Original Article
, Volume: 13( 2)Phytochemical Screening, Total Phenolics, Flavonoids Contents, Total Antioxidant Capacity and Antifungal Activity of Cymbopogon giganteus Extracts from Burkina Faso
- *Correspondence:
- Dabiré CM Laboratory of Applied Organic Chemistry and Physics (L.C.O.P.A.), Ouaga University I Pr. Joseph KI-ZERBO, UFR/SEA 03 BP 7021 Ouagadougou 03, Burkina Faso, Tel: +226 25 30 70 64; E-mail: dabireconst@yahoo.fr
Received Date: September 21, 2017 Accepted Date: November 30, 2017 Published Date: December 04, 2017
Citation: Bationo RK, Dabiré CM, Hema A, et al. Phytochemical Screening, Total Phenolics, Flavonoids Contents, Total Antioxidant Capacity and Antifungal Activity of Cymbopogon giganteus Extracts from Burkina Faso. Nat Prod Ind J. 2017;13(2):114
Abstract
Extracts obtained from roots, stems, leaves and flowers of Cymbopogon giganteus using hexane, dichloromethane (DCM), ethyl acetate and methanol were screened for their phytochemicals, total phenolics content, (TPC), total flavonoids content (TFC), total antioxidant content (TAC) and for their antifungal activity. Tannins, flavonoids, phenolic acids and alkaloids were mainly found in extracts, depending of the solvent used for extraction.
TAC were evaluated using Ferric Reducing Antioxidant Power (FRAP) and DPPH radical scavenging assays. Results from FRAP assay showed that methanolic flower extracts had highest TAC (112.94 ± 2.4 μg TE/mg of extracts), followed by leaves (68.56 ± 7.53 μg TE/mg of extract), roots (45.17 ± 4.28 μg TE/mg of extracts) and stems (39.21 ± 2.34 μg TE/mg of extract). The highest TPC were found in methanolic flowers extracts (171.17 ± 1.42 GAE/mg of extract) whereas DCM and ethyl acetate roots extracts exhibited the best TFC with respectively 348.34 ± 12.48 and 323.86 ± 31.55 μg of QE/mg of extract.
Only methanolic extracts of C. giganteus different parts were tested at 10 mg/mL on four local fungi. Results showed that methanolic roots extracts had an inhibitory effect of 16.16%, 19.15% and 10.79% respectively on the radial growth of Fusarium moniliforme, Phoma sorghina and Aspergillus flavius. Methanolic flowers extracts only inhibited Fusarium moniliforme radial growth at 19.4171% and Aspergillus flavius at 7.11%. None of plant parts extracts showed an inhibitory effect on Bypolaris orezae.
Keywords
Cymbopogon giganteus; Phenolics; Flavonoids; Antioxydant; Antifungal
Introduction
Fungi are responsible for agricultural losses, both while crops are growing and when they are later stored. During storage, fungi can make food crops unfit for consumption, by changing the nutritional value of seeds or producing mycotoxins that are harmful for human and animal health [1]. Many fungi are known to be indirectly responsible for allergic or toxic disorders among consumers because of the production of mycotoxins or allergens.
Phoma sorghina, Fusarium moniliforme, Aspergilus flavus, Bypolaris oryzae are common fungi that cause alterations during development stages including post-harvest of some of local plant such as sorghum, maize, millet, rice. Generally these phytopatogenic fungi are controlled by synthetic fungicides; however the use of these fungicides is increasingly restricted due the harmful effect of pesticides on human health and the environment [2]. The main toxic effect are carcinogenicity, genotoxicity, hepatotoxicity, reproductive disorders and immunosuppression [3,4]. A sizeable portion of world population living below poverty line in the developing countries are suffering from health problem associated with consuming mycotoxin contaminated grains or cereals [5]. Even though effective and efficient control of seed borne fungi of seeds can be achieved by the use of the synthetic chemical fungicides, the same cannot be applied to grains for reasons of toxicity [2]. Thus, there is a need to search for alternative approaches to store grains/cereals for human consumption without toxicity problems.
