Original Article
, Volume: 12( 1)Polyaniline - Zirconium Telluro Tungstate Composite Ion Exchanger: An Ecofriendly and Effective Solid Acid Catalyst for Sucrose Inversion
- *Correspondence:
- Nimisha KV Department of Chemistry, Sree Narayana College, Kerala-670007, India, Tel: +914742741793; E-mail: nimishavijil89@gmail.com
Received: August 13, 2016 Accepted: January 25, 2017 Published: January 30, 2017
Citation: Nimisha KV, Janardanan C. Polyaniline - Zirconium Telluro Tungstate Composite Ion Exchanger: An Ecofriendly and Effective Solid Acid Catalyst for Sucrose Inversion Inorg Chem Ind J. 2017;12(1):104
Abstract
Utility of hetero-polyacid salt based composite cation exchanger polyaniline-zirconium telluro tungstate (PANI-ZrTeW) synthesised by co-precipitation method as solid acid catalyst for sucrose inversion was analysed. Ion exchange capacity, pH titration, thermal stability were investigated to explore ion exchange behaviour of PANI-ZrTeW. The composite exchanger PANI-ZrTeW possess high cation exchange capacity (1.48 meq-1) due to the presence of exchangeable H+ ions, and the catalytic reactions were done with the PANI-ZrTeW and reaction progress were monitored by spectrophotometric methods. Inorder to optimise the sucrose inversion various factors that affect the % yield of the reaction such as reaction temperature, effect of catalyst dosage, pH effect and concentration of sucrose has been studied.
Keywords
Composite ion exchanger; Polyaniline; Solid acid catalyst; Sucrose inversion
Introduction
Traditionally, invert sugar is prepared from sucrose using mineral acids. Invert sugar find applications for many areas of food and pharmaceutical industries. Also, sugars are the key intermediates from biomass to chemicals. Biomass [1-3] is an alternative renewable and sustainable source to substitute the fossil resources in order to meet the problem of increasing energy demands together with global warming caused by CO2 emissions due to its rapid consumption. The hydrolysis of sucrose allows its conversion in to inverted sugars, i.e., glucose and fructose which are widely used in the food industry [4]. Enzymes and many mineral acids were the main industrial catalyst used for the hydrolysis of sucrose. However, they suffer from different drawbacks such as the production of waste effluent, low thermal stability and recyclability of catalyst and problems with separation and recovery of the catalyst from the product. The procedures are laborious and costly and this is why it appeared the necessity to use some new methods like heterogeneous acid catalyst. This alternative has impost itself because of its certain advantages comparing with both enzymatic and homogeneous method like the rigorous control of the reaction, the reduction of the secondary product content, easier separation of catalyst, absence of equipment corrosion, the possibility to pilot the catalytic processes at higher temperatures than in the case of enzyme processing which strongly favors the equilibrium displacement of reaction products [5].
Polymer based ion exchange resins and some inorganic materials with bronstead acid sites have been widely used as solid acid catalyst for many organic reactions including hydrolysis reactions. The hydrolysis of sucrose has been carried out over heterogeneous catalyst such as zeolites [6], Polystyrene with sulfonic acid group [7], sulfonated mesoporous silicas [8] silica supported heteropolyacids [9]. But they suffer certain limitations because of low mechanical and chemical strength of organic resins and while the use of inorganic ion-exchanger is limited by its non-reproducibility. Organic-inorganic hybrid, a new class of materials is attractive for creating high performance is expected to provide many possibilities. Due to the flexibility of the organic part these materials possess specific chemical reactivity and the presence of inorganic backbones make them thermally and mechanically more stable.
The present work reports the applicability of a heteropoly acid based composite cation exchanger PANI-ZrTeW as solid acid catalyst for the inversion of sucrose. The authors already reported the synthesis, characterisation and waste water detoxification analysis of the composite exchanger PANI-ZrTeW [10]. PANI-ZrTeW was used as heterogeneous catalyst because of their versatile properties, high exchange capacity and thermal stability. Also, optimum conditions for the catalytic reaction were developed by analysing the various controlling factors of hydrolysis.
Experimental Section
Reagents
The main reagents used for the synthesis of the composite material and catalytic studies were zirconium oxychloride (E. Merck), sodium tungstate (E. Merck), sodium tellurite (Lobachemie), aniline (E. Merck), ammonium persulphate (E. Merck). sucrose and glucose (Sisco) and dinitrosalicylic acid, sodium sulphite, phenol and sodium potassium tartarate (E. Merck). All other reagents and chemicals used were of analytical grade.
