Original Article
, Volume: 12( 12)Potentiality of Bacillus weihenstephanensis PKD5 Keratinase for Eco-Friendly Dehairing of Skins and Hide
- *Correspondence:
- Pradeep Kumar DM, Department of Microbiology, Vidyasagar University, Midnapore-721102, West Bengal, India, Tel: 09434410208; E-mail: pkdmvu@gmail.com
Received: November 25, 2016; Accepted: December 12, 2016; Published: December 15, 2016
Citation: Dipak Kumar S, Suman Kumar H, Hrudayanath T, et al. Potentiality of Bacillus weihenstephanensis PKD5 keratinase for Ecofriendly Dehairing of Skins and Hide. Biotechnol Ind J. 2016;12(12):119.
Abstract
Comparative dehairing efficiency of Na2S (1.5%), CaO (1.5%) and crude keratinase (15.3 U/ml) of Bacillus weihenstephanensis PKD5 was investigated in single and in combination on cow hide, goat and sheep skin. Complete dehairing was achieved by treatment with Na2S, CaO with Na2S, Na2S with crude keratinase and crude keratinase after 2, 2, 2 and 10 h respectively. Among all the treatments, the enzyme treated skin/hide was comparatively more white, smooth and shiny due to release of intact hair with follicle from skin/hide. Though chemical(s) and combination of chemical with enzyme dehair the skin/hide in relatively less time but the enzymatic dehairing improved the leather quality. So, the keratinase is produced using low cost poultry feather which can be employed for dehairing that is sustainable and potential to replace the chemical dehairing in leather industries.
Keywords
Bacillus weihenstephanensis; PKD5; Keratinase; Leather; Dehairing
Introduction
Leather industry has been a traditional industrial sector for the production of processed leather. It is a by product of meat industry which is prepared from skin and hide of animal. It was estimated that worldwide 5.5 million tons of wet salted raw hides were processed and 4.6 × 105 tons of heavy leathers was produced per year [1]. The leather processing involves various operations in a sequential manner from raw skin/hide to processes leather. Classical leather processing involves the major steps like soaking, dehairing, bating and tanning.
Dehairing or unhairing is an essential and unavoidable step to remove hair from skin and hide. After dehairing, the leather processed through many treatments like deliming, bating, degreasing, and pickling in a cascade manner; then it converts into a durable, long-lasting material for various uses including shoes, hats, jackets, skirts, trousers, belts, book binding, leather wallpaper, and as a furniture covering. Conventionally, lime (CaO) and sulphide (Na2S/NaSH) used concurrently for dehairing for a long time [2]. Lime as an alkali causes swelling of skins/hides by hydrolyzing asparagine and glutamine in collagen structure that helps to distort fiber bundles to remove hairs [3,4]. Whereas sulphide like Na2S/NaSH was used for dehairing, as the sulphide ion attack the disulphide bridges between cysteine residues present in keratin structure [5]. The production and quality of leather did not reach to optima due to lack of technology other than the use of lime and sodium sulphide.
The two main ingredients for dehairing was lime which produces a poisonous sludge whereas sulphide is highly toxic with obnoxious odour. They cause pollution in tannery’s wastewater which contaminates community water sources. They may also decline the efficiency of treatment plants to recycle the effluents [6]. Exposure to chemicals in air or in solution tanks in processing plants can be hazardous to workers and the symptoms are skin irritations, respiratory problems and dizziness. In this concern, the enzymatic dehairing approach, specifically protease is a better, greener and sustainable alternative to reduce environmental pollution, harmful health effects on workers and also improve the quality of leather [7-10].
Few reports on dehairing attested that employment of microbial proteases provide better quality leather in contrast with chemical dehairing agent [11-16]. Among the microbial sources, bacterial proteases gain much more appreciation and attention due to easy production through submerged fermentation, relatively higher yield, less production time and easy recovery of product. However commercial implementation of enzymatic dehairing is limited due to certain drawback like stability of enzyme in wide range of pH and temperature, consistent performance as well as production cost.
With the aim to search for a viable and eco-friendly alternative of the harmful chemicals, the present study was aimed to evaluate the dehairing efficiency of crude keratinolytic protease of Bacillus weihenstephanensis PKD5 along with Na2S and CaO (as chemical dehairing agent) in single and in combination.
Materials and Methods
Microorganism and keratinase production
A potent keratinase producing bacteria Bacillus weihenstephanensis PKD5 (GenBank accession no. JN897383) was previously isolated from soil sample of feather dumping area was employed in present study [17].
