Research
, Volume: 15( 6) DOI: 10.37532/tsbt.2019.15(6).198Qualitative Analysis of Rhododendron arboreum Leaves Extracts using HPLC-ESI-QTOF-MS
- *Correspondence:
- Deepak Sharma
Department of Biotechnology, School of Science, Kathmandu University, Nepal
E-Mail: deepakshrm59@gmail.com
Janardan Lamichhane
Department of Biotechnology, School of Science, Kathmandu University, Nepal
E-Mail: ljanardan@ku.edu.np
Received: December 16, 2019; Accepted: December 27, 2019; Published: December 31, 2019
Citation: Sharma D, Lamichhane J. Qualitative Analysis of Rhododendron arboreum Leaves Extracts using HPLC-ESI-QTOF-MS. Biotechnol Ind J. 2019;15(6):198.
Abstract
Nepal has the richest source of herbal medicinal plants and high plant biodiversity even within the altitudinal variation of every100 meter. Based on the high biodiversity report cell-mediated the national flower of Nepal, Rhododendron arboreum (Smith), was collected from Palpa district of Nepal with an altitude of 1350 and phytochemical analysis was performed from its methanol extract. Basic Phytochemical analysis showed the presence of Alkaloids, Flavonoids Coumarins, and Saponins. Based on primary research output and the importance of the basic compounds the samples were further reanalyzed with HPLC-ESI and QTOF-MS. Thirteen different chemically important compounds from R. arboreum were identified by HPLC-ESI-QTOF-MS. Among them, Rhodolatouside A and Rhodolatouside B were first time reported as new compounds in Rhododendron arboreum from Nepal whereas Coumaric acid, Gallic acid, Rhodolatouside A, Leuco-pelargonidin, Rhodolatouside B, Myricetin, Hyperoside, Quercetin, Maslinic Acid, Linoleic acid, 1-Naphthalenepropanol, Octadecanoic acid, and Betulinic Acid/Ursolic acid were observed as previously reported compounds. All the compounds were identified with their molecular formula.
Keywords
HPLC-ESI-QTOF-MS; Rhodolatouside A; Rhodolatouside B; Quercetin; 1-Naphthalenepropanol; Iridoids
Introduction
The stems or roots of R. arboreum are sometimes used in Ayurvedic medicine for few illnesses and there are other some cases quoted as well but they are rare and difficult to verify [1]. The plant has mildly sour-tasting flowers that occasionally utilized as a pickle (‘achar’ in Nepali) by the local inhabitants [2]. The flower having sweet, sour taste is said to be used in making good sub acid jelly. They, in some parts of Himalaya, are eaten by natives who according to Madden and Watt got intoxicated in case of a large volume of consumption. According to Jill Ronsley, from Himachal Pradesh of India, local people make Pickle (‘chutney’ in Hindi) and squash to mix with soda and water as a cold drink. Rhododendron arboreum was introduced to England from India in 1811 AD [3].
The scientific study of the Rhododendrons at this part of the world began only towards the end of the eighteen century along with the establishment of the Botanical Gardens of India in Calcutta by the British regime in 1972. In 1850 Sir Joseph Hooker introduced forty-five new species of the Rhododendron [4]. By the beginning of the twentieth century, about 300 new species of Rhododendron were known to Botanist [3] and by now more than 1200 species of Rhododendron are known. It has been found that R. arboreum leaves show toxic effects even though its flower petals were used to make squash [5], besides that it shows Anti-inflammatory and antinociceptive activity [6]. A flavonoid, quercetin, was isolated from ethyl acetate fraction of the methanol extract of flower petals of R. arboreum by repeated Sephadex LH-20 column chromatography technique [7,8]. Antifungal activities were also reported with bioactive constituents found in the bark of R. arboreum [9,10]. Antioxidant activity [11] and nitric oxide synthase activation property is also reported in the flower [12] which might be utilized as a promising source of therapeutics. Alcoholic extract of the leaves of R. arboreum was found to exhibit a significant reduction in humoral and cell-mediated immune responses [13,14].
In this research the plant sample of R. arboreum Smith. (Ericaceae) was collected from Palpa district of Nepal at an elevation of 1350 meters in the month of March 2017. The plant sample was identified by a botanist and deposited in the herbarium of Kathmandu University.