Apparatus and instruments
A glass column was used for column operations. ELICO LI613 pH meter was used for pH measurements. FT-IR spectra were recorded on a Thermo-Nicolet Avtar 370 instrument using KBr pellet of the samples at spectral resolution of 4 cm-1, X-ray Diffractometer Bruker AXS D8 Advance for X-ray diffraction studies with Cu Kα radiations, TG Perkin Elmer Diamond TG/DTA Analysis System for thermogravimetric/ derivative thermogravimetric analysis were used at a rate of 10ºC in nitrogen atmosphere. Jeol Model JSM-6390LV for SEM analysis, Jeol Model-Jed-2300 was used for Energy Dispersive Spectrometric analysis. UV-Visible Spectrophotometer model JASCO V660 was used for catalytic studies. Magnetic stirrer (Remi Equipments) was used for stirring purposes.
Synthesis of polyaniline zirconium (IV) tellurotungstate composite cation exchanger
Polyaniline gels were prepared by mixing of the acidic solutions of 0.05 M aniline and 0.1 M ammonium persulfate in different volume ratios with continuous stirring by a magnetic stirrer keeping the temperature below 10°C for half an hour.
The salt was prepared by mixing boiling aqueous solutions of Na2WO4·2H2O and NaTeO3 upon vigorous stirring. pH was adjusted to acidic by adding 1.0 M HNO3 drop wise, the gelatinous precipitate locally formed disappears upon stirring. After boiling for a few minutes, the clear solution was precipitated by addition of an aqueous solution of zirconium oxychloride. A white gelatinous precipitate was formed.
Zirconium (IV) tellurotungstate incorporated polyaniline composite was prepared by simple stirring method. The ex-situ polymerized gels of polyaniline were added to the white inorganic precipitate of zirconium (IV) tellurotungstate and mixed thoroughly with constant stirring, the resultant mixture turned slowly into green colored slurry. The green colored gel was kept for 24 h at room temperature (25 ± 2 ºC) for digestion. The supernatant liquid was decanted and the gel was filtered. The excess acid was removed by washing with DMW and the material was dried in an air oven at 30 ºC. The dried products were immersed in DMW to obtain small granules. They were converted to H+ form by treating with 1.0 M HNO3 for 24 hrs with occasional shaking, intermittently replacing the supernatant liquid with fresh acid. The excess acid was removed after several washings with DMW and then dried at 30ºC. The particles size of the range (125 ?m) was obtained by sieving and was kept in desiccators.
Chemical characterizations of catalyst
Stability of catalyst: About 250 mg portion of the composite cation exchanger (H+ form) was treated with 20 mL of different common acids (HCl, HNO3, H2SO4), bases (NaOH, KOH, NH4OH), sodium nitrate solution, and few organic solvents (DMSO, DMF, acetone, THF, CHCl3, CCl4) and also with demineralized water for 24 hrs with occasional shaking. Changes in color, nature and weight of the catalyst were noted.
Ion-exchange capacity (IEC): The ion exchange capacity of the material was determined by column chromatographic method. 1.0 g of the exchanger in H+ form was taken in a glass column of 1.1 cm diameter. The H+ ions were eluted by percolating 100 ml of 1.0 M NaCl solution. The effluent was collected and titrated against standard sodium hydroxide solution. The ion exchange capacity in meqg-1 was calculated using the formula,
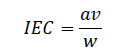
Where, a is the molarity, v is the volume of alkali used during titration and, w is the weight of the exchanger taken [11].
Thermal effect and Effect of ionic radii on IEC: The effect of temperature on ion exchange capacity was studied by heating the material at different temperature for 3 hrs and the Na+ ion exchange capacity was determined by column process after cooling them at room temperature. The effect of the size and charge of the exchanging ions on the ion-exchange capacity was determined by using other alkali and alkaline earth metal solution as eluent in addition to NaCl.
pH-titration: A pH-titration study of PANI-ZrTeW was performed by the method of Topp and Pepper [12]. 500 mg of the cation-exchanger in the H+-form were placed in several 250 ml conical flasks, followed by the addition of equimolar solutions of alkali metal chlorides and their hydroxides in different volume ratio. The final volume is kept 50 ml to maintain the ionic strength constant. The pH of each solution was recorded [13-15] after every 24 h until equilibrium was attained, which needed ∼5 days and pH at equilibrium was plotted against the milli equivalents of OH- ions added.