The strain was grown in 250-ml Erlenmeyer flasks in optimized condition (g/l): NaCl 5.0, MgSO4 1.0, K2HPO4 1.0, (NH4)2SO4, 2.0, white chicken feather 10.0, at pH 8.0, 2% inoculum (109 cells/ml), 40°C, 120 rpm for 7 days [17]. Thereafter the fermented broth was centrifuged (at 5590×g for 5 min) and the supernatant was considered as crude enzyme.
Enzyme assay
The keratinolytic activity of crude enzyme was assayed with keratin powder (Hi-Media, India) as a substrate with the modified method of Gradisar et al. [18]. One unit of keratinase activity was defined as the amount of enzyme required to liberate 1 μg of tyrosine per minute under the experimental conditions.
Dehairing assay
Fresh fleshed skin of goat; sheep and cow hide were collected from local slaughter house, washed sequentially with sodium chloride solution and water to remove impurities then dried in hot air oven at 50°C. The skins were cut into pieces and incubated with 40 ml solution in 100 ml beaker (dip method) of different agents viz. 1.5% sodium sulphide (Na2S), 1.5% lime (CaO) and crude enzyme (15.3 U/ml) in single and in combination (1.5% CaO+1.5% Na2S, 1.5% lime+15.3 U/ml keratinase and 1.5% Na2S+15.3 U/ml keratinase) at room temperature. Skin/hide treated with distilled water was considered as negative control. Skin/hide were withdrawn at regular interval and examined