Materials and Methods
Sample collection and sample preparation
The extract R. arboreum was used as a sample collected from Papua Tansen of Nepal. Cold extraction was performed using methanol as a solvent. The crude extracts were tested for phytochemical analysis using the stablished protocol of alkaloids, phenolic compounds [4] saponins, glycosides, tannins, flavonoids, and coumarins [11] which matches the resulted presented by previous researches in extract (TABLE 1).
| Properties | Coumarins | Saponins | Glycosides | Alkaloids | Sterols | Reducing sugars | Flavonoids |
|---|---|---|---|---|---|---|---|
| Sample | |||||||
| Palpa1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Palpa2 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Palpa3 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Palpa4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Palpa5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
TABLE 1. Phytochemical Screening of R. arboreum.
Few samples with high microbial and antibacterial activity were further analyzed by HPLC ESI-QTOF-MS to identify the active compounds present in the sample. HPLC-ESI-QTOF-MS analysis was performed on an Agilent 6520 Quadrupole Time-of-Flight (QTOF) mass spectrometer connected with the Agilent 1200-HPLC system via Dual electrospray ionization (ESI) interface (Agilent Technologies, USA). The Agilent 1200-HPLC system consisted of a quaternary pump (G1311A), an online vacuum degasser (G1322A), autosampler (G1329A), and a diode array detector (G1315D). The HPLC separation was carried out on a Waters XBridge C18 column (50 mm × 4.6 mm, 3.5 