Plants extracts have been reported to exhibit antibacterial, antifungal and insecticidal properties [6,7]. Furthermore, many plants are known to contain a number of secondary metabolites like phenols, flavonoids, quinines, essential oils, alkaloids, saponins and steroids. Previous works showed that there is an important link between phenolic compounds, mainly flavonoids and most of the biological activities such as antioxidant, anti-inflammatory and antifungal activities [8,9]. On the other hand, several works demonstrated in laboratory trials that plant parts, such as roots, leaves, seeds, stems and flowers possess inhibitory properties against bacteria, fungi and insects [10]. The present work aimed to investigate for antifungal biological activity of extracts from different parts of Cymbopogon giganteus from Burkina Faso. This plant was selected according to reported data on its biological activities [11]. Cymbopogon giganteus is used in local African medicine to cure diseases such as rheumatism, fever, cough, skin disorders (decoctions of leaves and flowers) and arterial hypertension [12]. Furthermore, previous works only focused on essential oil (volatile compounds) of Cymbopogon giganteus [11] from Burkina Faso, but no study has been undertaken on non-volatile compounds in our knowledge. Because of the possible link between phenolic compounds and biological activities, all the extracts obtained from roots, leaves, stems and flowers of this plant were first screened for their phytochemicals, phenolics, flavonoids and total antioxidant contents; subsequently, the most potent extracts were tested for their antifungal activity.
Materials and Methods
Materials
Plant material: Plant material (roots, stems, leaves and flowers) of Cympobogon giganteus were collected in 2015, in an experimental field, at Applied Science and Technology Research Institute, Natural Products Department (Ouagadougou, Burkina Faso). Plant has been identified and described by the Laboratory of Vegetal Biology and Ecology of University of Ouagadougou, where a specimen was filed. All the samples were shade dried for 15 days. After drying, plant materials were crushed.
Biological material: Four fungi (Phoma sorghina, Fusarium moniliforme, Bipolaris oryzae and Aspergillus flavus) were produced from grains or leaves of sorghum, maize, millet and rice collected in Burkina Faso. Once infested grains identified, each fungus was removed using a needle and placed in a petri dish containing a culture medium (malt agar). The petri dish was then incubated at 25°C under a UV lamp light, for at least 5 days. The fungi were identified and isolated by the laboratory of Phytopathology of Nazi Boni University of Bobo.
Methods
Preparation of plant extracts: 50 g of each plant part were successively extracted (three times) with 300 mL of hexane, dichloromethane, ethyl acetate and methanol by cold maceration under magnetic stirring for 24 h. The solutions were filtered, concentrated under reduced pressure to yield hexane extract (HE), dichloromethane extract (DCME), ethyl acetate extract (EAE) and methanol extract (ME). Thus, sixteen extracts were prepared.
Phytochemical screning: All the extracts prepared were screened for several chemical groups: tannins, alkaloids, flavonoids, saponins, anthracenosids, phenolic acids, using the methods described by Wagner et al. [13]
Determination of total phenolics content (TPC): Total phenolics content was determined by the Folin-Ciocalteu method as described by Nihal et al. [14]. Gallic acid was used as standard. A calibration curve was first established using gallic acid solutions at concentrations ranging from 0, 25 μg/mL, 50 μg/mL, 75 μg/mL and 100 μg/mL. Then 60 μL of the Folin-ciocalteu reagent (FC-R 1:10 dilution), were added to the solutions of gallic acid. The mixtures were left to stand at room temperature for 8 min and then, 120 μL of sodium carbonate solution (Na2CO3), 7.5% were added to neutralize the residual reagent. The absorbance was recorded at 700 nm after incubation for 30 min at 37°C. After that, 60 μL of each extract dissolved in DMSO, suitably diluted and were added to 60 μL of FC-R, instead of gallic acid. The total phenolics contents, determined from the equation of the calibration curve, were expressed as μg of gallic acid equivalent/mg extract (μg GAE/mg).
Total flavonoids content (TFC) measurement: Total flavonoids content was measured using aluminum chloride colorimetric assay as described by Zhishen J et al. [15] with slight modifications (adapted for microplates). A calibration curve was established using different concentrations (0, 25 μg/L, 50 μg/L, 75 μg/L and 100 μg/L) of Quercetin solutions. Briefly, 25 μL of plant extracts (properly diluted) or standard solution of Quercetin at different concentration was added to 150 μL of distilled water. Then 10 μL of 5% NaNO2 was added. After 5 min, 10 μL of 10% AlCl3 were added. After 6 min, 50 μL of NaOH 1 M were added. After incubation for 30 min at 37°C, absorbance of the mixture was recorded at 415 nm. Tests were carried out in triplicates and results were expressed as μg of Quercetin equivalent/mg of extract (μg QE/mg).