Catalytic experiments: The catalytic experiments were carried out in a round bottom (R.B) flask at 50ºC. In this experiment, the reactor was loaded with 1000 ml of 0.5 M sucrose. Reactions were started by adding 0.50 g of catalyst. In all experiments the stirrer speed was kept constant at 500 rpm. After the hydrolysis catalyst was separated from the reaction mixture, washed with water and dried at 30ºC. A portion of the solution was taken out at known time intervals and was analyzed by spectrophotometer by DNSA (Dinitrosalicilic acid) method. According to this method, the reductant sugar reduces DNSA, which produces forming orangeish colour complexes, which can be quantified by UV-Visible spectrophotometric method with an absorption band at 540 nm. A standard curve containing glucose allows the association of absorbance with the concentration of hydrolysis reaction products [16].
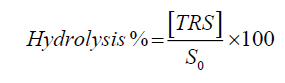
Where, [TRS] is the total reductant sugar (glucose+fructose) and S0 is the initial sucrose concentration.
Results and Discussion
Characterization of catalyst
Various samples of cation exchange composite material have been developed by the incorporation of the heteropoly acid ZrTeW into the polymeric matrix of conducting polymer poly aniline under different conditions. PANI-ZrTeW appeared as greenish dark crystals prepared under different composition having maximum ion exchange capacity of 1.48 meqg-1 was selected for detailed study. This comparatively high ion exchange capacity than its inorganic counter parts is attributed due to the presence of freely movable H+ ions and H2O molecules situated in the cavities of the polymeric matrix. Due to its high cation (H+ ion) exchange capacity sample was selected for catalytic studies.
Mass % of C-8.09%, N-0.35%, O-7.6%, Zr-26.99%, Te-22.36%, W-34.61% was found from EDS analysis.
The catalyst was found to be quite stable in different concentrations of mineral acids such as 10 M HNO3, 5.0 M H2SO4 and 12 M HC1, 0.05 M solutions of bases and organic solvents like ethanol, acetone, CCl4,DMSO,DMF etc.
Thermal effect on IEC in Figure. 1 shows a gradual decrease in ion exchange capacity with increase in temperature for PANIZrTeW. It is evident from the data that composite material PANI-ZrTeW retains 80% of its IEC up to 250°C. After this temperature the decrease in IEC may be due to decomposition of organic moieties from the composite exchanger.
The effect of size and charge on ion exchange capacity for PANI-ZrTeW were studied for alkali metals and alkaline earth metal cations. The order was found to be Li+ < Na+ < K+; Mg2+ < Ca2+< Ba2+ (Figure. 2).
This sequence is in accordance with the hydrated radii of the exchanging ions [17]. The ions with smaller hydrated radii easily enter the pores of the exchanger, resulting in higher sorption [18]. pH titrations were performed for NaCl/NaOH, KCl/KOH systems (Figure. 3).
It shows two inflection point which indicated the nearly bifunctional behavior for the exchanger due presence of two ionizing groups, i.e., strong and weak acidic H+ ions present in the composite material ionize. The exchange capacity obtained from the curve is in agreement with that obtained by the experimental method. It appears to be a strong cation exchanger as indicated by a low pH (~2) of the solution when no OH- ions were added to the systems. The solution is progressively neutralized with the addition of hydroxide ions and at the same time ion exchange process is going to completion. Thus, the curve shows gradual rise of pH with the addition of OH-and become constant after complete neutralization of the cation exchanger.
FTIR spectrum of ZrTeW in Figure. 4a shows a broad band in the region 3200–3500 cm-1 and a sharp peak in the region 1600 cm-1. These bands justify the presence of – OH stretching and bending mode. A sharp peak at 1387 cm-1 is attributed to W-O bond. The band observed in the 650-967 cm-1 region is due to the symmetric and asymmetric stretching of M-O-H bond. In the IR spectrum of PANI-ZrTeW (Figure. 4b), an assembly of bands in the region 1400–1600 cm-1 may be ascribed to the stretching vibration frequency of C–N bonds and a band around 3100 cm-1 may be related to the stretching of NH bonds of benzenic and quinonic rings present in the material [19]. A broad band around 3000 cm-1 results from symmetric and assymetric stretching vibration of H2O molecule. A peak observed at 1289 cm-1 was attributed to stretching vibration of C-H in the benzenoid ring and that observed at 1474 cm-1 was attributed to C=N stretching vibration of quinoid ring [20]. A band at 1576 cm-1 indicates the C-C stretching vibration of quinoid ring [21]. Also, a broad band in the region 2371 cm-1 was characteristics of N-H bending vibration. A sharp band at 790 cm-1 shows the presence of para substituted aromatic rings, indicating polymer formation. Other characteristic band associated with inorganic precipitate is reflected with slight shift in the composite material. It gives an evidence for the encapsulation ZrTeW in the polymeric matrix of polyaniline and forms PANI-ZrTeW composite material.