for dehairing by gently scraping with blunt scalpel and the process is continued up to 10 h. Skin pieces and hair were analyzed through stereomicroscope (Magnus MS24). The experiment was carried out in triplicate. After one batch, the used enzyme (single and in combination) also employed upon fresh skins to see its dehairing efficiency.
Results
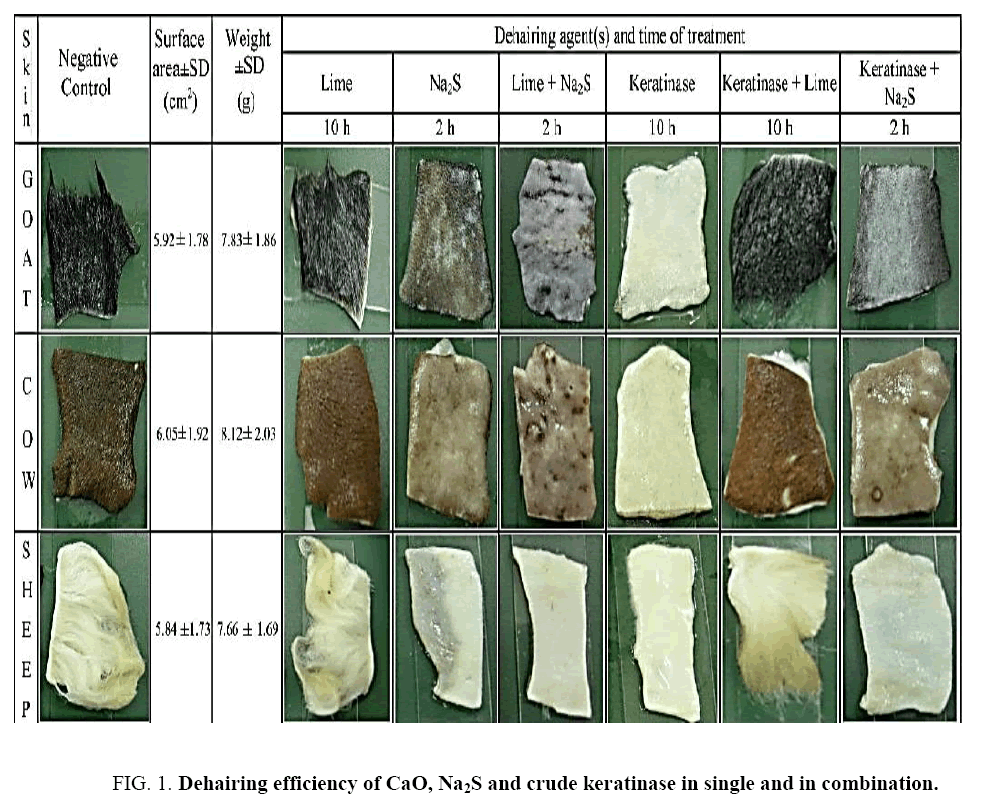
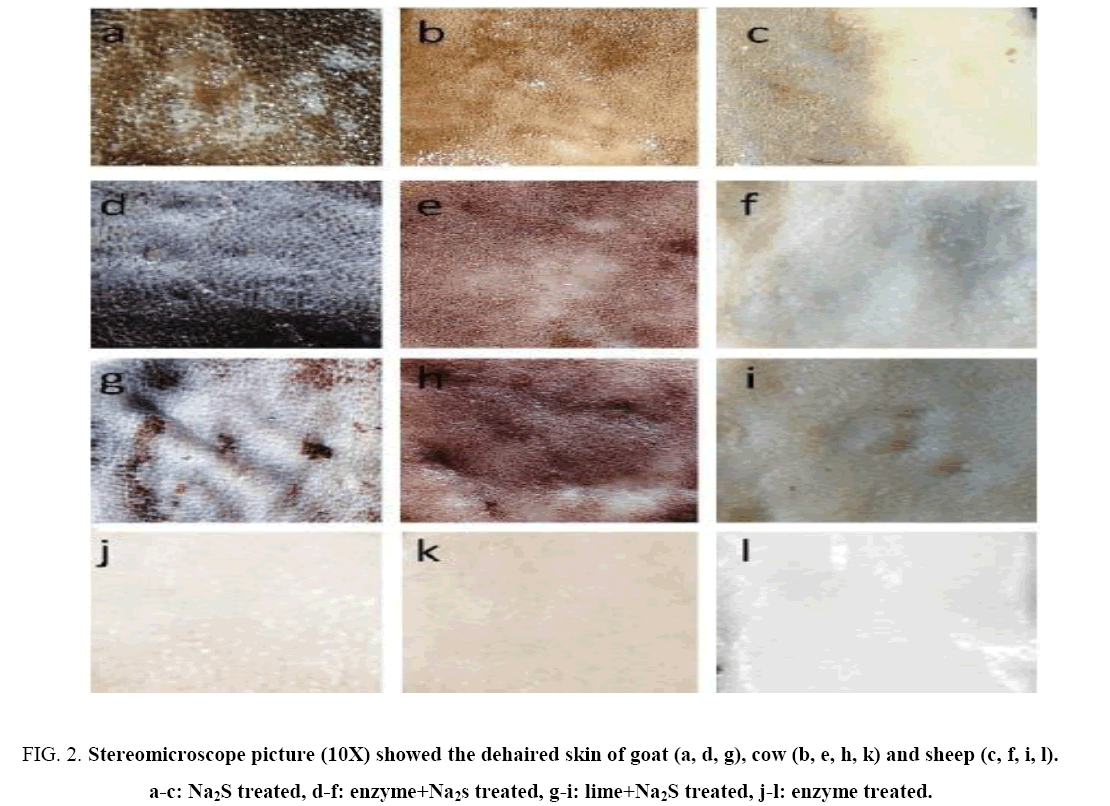
Effect of different agent(s) on dehairing of skins/hide was investigated in time dependent manner and the appearance of dehaired skins and hide was shown in Figure 1 . Complete dehairing was noticed after treatment with Na2S (1.5%), lime+Na2S (1.5%) and Na2S+ crude keratinase within 2 h of incubation in all the three types of skin/hide. On the other hand in crude enzyme treatment, complete dehairing was achieved within 10 h ( Figure 1 ). The visual and stereomicroscopic examination revealed that the proteolytic enzyme removed the hairs with follicles as a result the skins become smooth, white and shiny. On the other hand, the chemically treated skins were hard, dark brown and wrinkled ( Figure 2 ).
Figure 2: Stereomicroscope picture (10X) showed the dehaired skin of goat (a, d, g), cow (b, e, h, k) and sheep (c, f, i, l). a-c: Na2S treated, d-f: enzyme+Na2s treated, g-i: lime+Na2S treated, j-l: enzyme treated.