μ). The mobile phase consisted of 0.1% formic acid aqueous solution (A) and Acetonitrile (B) with flow rate of 0.4 ml/min under the gradient program of 10% to 80% (B) for initial 20 min, then 90% (B) from 20 min to 25 min, hold at 90% (B) from 25 min to 30 min and finally back to initial condition 90% to 10% (B) from 30 to 40 min. The sample injection volume was 10 μL.
The diode array detector recorded UV spectra in the range 190 nm to 400 nm and was set to monitor at 254 nm and 280 nm. The mass spectrometric analysis was performed on Agilent 6520 QTOF mass spectrometer in positive ESI mode. The resolving power of the QTOF analyzer was set above 10,000 (FWHM, full width at half maximum) and spectra were acquired within a mass range of m/z 100-1500. Nitrogen was used as nebulizing, drying and collision gas. The capillary temperature was set to 350°C and nebulizer pressure to 42 psi and the drying gas flow rate was 12 L/min. Ion source parameters such as Vcap, fragment, skimmer, and octupole radiofrequency (rf ) peak voltage were set to 3500 V, 150 V, 65 V and 750 V, respectively [13].
Results and Discussion
Results of peak chromatogram
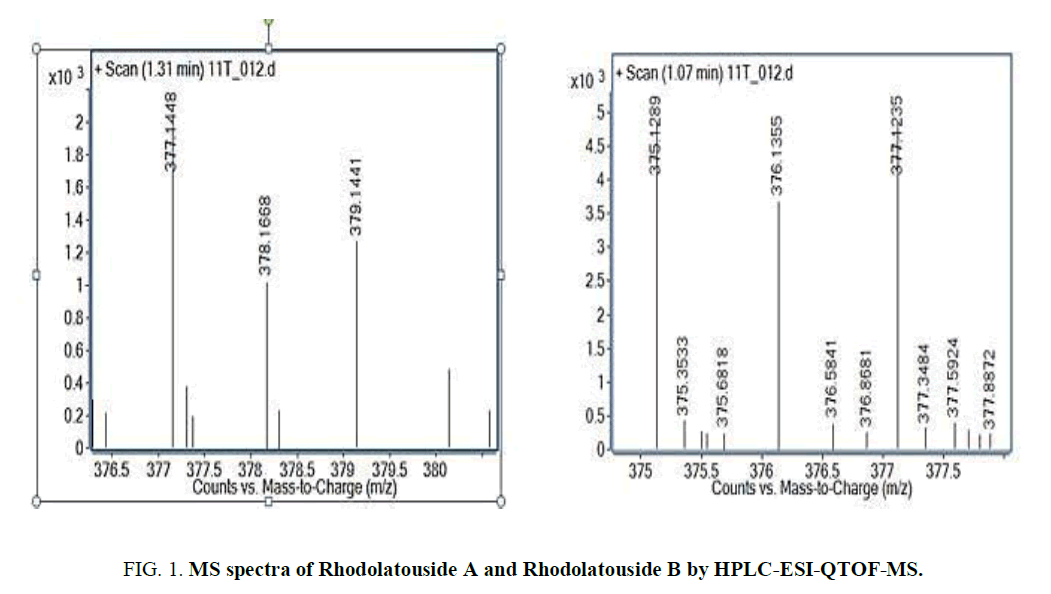
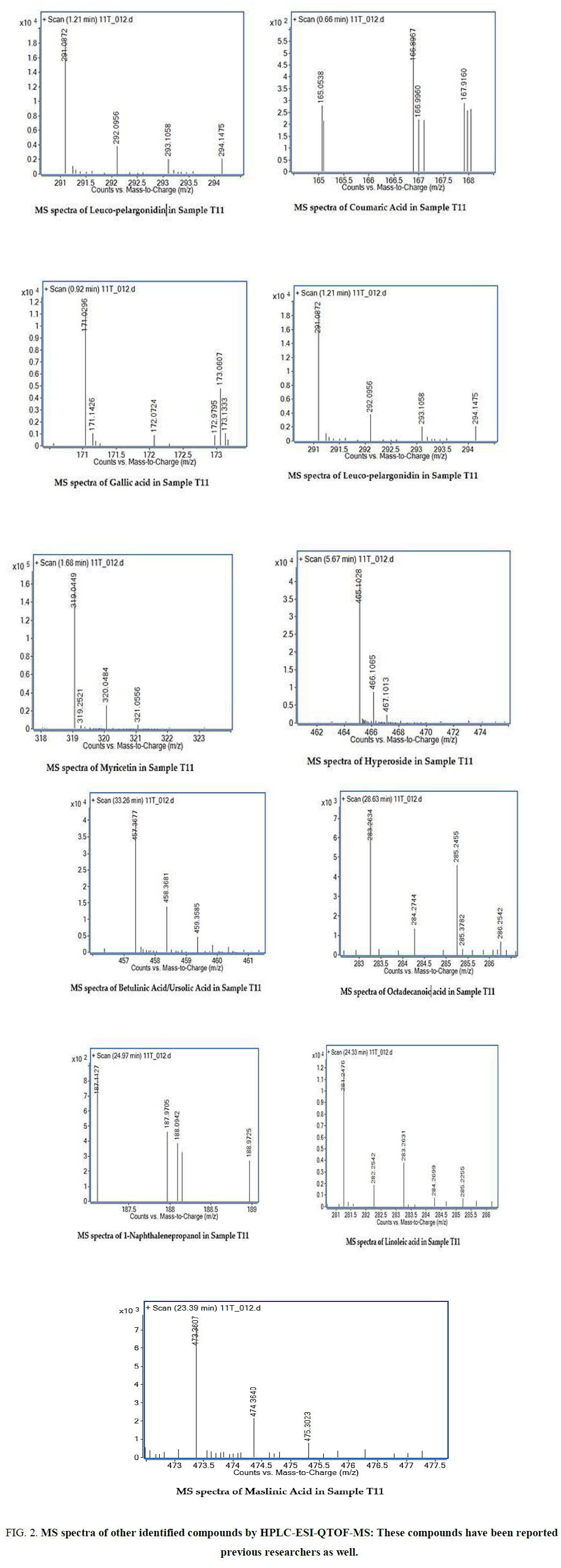
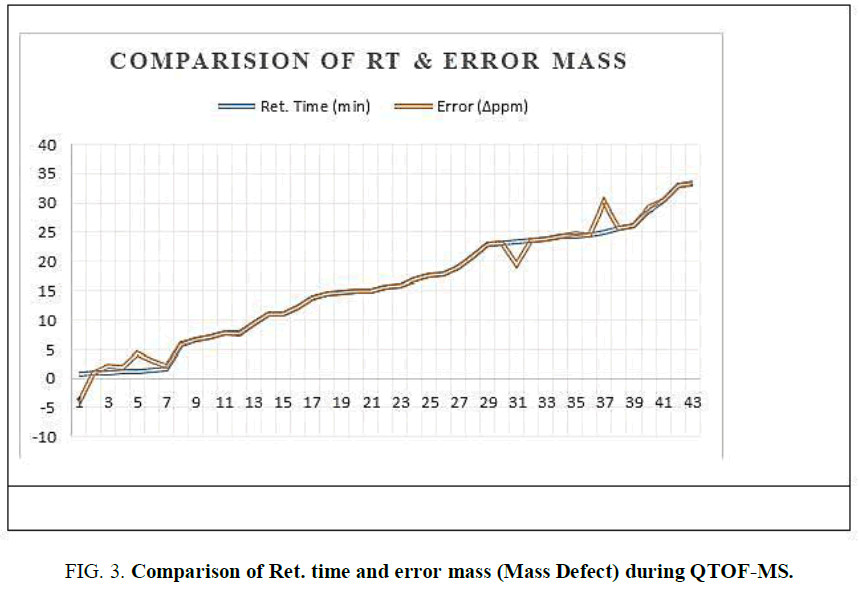
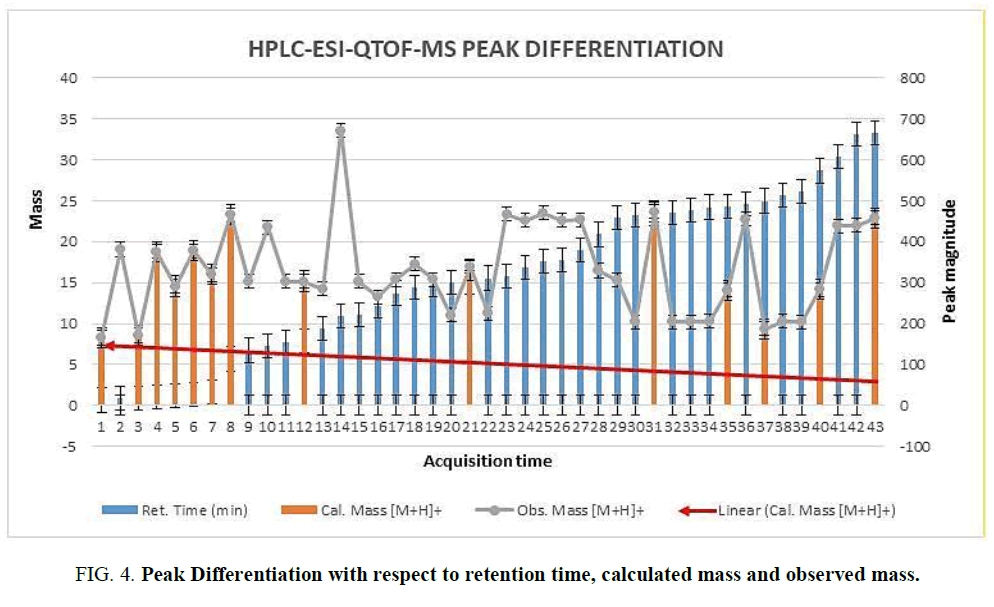
The two new constituents of iridoids, Rhodolatouside A and Rhodolatouside B, with a molecular mass of [M+H]+ 375.1289 and 377.1448 (FIG. 1) were observed with a mass defect of 0.8 and 1.6 respectively including the previously reported compounds found in R. arboreum Smith by HPLC-ESI-QTOF-MS method. The