Thermogram of ZrTeW in Figure. 5a shows 16% loss of weight at around 102°C due to the evaporation of external water molecules and condensation of structural hydroxyl groups. Above this, heat energy changes without any appreciable weight loss. This may be due to phase changes of the material. As the temperature increases, it shows a gradual weight loss and about 77% of the weight is lost when the temperature reaches 900°C. From the TG scans of PANI-ZrTeW (Figure. 5b) it is evident that the composite material also shows the same decomposition pattern as its inorganic counterpart up to 500°C, after increasing heat energy up to 900ºC the thermogram shows that 46% of the material is retained. This clearly reveals that the composite material is quite stable at high temperatures than ZrTeW and can be used in high temperature applications. XRD pattern of the ZrTeW and PANI-ZrTeW is given in Figure. 6.
SEM micrographs of ZrTeW and PANI- ZrTeW in (Figure. 7a and 7b) and (Figure. 7c and 7d) shows quite similar morphology confirmed the development of particles with almost same shapes and dimensions. Both samples have voids and can be attributed to transportation of ions between solid exchanger and external solution. The particles were broad in size range, having an irregular shape and no sign of crystalline structure.
Catalytic experiments
In order to evaluate the catalytic activity of the composite material sucrose hydrolysis was selected as model reaction due to its high ion exchange capacity. For the optimization of reaction conditions such as catalyst loading, initial concentration of sucrose on the hydrolysis, temperature effect and pH effect a detailed experiment was conducted.
Figure. 8a summarizes the optimum pH values for the maximum hydrolysis of sucrose by PANI-ZrTeW. It can be seen that maximum hydrolysis of 91% was attained in 30 minutes at a pH value of 2. It can also observe that with increase in pH% hydrolysis of sucrose was decreased; therefore pH 2 was selected as an optimum value. After adding the PANI-ZrTeW composite catalyst, pH of the sucrose solution was shifted to acidic region due to the presence of high H+ ions present in the catalyst. The composite material contains functional group such as -NH2, -NH, -OH with the change in the solution pH behavior of these functional group also changes. At low pH values most of these functional groups are present in protonated form and can furnish large number of H+ ions in aqueous solution, which helps to increase the rate of inversion of sucrose.
Figure 8: (a) pH effect (b) Temperature effect (c) Effect of conc. of sucrose (d) Catalyst dosage effect on sucrose inversion by PANI-ZrTeW.
Temperature of the solution is an important parameter that affects the rate of inversion of sucrose; an increase or decrease of temperature of will change the % hydrolysis of sucrose by composite catalyst and is depicted in Figure. 8b. It seems that there is an increase in rate of inversion of sucrose with increase in temperature. Also, shows a net gain 37% yield in the reaction when the temperature increases from 30°C to 50°C. Thus 50°C was selected as the optimum temperature for the reaction by PANI-ZrTeW.
In order to study the effect of concentration of sucrose on % yield of TRS a reaction with a temperature of 50°C, pH of 2 and catalyst dosage of 1.0 g was chosen and is depicted in Figure. 8c. About 94% yield of TRS was observed within 60 minutes by using 0.5 M sucrose concentration and was preferred as optimum concentration of sucrose. Further increase of concentration has not that much increase in the yield because still the catalyst amount was 1.0 g which already releases its maximum H+ ions in solution.
Effect of catalyst dosage on the hydrolysis reaction of 0.5 M sucrose was shown in Figure. 8d by keeping a temperature of 50°C and a pH of 2. Which shows an increase in % hydrolysis with increase of amount of catalyst. This means that acid concentration played an important role in hydrolysis reaction because use of larger amount of composite catalyst resembles the use of larger amount of soluble strong acid. An approximate gain of 40% was observed when the catalyst dosage was increased from 0.2 g to 1.0 g.
Reusability of the catalyst
After catalytic experiments the catalyst was separated and regenerated by acidifying with 1.0 M HNO3. Its reusability as heterogeneous catalyst has been tested in six cycles.
Figure. 9, a gradual decrease in catalytic activity of PANI-ZrTeW was observed from first cycle to sixth cycle, which may be due to the loss of some amount of catalyst in washing after every cycle. The total loss of catalyst activity after six runs was 8% of the starting amount of PANI-ZrTeW.
