : 40°C
Sample Temperature : Ambient
Injection volume : 20 μL
Detection wavelength : UV 299 nm
Needle wash : Methanol
Mobile Phase A (buffer preparation): Weigh about 1.36 g of potassium dihydrogen phosphate and 1.0 g of sodium-1-octane sulphonic acid into 1000 ml water; and pH adjusted to 6.0 with dilute potassium hydroxide solution [17].
Mobile phase B: Acetonitrile.
Diluent: DMSO and Methanol.
Gradient program
Standard stock solution: Weigh and transfer 10 mg standard of carboxamide impurity into 100 ml volumetric flask, dissolve and dilute up to the mark with methanol. Transfer 1 ml of this solution into 100 ml volumetric flask and dilute with methanol.
Standard solution (10 ppm w.r.t. sample concentration): Transfer 2 ml of standard stock solution into 20 ml volumetric flask, add 2 ml of DMSO and dilute up to the mark with methanol [18].
Sample preparation: Weigh 100 mg of Dasatinib drug substance into 10 ml volumetric flask, dissolve in 1 ml of DMSO and dilute up to the mark with methanol.
Procedure: Set the HPLC as per above chromatographic conditions and set wavelength at 299 nm and wait to get a stable base line. Inject blank into the chromatography by following the test method conditions. Inject six replicates of standard solution into the chromatograph by following the test method conditions and evaluate system suitability.
System suitability test: The USP plate count for carboxamide peak from standard solution should not less than 5000 [19]. The relative standard deviation of area of carboxamide form six replicate injections from standard solution should not more than 5.0%.
Calculation: Calculate the carboxamide impurity in Dasatinib drug substance by following formula-
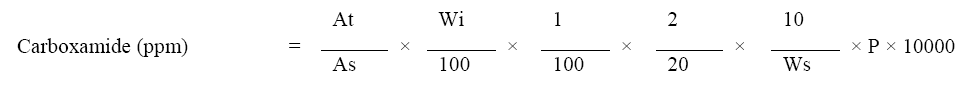
Where,