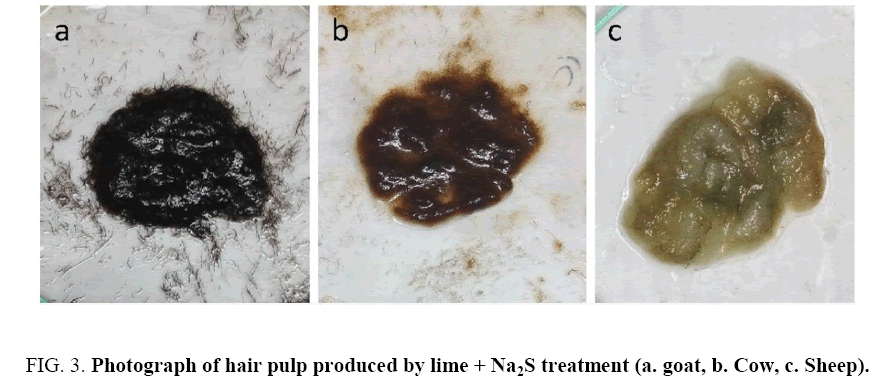
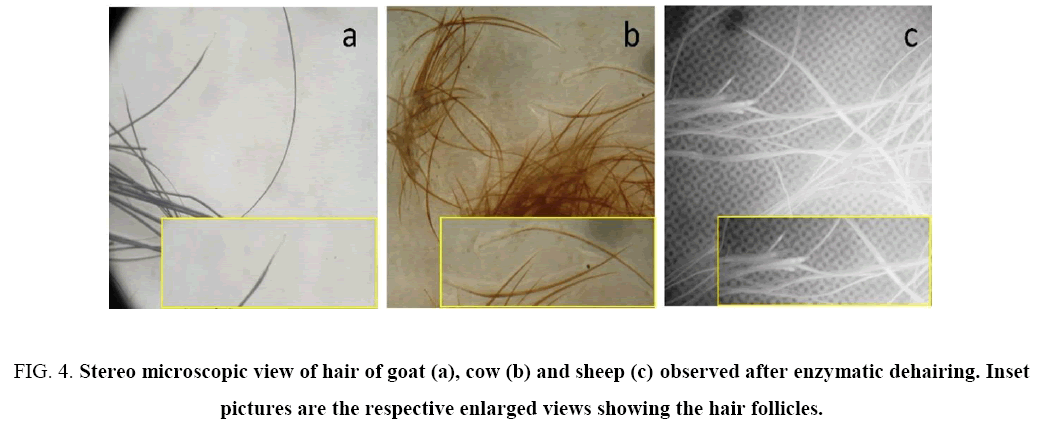
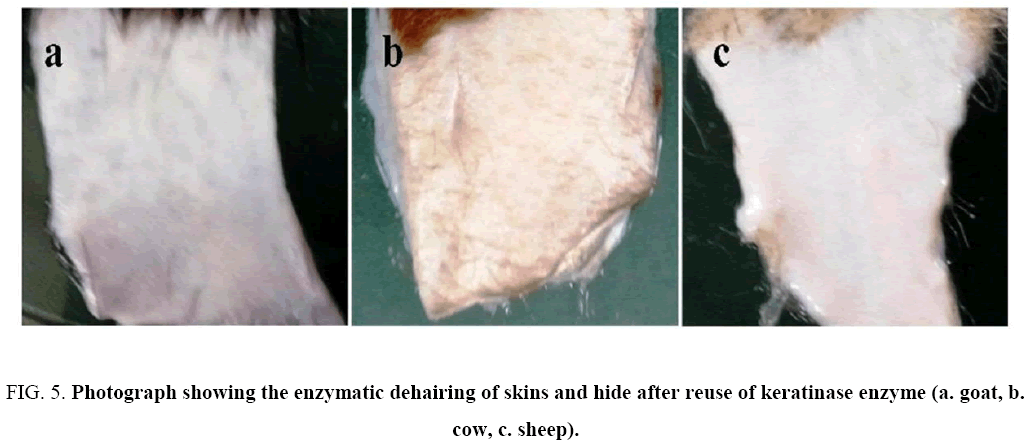
Hair collected from traditional dehairing through the lime-sulfide process was found to be gelatinized and subsequently converted into pulp ( Figure 3 shows Na2S+lime treatment only), whereas hairs collected from enzymatic treatment was intact with follicle ( Figure 4 ). The used enzyme after one batch could also dehaired all types of skins/hide efficiently within 14 h of incubation ( Figure 5 ) with mild obnoxious odour.
Figure 4: Stereo microscopic view of hair of goat (a), cow (b) and sheep (c) observed after enzymatic dehairing. Inset pictures are the respective enlarged views showing the hair follicles.
Figure 5: Photograph showing the enzymatic dehairing of skins and hide after reuse of keratinase enzyme (a. goat, b. cow, c. sheep).