molecular formula of Rhodolatouside A and Rhodolatouside B was found to have C16H22O10 and C16H24O10 respectively (TABLE 2). Along with these two compounds previously reported compounds Myricetin [M+H]+ 319.0449 (C15H10O8), Hyperoside [M+H]+ 465.1028 (C21H20O12), Quercetin [M+H]+ 303.0504 (C15H10O7), Maslinic acid [M+H]+ 473.3607 (C30H48O4), Linoleic acid [M+H]+ 281.2476 (C18H32O2), Coumaric acid [M+H]+ 165.0538 (C9H8O3), Gallic acid [M+H]+ 171.0295 (C7H6O5), Leuco-pelargonidin [M+H]+ 291.0672 (C15H14O6), 1-Nephthalenepropanol [M+H] + 187.1127 (C13H14O), Octadecanoic acid [M+H]+ 283.2634 (C18H34O2) and Betulinic acid/Ursolic acid [M+H]+ 457.3677 (C30H48O3) were also observed with their respective mass peaks and molecular formula (FIG. 2 and TABLE 2) with their mass defects (FIG. 3). The reported data from HPLC-ESI-QTOF-MS supports the components putatively signaled by phytochemical screens [11] reported for each sample of R. arboreum obtained from different locations of Palpa district Nepal. The mass peaks observed are in the sequence of small molecules detected first followed by the larger molecules in different acquisition time (red line arrow) indicating the time of flight of differently mass molecules in MS spectroscopy. The orange color of the line indicated as a calculated mass of known compounds in increasing order which coincides with its observed mass as blue lines (FIG. 4). The literature survey result of the reported compounds from this research showed to have potent herbal drug resources [15]. This emphasizes the plant's importance in homeopathic alignment as a healthcare product.