At=Area of carboxamide peak in the sample
As=Average area of carboxamide peak in the standard solution.
Wi=Weight taken of carboxamide in standard solution
Ws=Weight of Dasatinib drug substance taken.
P=Potency of carboxamide impurity
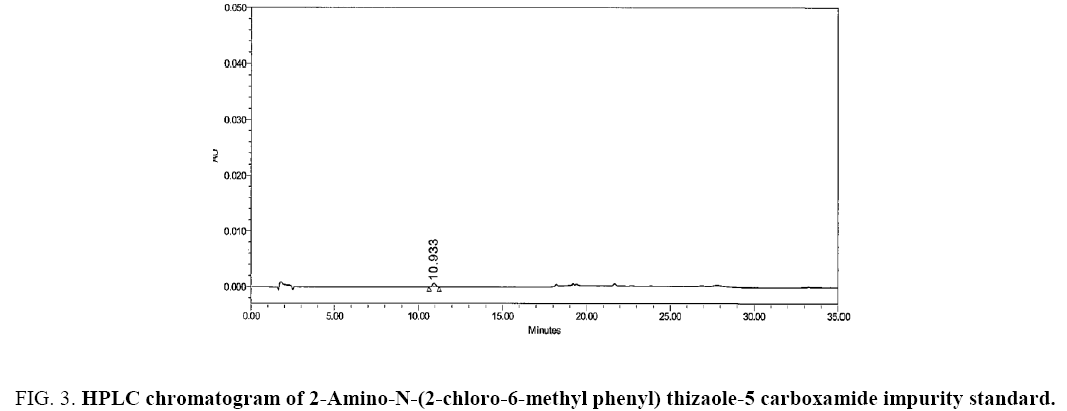
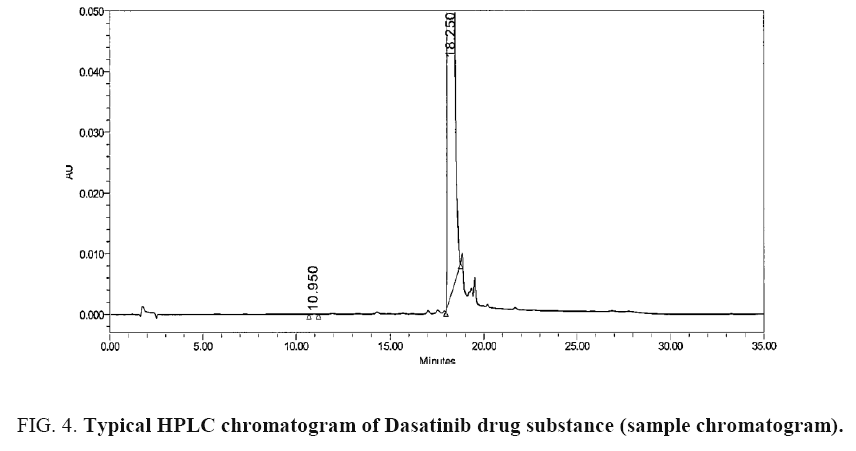
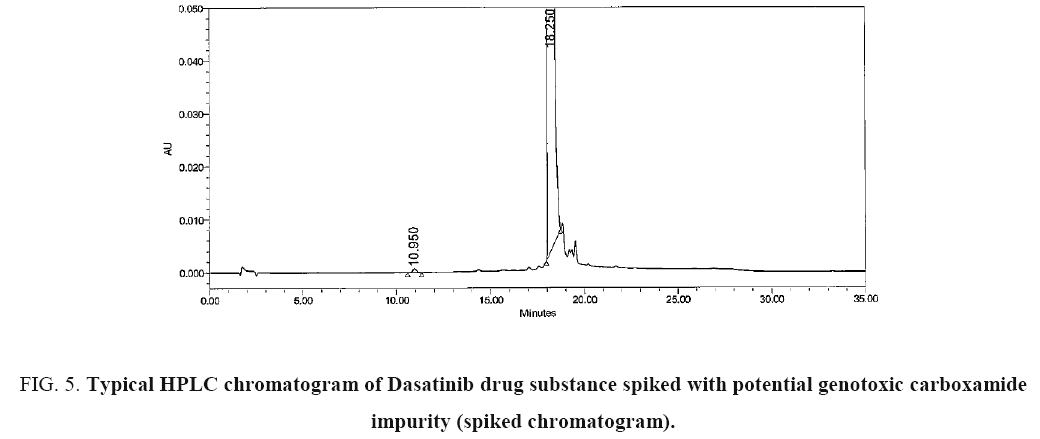
The chromatograms blank (diluent), carboxamide impurity standard (individual), Dasatinib sample and Dasatinib drug substance spiked with carboxamide impurity using the proposed method is shown in Figure 2-5 respectively. System suitability results of the method are presented in Table 1.
| S No | System suitability parameters | Result | Acceptance criteria |
|---|---|---|---|
| 1 | USP plate count impurity peak | 15420 | NLT 5000 |
| 2 | %RSD for six replicate injections of impurity peak area. | 1.35% | NMT 5% |
Table 1: System suitability report.
Figure 3: HPLC chromatogram of 2-Amino-N-(2-chloro-6-methyl phenyl) thizaole-5 carboxamide impurity standard.
Figure 5: Typical HPLC chromatogram of Dasatinib drug substance spiked with potential genotoxic carboxamide impurity (spiked chromatogram).
Validation of the developed method
The method was validated as per the ICH guideline for system suitability, specificity, system precision, method precision, detection limit, quantitation limit, linearity and range, recovery/accuracy, intermediate precision, robustness and solution stability for sample [20,21].
Specificity
Specificity of the method shall be demonstrated by identification of genotoxic impurity 2-Amino-N-(2-chloro-6-methyl phenyl) thiazole-5-carboxamide with respect to retention times (individually and in mixture) and the peak purity of carboxamide under normal conditions.