Discussion
In our previous assignment, we explored a potent keratinase producing bacteria B. weihenstephanensis PKD5 which capable to produce the enzyme by utilizing low cost chicken feather [17] that will be highly acceptable in industrial sector for large scale production. The enzyme was found stable in wide range of pH (6-9) and temperature (30°C to 60°C) which encourage its application in leather processing [17]. In the present study, the crude keratinase of B. weihenstephanensis PKD5 takes about 10 h for dehairing (at 15.3 U/ml enzyme concentration, pH 8.0, room temperature) that is comparatively better than the reported protease of Bacillus subtilis [19], Pseudomonas stutzeri strain K4 [20], Bacillus safensis [21], Paenibacillus woosongensis (TKB2) [22] which take 24 h, 20 h, 16 h, 14 h respectively (Table 1). Though, the purified enzyme of Brevibacillus brevis US575 takes about 10 h for dehairing [23], but the purification process is costly, time consuming and sensitive. Therefore, the application of crude enzyme eliminates the cumbersome purification process. Moreover, the presence of other proteolytic enzymes in crude preparation may enhance or they may act synergistically with keratinase which in turn hasten the dehairing efficiency.
| Bacterial strain | Enzyme (Purified /Partially purified/ Crude) and its concentration/amount | Dehairing condition | Type of skin/hide | References |
|---|---|---|---|---|
| PaenibacilluswoosongensisTKB2 | Crude enzyme (3:1 w/v) | 14 h, pH 8-9 | Goat | [22] |
| Pseudomonas stutzeri strain K4 | Crude enzyme (40 U/ml) | 20h, pH 8.0, 30°C | Goat | [20] |
| Bacillus subtilis | Crude enzyme (59 U/ml) | 24 h, pH 8.0, 37°C | Goat | [19] |
| Bacillus safensis | Crude enzyme (35.4U/mlto 50.4 U/ml) | 16 h, 30°C ± 2°C | Goat | [21] |
| Brevibacillus brevis US575 | Purified (2,000 U/ml) | 10 h, 37°C | Rabbit, goat, sheep, bovine | [23] |
| Bacillus cereus strainAT | Crude enzyme (4813U/g ± 62 U/g) | 18 h, pH 9.0, 30°C | Goat | [24] |
| Pseudomonas aeruginosa MCM B-327 | Partially purified (1%, w/w in 20% water) | 16-21 h, pH 7.0, 26°C to 30°C | Buffalo | [25] |
| Exiguobacteriumsp.DG1 | Crude enzyme | 24 h | Sheep | [26] |
| Bacillus circulans | Purified (2mg/50 ml) | 16 h, pH 11.0, 35°C | Goat | [27] |
| Bacillus weihenstephanensis PKD5 | Crude enzyme (15.3 U/ml) | 10 h, pH 8.0, room temperature | Goat, cow, sheep | Present study |
Table 1: Comparison of dehairing conditions of B. weihenstephanensis PKD5 with reported bacterial keratinases/proteases.
The chemicals and the cocktail of enzyme with chemical synergistically accelerate the dehairing process though enzymatic dehairing removes intact hair whereas chemical dehairing produces hair-pulp. So, enzyme-based dehairing significantly reduces the chance of pollution, eliminates the use of toxic chemicals as well as improves the leather quality. Moreover, the intact hair (especially sheep) recovered from enzymatic dehairing may use in textile industry.
Conclusion
In this study, an eco-friendly enzymatic dehairing process was developed using keratinolytic protease of B. weihenstephanensis PKD5 produced by utilizing low cost chicken feather, which is more effective in various classes of skins/hide in addition to decrease pollution load and improve leather quality. Worldwide, search is going on for a stable enzyme which will be more effective on various types of hide and skin. In this concern, large scale enzyme production and subsequent application for dehairing may cope with the scale of normal leather production at industrial level.
References
- Paul T, Jana A, Mandal AK, et al. Bacterial keratinolytic protease, imminent starter for Next Gen leather and detergent industries. Sustainable. Chem Pharm. 2016;3:8-22.
- Saravanabhavan S, Thanikaivelan P, Rao JR, et al. Silicate enhanced enzymatic dehairing: a new lime-sulfide-free process for cowhides. Environ Sci Technol. 2005;39:3776-83.