Conclusion
In the present study, an ambient reaction condition was developed to encapsulate zirconium (IV) tellurotungstate in the matrix of polyaniline for composite catalyst preparation. Spectral analysis confirmed the composite formation. The composite material is stable at high temperature and exhibiting promising ion exchange capacity. Due to high cation exchange capacity of the material, it can be successfully used as a heterogeneous catalyst for sucrose inversion.
Acknowledgment
Author gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award senior research fellowship. The author is also gratefully acknowledged to STIC, Cochin for instrumental support.
References
- Klass D L. Biomass for Renewable Energy, Fuels, and Chemicals, Academic Press; San Diego, Entech International, Inc. 1998;193-212
- Lange J P, Heide E, Buijtenen J, et al. Furfural a promising platform for lignocellulosic biofuels. Chem Sus Chem. 2012;9;5(1):150-66.
- D. Hayes. An examination of biorefining processes, catalysts and challenges. Catal. Today. 2009;145,138-51.
- Corma A, Iborra S and Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007;107(6):2411-502.
- Chambré D, Cornelia I and Maria-Raluca S. The reaction conditions influence on sucrose acid hydrolysis studied by means of DSC method. J. Therm.Anal.Calorim. 2017;88(3)681-6.
- Moreau C, Durand R, .Aliès F, et al. Hydrolysis of sucrose in the presence of H–form zeolites, Ind. Crops Prod.2000;11;237- 42.
- Masroua A, Revillon X, Martin JC,et al. Bull. Soc. Chim. Fr.1988;3,561.
- Dhepe PL, Ohashi M, Inagaki S,et al. Hydrolysis of sugars catalyzed by water-tolerant sulfonated mesoporous silicas. Catal. Lett. 2005;102(3)163-9.
- Iloukhani H, Azizian S and Samadani N. Hydrolysis of sucrose by heterogeneous catalysis. Phys. Chem. Liq. 2002;40;159- 65.
- Nimisha KV, Aparna M and Janardanan C, Efficacy of Zirconium(Iv) Tellurotungstate Encapsulated in The Matrix of Polyaniline for Heavy Metal Ion Separation Res. J. Rec. Sci. 2014;3(12)60-66.
- Vogel AI. A text book of quantitative inorganic analysis. Longman Group Limited; London, (1975).
- Topp NE and Pepper KW. Properties of ion-exchange resins in relation to their structure. Part I. Titration curves J. Chem. Soc. 1949;690, 3299-303.
- Helfrich H, “Ionites”, Foreign Literature;Moscow ,(1962).
- Grissbach,R. Theory and Practice of the Ion Exchange. Foreign Literature;Moscow,( 1963).
- Gregor H, Hamilton MJ and Becher J. Studies on Ion Exchange Resins. XIV. Titration, Capacity and Swelling of Methaerylic Acid Resins. J. Phys. Chem.1955;59(9);874-81.
- Cláudia A, Luciares C, Andressa M, et al. Sucrose hydrolysis catalyzed by auto-immobilized invertase into intact cells of Cladosporium cladosporioides, Electron J Biotechnol.2005;8(1)55-62.
- Preetha B, Janardanan C. UV -Visible Diffuse Reflectance spectroscopic studies on Mn and Cu ion exchange of newly synthesized cerium zirconium antimonate and its application in dye degradation. Res.J.Recent.Sci. 2012;1(ISC-2011),85- 92.
- Qureshi M, Kumar R, Kaushik R.C. Synthesis and Ion-Exchange Properties of Reproducible Stannic Molybdoarsenate. Separations of Ba2+–La3+, Mg2+–La3+, Sr2+–Y3+, and Sr2+–La3+ Sci. Technol. 1978;13(2) 185-92.
- Sen S, Dipak D, and Aninda J. Ultra-small sulphur nanoparticles configured inside a flexible organic mixed conducting network as a cathode for lithium–sulphur batteries. J.Mater. Chem A. 2015;3(42)20958-65.
- Dutta D, Sarma TK, Chowdhury D et al. A polyaniline-containing filter paper that acts as a sensor, acid, base, and endpoint indicator and also filters acids and bases. J.colloid interface sci. 2005;283(1)153-9.
- Gope S, Dutta D, Bhattacharyya AJ. Confining Sulfur within a Zeolite Host Wrapped inside Conducting Polymer Sheaths as Cathode for Li?S Battery. Chemistry Select. 2016;1(4)728-735.