Conclusion
Retention time of genotoxic carboxamide impurity obtained individually and in mixture are comparable. The peak purity data of genotoxic carboxamide peak in individual and mixture shows that carboxamide impurity peak is homogeneous and there are no co-eluting peaks indicating that the method is specific
Linearity and Range
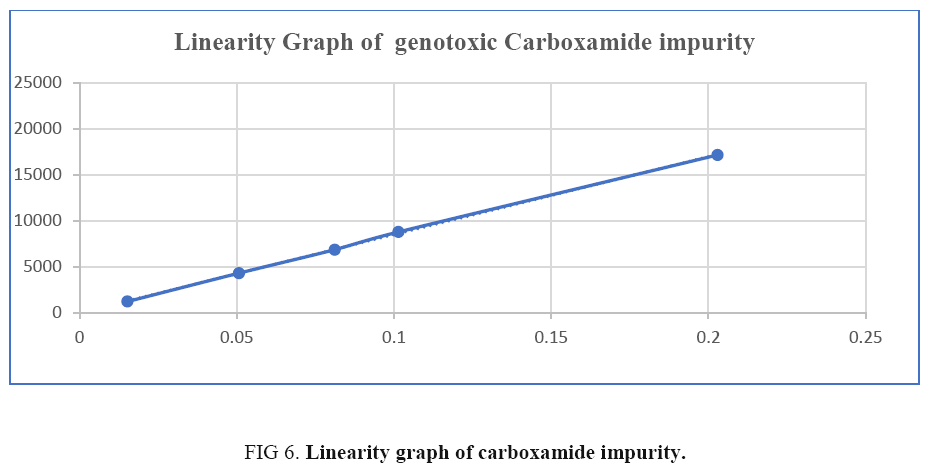
Linearity of genotoxic carboxamide impurity determined by preparing a series of solutions at concentrations ranging from LOQ (limit of quantitation) to 200% of specification level i.e., 1.5 ppm to 20 ppm and injected into HPLC in triplicate by following the test method conditions. The RSD for area response of genotoxic carboxamide impurity at each concentration and plotted the linearity graph by average area vs. concentration. The linear regression, slope and correlation coefficient also calculated. Calculated limit of detection (LOD) and limit of quantitation (LOQ) by slope method. The correlation coefficient was found to be 0.999 in the given concentration range of 1.5 ppm to 20 ppm. So, the method is said to be linear, presented in Figure 6 and linearity data in Table 2.
| Concentration | Area | Avg. area | % RSD | Slope | Steyx | *Corrl | ||
|---|---|---|---|---|---|---|---|---|
| ppm* | Inj-1 | Inj-2 | Inj-3 | |||||
| 1.5 | 1123 | 1345 | 1203 | 1224 | 9.19 | 84913 | 118 | 0.9999 |
| 5.0 | 4314 | 4265 | 4371 | 4317 | 1.23 | |||
| 8.0 | 6812 | 6907 | 6880 | 6866 | 0.71 | |||
| 10.0 | 8853 | 8676 | 8863 | 8797 | 1.20 | |||
| 20.0 | 17268 | 17031 | 17211 | 17170 | 0.72 | |||
| *Corrl=Correlation coefficient | ||||||||
Table 2: Linearity Data.
Conclusion: The correlation coefficient is more than 0.99 for genotoxic carboxamide impurity. Hence, the response is linear from LOQ to 200% of specification.
LOD and LOQ
The minimum concentration level at which the analyte can be reliably detected (LOD) and quantified (LOQ) were found to be 0.5 ppm and 1.5 ppm respectively with respect to specification level from linearity by slope method [22]. The relative standard deviation for area response of genotoxic carboxamide impurity from six LOD and LOQ injections are well within acceptance criteria (Tables 3, 4 and 5).
| Level | Prep. No | Amount in sample (µg/ml) |
Amount Spiked (µg/ml) |
Amount Recovered (µg/ml) |
% Recovery | % RSD |
|---|---|---|---|---|---|---|
| LOQ | 1 | 0.0048 | 0.0166 | 0.0185 | 100.83 | 1.62 |
| 2 | 0.0048 | 0.0166 | 0.0187 | 112.39 | ||
| 3 | 0.0048 | 0.0166 | 0.0181 | 108.82 | ||
| 100 | 1 | 0.0048 | 0.1041 | 0.0995 | 95.65 | 3.32 |
| 2 | 0.0048 | 0.1041 | 0.1064 | 1002.22 | ||
| 3 | 0.0048 | 0.1041 | 0.1026 | 98.93 | ||
| 200 | 1 | 0.0048 | 0.2081 | 0.2018 | 96.95 | 0.54 |
| 2 | 0.0048 | 0.2081 | 0.1996 | 95.90 | ||
| 33 | 0.0048 | 0.2081 | 0.2008 | 96.49 |
Table 3: Accuracy data.
| Robustness parameter | Retention time of impurity | % RSD | USP plate count |
|---|---|---|---|
| Wavelength-297 nm | 11.03 | 0.85 | 15521 |
| Wavelength-301 nm | 11.03 | 0.73 | 15432 |
| Mobile phase flow rate-0.90 ml/min | 11.80 | 1.66 | 17464 |
| Mobile phase flow rate-1.10 ml/min | 10.37 | 2.45 | 14874 |
| Column temperature 35°C | 11.32 | 1.42 | 16103 |
| Column temperature 45°C | 10.60 | 2.23 | 13574 |
Table 4: System suitability data.