- Sivasubramanian S, MuraliManohar B, Rajaram A, et al. Ecofriendly lime and sulfide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemosphere. 2008;70:1015-24.
- Heidemann E. Newer developments in the chemistry and structure of collagenous connective tissues and their impact on leather manufacture. J Soc Leath Tech Ch. 1982;66:21-9.
- Choudhary RB, Jana AK, Jha MK. Enzyme technology applications in leather processing. Ind J Chem Technol. 2004;11:659-71.
- Davies RM. Setting of consent limits for tanning industry trade effluents.J Soc Lea Technol Chem. 1997;8:32-6.
- Kida K, Morimura S, Noda J, et al. Enzymatic hydrolysis of the horn and hoof of cow and buffalo. J Bios Bioeng. 1995;80:478-84.
- Macedo AJ, wobd DS, Gava R, et al. Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl Environ Microbiol. 2005;71:594-6.
- Priya S, Rajaram A, Rajaram R, et al. Depilation of skins by pure enzymes. J Soc Leath Tech Ch. 2008;92:214-21.
- Verma A, Pal HS, Singh R, et al. Potential of alkaline protease isolated from Thermoactinomyces sp. RM4 as an alternative to conventional chemicals in leather industry dehairing process. Int J Agric Environ Biotech. 2011;4:173-8.
- Mala M, Srividya S. Partial purification and properties of a laundry detergent compatible alkaline protease from a newly isolated Bacillus species Y. Ind J Microbiol. 2010;50:309-17.
- Chauhan PS, George N, Sondhi S, et al. An overview of purification strategies for microbial mannanases. Int J Pharm Bio Sci. 2014;5:176-92.
- Mande SS, Gupta N, Ghosh A, et al. Homology model of a novel xylanase: molecular basis for high-thermostability and alkaline stability. J BiomolStrDyn. 2000;18:137-44.
- Chauhan PS, Puri N, Sharma P, et al. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol. 2012;93:1817-30.
- Kandasamy N, Velmurugan P, Sundarvel A, et al. Eco-benign enzymatic dehairing of goatskins utilizing a protease from a Pseudomonas fluorescens species isolated from fish visceral waste. J Clean Prod. 2012;25:27-33.
- Kumar VE, Srijana M, Kumar K, et al. A novel serine alkaline protease from Bacillus altitudinis GVC11 and its application as a dehairing agent. Bioprocess Biosyst Eng. 2011;34:403-9.
- Sahoo DK, Das A, Thatoi H, et al. Keratinase production and biodegradation of whole chicken feather keratin by a newly isolated bacterium under submerged fermentation. Appl Biochem Biotechnol. 2012;167:1040-51.
- Gradisar H, Kern S, Friedrich J. Keratinase of Doratomycesmicrosporus. Appl Microbiol Biotechnol. 2000;53:196-200.
- Kazi YF, Kumar P, Soomro IH. Characterization of the keratinolytic activity of indigenous Bacillus subtilis keratinase. J Chem Pharm Res. 2015;7:800-9.
- Chaturvedi V, Bhange K, Bhat R, et al. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. Biocatal Agric Biotechnol. 2014;3:167-74.
- Lateefa A, Adelerea IA, Gueguim-Kanac EB. Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnol Biotechnol Equip. 2015;29:54-63.
- Paul T, Das A, Mandal A, et al. Effective dehairing properties of keratinase from PaenibacilluswoosongensisTKB2 obtained under solid state fermentation. Waste Biomass Valor. 2014;5:97-107.
- Jaouadi NZ, Rekik H, Badis A, et al. Biochemical and molecular characterization of a serine keratinase from Brevibacillus brevis US575 with promising keratin-biodegradation and hide-dehairing activities. PloS One. 2013;8:1-17.
- Vijayaraghavan P, Lazarus S, Vincent SGP. De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: Biosynthesis and properties. Saudi J Biol Sci. 2014;21:27-34.
- Zambare VP, Nilegaonka S, Kaneka P. A novel extracellular protease from Pseudomonas aeruginosa MCM B-327: enzyme production and its partial characterization. New Biotechnol. 2011;28:173-81.
- Gumilar J, Triatmojo S, Yusiati LM, et al. Isolation, identification and dehairing activity of Indonesian native keratinolytic bacteria Exiguobacteriumsp. DG1.Pak J Biotechnol. 2015;12:41-8.
- SubbaRao CH, Sathish T, Ravichandra P, et al. Characterization of thermo-and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem. 2009;44:262-8.