Figure 2. MS spectra of other identified compounds by HPLC-ESI-QTOF-MS: These compounds have been reported by previous researchers as well.
| Peak No. | Ret. Time (min) | Cal. Mass [M+H]+ | Obs. Mass [M+H]+ | Error (∆ppm) | Molecular Formula | Identification |
|---|---|---|---|---|---|---|
| 1. | 0.66 | 165.055 | 165.054 | -4.8 | C9H8O3 | Coumaric Acid |
| 2. | 0.87 | - | 381.081 | - | NA* | |
| 3. | 0.89 | 171.029 | 171.03 | 1.2 | C7H6O5 | Gallic acid |
| 4. | 1.07 | 375.129 | 375.129 | 0.8 | C16H22O10 | Rhodolatouside A |
| 5. | 1.21 | 291.086 | 291.087 | 3.1 | C15H14O6 | Leuco-pelargonidin |
| 6. | 1.31 | 377.144 | 377.145 | 1.6 | C16H24O10 | Rhodolatouside B |
| 7. | 1.68 | 319.045 | 319.045 | 0.3 | C15H10O8 | Myricetin |
| 8. | 5.67 | 465.103 | 465.103 | 0.2 | C21H20O12 | Hyperoside |
| 9. | 6.73 | - | 303.051 | - | NA* | |
| 10. | 7.21 | - | 435.094 | - | NA* | |
| 11. | 7.78 | - | 303.051 | - | NA* | |
| 12. | 7.83 | 303.051 | 303.05 | -0.3 | C15H10O7 | Quercetin |
| 13. | 9.42 | - | 285.06 | - | NA* | |
| 14. | 10.9 | - | 671.164 | - | NA* | |
| 15. | 11.1 | - | 303.051 | - | NA* | |
| 16. | 12.1 | - | 264.232 | - | NA* | |
| 17. | 13.7 | - | 307.154 | - | NA* | |
| 18. | 14.4 | - | 345.06 | - | - | NA* |
| 19. | 14.7 | - | 307.153 | - | - | NA* |
| 20. | 15 | - | 221.19 | - | - | NA* |
| 21. | 15 | 329.156 | 339.156 | |||
| 22. | 15.5 | - | 225.196 | - | - | NA* |
| 23. | 15.7 | - | 467.316 | - | - | NA* |
| 24. | 16.9 | - | 451.323 | - | - | NA* |
| 25. | 17.6 | - | 469.331 | - | - | NA* |
| 26. | 17.8 | - | 451.32 | - | - | NA* |
| 27. | 18.9 | - | 453.338 | - | - | NA* |
| 28. | 20.9 | - | 329.269 | - | - | NA* |
| 29. | 22.9 | - | 305.176 | - | - | NA* |
| 30. | 23.3 | - | 203.18 | - | - | NA* |
| 31. | 23.4 | 473.363 | 473.361 | -3.8 | C30H48O4 | Maslinic Acid |
| 32. | 23.5 | - | 203.18 | - | NA* | |
| 33. | 23.8 | - | 203.179 | - | NA* | |
| 34. | 24.2 | - | 205.195 | - | NA* | |
| 35. | 24.3 | 281.248 | 281.248 | 0.4 | C18H32O2 | Linoleic acid |
| 36. | 24.6 | - | 455.352 | - | NA* | |
| 37. | 25 | 187.112 | 187.113 | 5.3 | C13H14O | 1-Naphthalenepropanol |
| 38. | 25.6 | - | 205.195 | - | NA* | |
| 39. | 26.1 | - | 203.18 | - | NA* | |
| 40. | 28.6 | 283.263 | 283.263 | 0.7 | C18H34O2 | Octadecanoic acid |
| 41. | 30.4 | - | 437.34 | - | NA* | |
| 42. | 33.1 | - | 439.357 | - | - | NA* |
| 43. | 33.3 | 457.368 | 457.368 | 0.2 | C30H48O3 | Betulinic Acid/Ursolic Acid |
TABLE 2. HRMS of peaks identified in the sample using HPLC-ESI-QTOF-MS analysis.