| Level | Prep. No | Amount in sample (µg/ml) |
Amount spiked (µg/ml) |
Amount recovered (µg/ml) |
% Recovery | % RSD |
|---|---|---|---|---|---|---|
| LOQ | 1 | 0.0048 | 0.0166 | 0.0185 | 100.83 | 1.62 |
| 2 | 0.0048 | 0.0166 | 0.0187 | 112.39 | ||
| 3 | 0.0048 | 0.0166 | 0.0181 | 108.82 | ||
| 100 | 1 | 0.0048 | 0.1041 | 0.0995 | 95.65 | 3.32 |
| 2 | 0.0048 | 0.1041 | 0.1064 | 1002.22 | ||
| 3 | 0.0048 | 0.1041 | 0.1026 | 98.93 | ||
| 200 | 1 | 0.0048 | 0.2081 | 0.2018 | 96.95 | 0.54 |
| 2 | 0.0048 | 0.2081 | 0.1996 | 95.90 | ||
| 33 | 0.0048 | 0.2081 | 0.2008 | 96.49 |
Table 5: Accuracy data.
System precision
The relative standard deviation of carboxamide peaks areas of six injections of the standard solution and relative standard deviation of the content of impurity were 1.6% and 2.8% respectively which shows that proposed method is precise.
Accuracy
The percent (%) recovery of carboxamide impurity ranged from 80% to 120%. From data obtained, it was observed that the recovery was found within acceptance criteria. Accuracy data is presented in Table 5.
Conclusion: The method has an acceptable level of accuracy for the determination of genotoxic carboxamide impurity in Dasatinib drug substances from LOQ to 200% of Specification.
Intermediate precision/Ruggedness
The intermediate precision or ruggedness of the method was evaluated by the different analyst, column and by using different equipment, % RSD’s were within 5%, precision (method precision) is meeting the acceptance criteria for genotoxic carboxamide impurity with 2nd analyst, 2nd column on 2nd day, confirming the precision of the method.
Robustness
The optimum chromatographic conditions set for this method have been slightly modified to evaluate the method robustness. The small, but deliberate variations made in method parameter such as wavelength, flow rate, column temperature and. Injected standard solution in replicate (six time) and evaluate system suitability test; the system suitability results were meeting the acceptance criteria after individually changing the conditions parameters (acceptance criteria the USP plate count for carboxamide should not less than 5000 and the relative standard deviation of area of carboxamide peak should not more than 5%).
The content of impurity from normal and modified conditions met the acceptance criteria. Hence, the method was considered robust. System suitability data is presented in Table 6.
| Robustness parameter | Retention time of impurity | % RSD | USP plate count |
|---|---|---|---|
| Wavelength-297 nm | 11.03 | 0.85 | 15521 |
| Wavelength-301 nm | 11.03 | 0.73 | 15432 |
| Mobile phase flow rate-0.90 ml/min | 11.80 | 1.66 | 17464 |
| Mobile phase flow rate-1.10 ml/min | 10.37 | 2.45 | 14874 |
| Column temperature 35°C | 11.32 | 1.42 | 16103 |
| Column temperature 45°C | 10.60 | 2.23 | 13574 |
Table 6: System suitability data.
Solution stability
The solution stability of spiked solution of Dasatinib sample with potential genotoxic carboxamide impurity was carried out by leaving spiked sample solution at room temperature for 24 h. Observed no degradation in spiked impurity solution result at regular interval injection till 24 h. From this study of solution stability; sample solution was stable up to 24 h. The cumulative relative standard deviation of impurity peak is presented in Table 5.
Conclusion
A rapid, sensitive, cost-effective RP-HPLC method was successfully developed for quantitative of potentially genotoxic carboxamide impurity [2-Amino-N-(2-chloro-6-methyl phenyl) thiazole-5-carboxamide] of Dasatinib drug substance. The method was found to be precise, linear and accurate with decent and constant recoveries. The validated method may be used for the regular analysis of the determination of potential genotoxic carboxamide impurity of Dasatinib drug substance.
Acknowledgement
Author is thankful to Oncogen pharma (Malaysia) Sdn Bhd for providing Dasatinib drug substance sample, reference standard of carboxamide impurity and other necessary facilities to conduct this development work.
References
- Silvia DF, Antonio DA, Francesca DM, et al. New HPLC-MS method for the simultaneous determination of the antileukemia drugs imatinib, dasatinib and nilotinib in human plasma. J Chromatogr B. 2009; 877:1721-6.
- Rao KNV, Srivani P, Raja MA, et al. Analytical method development and validation of dasatinib in its pharmaceutical dosage form by UPLC with forced degradation studies. Int J Pharm Res Scholars. 2013;2:221-7.
- Mhaske DV, Dhaneshwar SR. Stability indicating HPTLC and LC determination of dasatinib in pharmaceutical dosage form. Chromatographia. 2007;66:1-2.
- Lankheet NAG, Hillebrand MJX, Rosing H, et al. Method development and validation for the determination of dasatinib, erlotinib, gefitinib, imatinib, lapatinib, nilotinib, sorafenib and sunitinib in human plasma by liquid chromatography coupled with tandem mass spectrometry. Biomedical Chromatography. 2012; 27(4):466-476.
- Furlong MT, Agrawal S, Hawthorne D, et al. A validated LC-MS/MS assay for the simultaneous determination of the anti-leukemic agent dasatinib and two pharmacologically active metabolites in human plasma: Application to a clinical pharmacokinetic study.J Pharm Biomed Anal. 2012 25;58:130-5.
- Arun Kumar K, AnantaRaoB,Yaswanth A, et al. Development and validation of RPHPLCmethod for estimation of Dasatinib in bulk and its pharmaceutical formulation. Am JPharm Tech Res. 2012;2 (4):863-872.
- N Balaji, Sayeeda S. Trace level determination and determination of potentially genotoxic impurities in dasatinib drug substance by UHPLC/infinity LC. Int J Pharm Pharm Sci. 2016;8(10):209-216.
- Eva K, Jurij T, Tadej P,et al.Simultaneous measurement ofImatinib, Nilotinib and Dasatinib in Dried blood spot by ultra-highperformance liquid chromatography tandem mass spectrometry. JChromatogr B. 2012; 903:150-156.
- Mohammed GK, Essam E, Hesham MK, et al. A high-performance liquid chromatographic method for the determination of dasatinib in rabbit plasma using fluorescence detection and its application to a pharmacokinetic study. J Chromatogr B. 2013;939:73-9.
- Michael TF, Shruti A, Dara H, et al. A validated LC-MS/MS assay for the simultaneous determination of the anti-leukemic agent dasatinib and two pharmacologically active metabolites in human plasma: Application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2012; 58:130-5.
- Ramachandra B, Suguna B, Sivarami Reddy P, et al. Development of a UV-visible spectrophotometric method for determination of dasatinib in pharmaceutical formulation and biological samples. Int J Pharm Sci Res. 2015;6:293-303.
- Sreedevi A, LakshmanaRao A. Development and validation of novel HPLC method for the estimation of dasatinib in bulk and pharmaceutical dosage forms. Int J Res Pharm Chem. 2013;3:724-9.
- Vivekananda RD, Sreelatha P, Rama Devi B. A novel stability indicating assay method development and validation of dasatinib tablets formulations. ChemSci Rev Lett. 2014;3:747-58.
- Alagar Raja M, Namratha CH, David Banji, et al. RP-HPLC method for estimation of dasatinib in active pharmaceutical ingredient and pharmaceutical dosage form as per ICH guidelines. Asian J Pharm Anal Med Chem. 2015;3:109-16.
- http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. [Last accessed on 10 Jun 2016].
- http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1B/Step4/Q1B_Guideline.pdf. [Last accessed on 10 Jun 2016].
- http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_Step_4.pdf. [Last accessed on 10 Jun 2016].
- http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021986s000_Sprycel__ClinPharmR.pdf. [Last accessed on 10 Jun 2016].
- http://www.drugbank.ca/drugs/DB01254. [Last accessed on 10 Jun 2016].
- Lombardo LJ, Lee FY, Chen P. Discovery of N? (2?chloro?6?methyl? phenyl)?2?(6?(4?(2?hydroxyethyl)?piperazin?1?yl)?2?methylpyrimidin?4?ylamino)thiazole?5?carboxamide, A dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of Medicinal Chemistry. 2004;47: 6658- 61.
- NIOSH Alert: Preventing occupational exposures to Antineoplastic and other hazardous drugs in healthcare settings. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2. html.