These results should be further validated by more rigorous scientific studies and structure stereochemistry of secondary metabolites which may define their importance as future drugs. Further DNA based authentication and its conservation are required for its identity and indigenousness.
In approval of its high utility, the plant can be a good economic source for the poor people who can domesticate the plant on a large scale and market it as a medical value-added plant that will help to the financial upliftment of the community [16,17].
Acknowledgments
The work was financially supported by the University grant commission Nepal and NAST Nepal for publication support, HPLC-ESI-QTOF-MS analysis was done in Central Drug Research Institute Lucknow. We are thankful to Tirtha Maiya Shrestha for the taxonomic identification of the samples.
Conflict of Interest
The authors declare that they have no conflict of interests.
1. Author: Prof. Janardan Lamichhane
Work Contribution: As a Supervisor provides full guidance to execute the research activity including all the facilities required for research in the laboratory.
2. Author : Deepak Sharma
Work Contribution: As a student working in the laboratory for the research activity within the laboratory as a full-time researcher including Sample collection, phytochemical analysis, and research execution.
References
- Paula CC, Martins DTO, Arunachalam K, et al. Antimicrobial screening of medicinal plants popularly used in mato grosso for treating infections: Advances on the evaluation of Conyza bonariensis (L.). Pharmacogn J. 2018;10(6):152-66.
- Rebecca RJ. Phenolic acids in foods: An overview of analytical methodology. J Agricult Food Chem. 2003;51:2866-87.
- Koirala R. Magical uses of Rhododendron national flower of Nepal. 2011.
- Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenoli compounds. Molecules. 2013;18(2):2328-75.
- Bhatt M, Abrol G, Kumar S, et al. Preparation and evaluation of functionally enriched squash from Rhododendron arboreum Sm. flowers. Intl J Food Techno. 2017;7(1):191-96.
- Nostro A, Germano MP, D'angelo V, et al. Extraction methods and bio autography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379-84.
- Bhandari L, Rajbhandari M. Isolation of quercetin from flower petals, estimation of total phenolic, total flavonoid and antioxidant activity of the different parts of Rhododendron arboreum smith.Scientific World. 2014;12(12):34-44.
- MR Bhandari, Kawabata J.Antidiabetic activity of laligurans (Rhododendron arboreum Sm.) flower. J Food Sci Technol. 2008;4:61-64.
- Ved Prakash V, Rana S, Sagar A. Studies on antibacterial activity of leaf extracts of Rhododendron arboreum and Rhododendron campanulatum. Int J Curr Microbiol App Sci. 2016;5(4):315-22.
- Nisar M, Ali S, Qaisar M, et al.Antifungal activity of bioactive constituents and bark extracts of Rhododendron arboretum.Bangladesh J Pharmacol. 2013;8:218-22.
- Sharma D, Shrestha T, Naresh R, et al. Screening and bioactivity measurement of high altitude medicinal plants of Nepal. Vegetos. 2016;29:4.
- Verma N, Singh AP, Amresh G, et al. Anti-inflammatory and anti-nociceptive activity of Rhododendron arboretum. J Pharm Rese. 2010;3(6):1376-80.
- Acharya K, Khatua S, Roy T. Antioxidant and free radical scavenging capacity of phenolic extract from Russula laurocerasi.Int J Pharm Tech Res. 2013;3(2);757-62.
- Kumar P. R. arboreum (Ericaceae): Immunomodulatory and related toxicities studies. Orient Pharm Exp Med. 2012;1(4).
- Rawat P, Bachheti RK, Kumar N, et al. Phytochemical analysis and evaluation of in vitro immunomodulatory activity of Rhododendron arboreum leaves. Asian J Pharm Clinical Res. 2018;11(8);123-28.
- Pallavi Shrisvastava. Rhododendron arboretum: An overview. J Appl Pharmal Sci. 2012;2(1):158-62.
- Swaroop A, Prakash A, Sinha K. Simultaneous determination of quercetin, rutin and coumaric acid in flowers of Rhododendron arboreum by HPTLC 1.Chromatographia. 2005;62(12):649-52.