Evaluation of total antioxidant content (TAC): TAC of extracts were determined using two (02) methods: 2, 2-diphenyl-1-picryhydrazyl (DPPH) radical scavenging assay and Ferric Reducing Antioxidant Power (FRAP). Trolox was used as reference. All the tests were carried out using a microplate reader (spectrophotometer MP96, SAFAS) and were performed in triplicates.
DPPH radical scavenging test: This test was performed according to the method described by Lamien-Meda et al. [16] with slight modifications and adapted for microplates. A calibration curve was first established using standard solutions of Trolox at different concentrations (10 μg/mL to 50 μg/mL). For this purpose, briefly, 200 μL of DPPH solution 10-4 M were added to 50 μL of Trolox solutions. The mixtures were allowed to stand for 10 min in the dark at room temperature and then, absorbance was recorded at 517 nm.
After that, 50 μL of each extract suitably diluted were mixed with 200 μL of DPPH solution. The absorbance was recorded at 517 nm, 10 min after the start of the reaction. Total antioxidant capacity was determined from the equation of calibration curve and results were expressed as μg of Trolox equivalents/mg extract (μg TE/mg).
Ferric Reducing Antioxidant Power (FRAP) assay: In this method, 2, 4, 6-Tripyridyl-s-Triazine (TPTZ) was used as oxidant substratum [17]. The FRAP reagent was prepared by mixing 1 mL TPTZ (10 mM), 10 mL of sodium acetate buffer (pH=3.6) and 1 ml of solution of ferric (III) chloride, 20 mM. As in DPPH scavenging assay, a calibration curve was established using Trolox solutions at different concentrations (10 μg/mL to 50 μg/mL). Then, 200 μL of FRAP reagent were added to 50 μL of each of the previous solutions of Trolox, followed by 30 μL of distilled water. The absorbance of the intense blue color mixture was read after 10 min at 595 nm.
For the extract, 50 μL of each sample properly diluted was used instead of Trolox. Total antioxidant capacity was determined from the equation of calibration curve and results were expressed as μg of Trolox equivalents/mg extract (μg TE/mg).
Antifungal activity of the extracts: This test was performed using the method described by Bonzi et al [1]. Growing fungi and their isolation: Fungi to be tested were grown in a fresh malt agar medium contained in petri dishes of 9 cm diameter. From a l0 days-old colony four mycelium explants of identical size (5 mm) were placed in the center of a petri dish containing culture media with or without extract to be tested. All the extracts were dissolved in DMSO 1% and tested at 10 mg/mL. Calcio C was used as positive control and was tested at 1 mg/mL. The inoculated plates were incubated in the heat chamber at 28°C and keep in the dark for 10 days (Bonzi et al., 2012).
Evaluation of antifungal activity: The evaluation of the radial growth involved tracing on the lid of the petri dish two perpendicular lines through the center of the explant. The diameters of the mycelium colonies (in cm) were measured every two (02) days for 10 days after incubation. The tests were performed in triplicate. The percentage inhibition of each sample is calculated using the following formula:
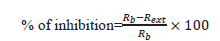
Where, Rb: radius of the blank (without extract);
Rext: radius of the extract.
Results and Discussion
Phytochemical screening
Results are presented in Table 1; the presence or absence of chemical groups depends on the plant tissue and the solvent used for extraction.
| Extracts | Tannins | Flavonoids | Alkaloids | Anthracenosides | Saponins | Phenolic acids | |
|---|---|---|---|---|---|---|---|
| Hexane | - | + | + | - | - | + | |
| Flowers | DCM | - | - | - | - | - | - |
| Eth. Ac. | - | + | + | + | - | + | |
| MeOH | + | + | + | + | - | + | |
| Hexane | - | + | + | - | - | + | |
| Leaves | DCM | - | - | - | - | + | - |
| Eth. Ac. | - | - | - | - | - | - | |
| MeOH | + | + | - | + | + | + | |
| Hexane | - | - | + | - | - | - | |
| Stems | DCM | - | - | + | - | + | - |
| Eth. Ac. | - | + | - | + | - | - | |
| MeOH | + | + | - | + | - | + | |
| Hexane | - | - | + | - | - | - | |
| Roots | DCM | - | - | + | + | + | - |
| Eth. Ac. | - | - | - | + | - | - | |
| MeOH | + | + | - | + | + | + | |
+: presence ; -: absence; Eth. Ac.: Ethyl Acetate ; DCM: Dichloromethane ; MeOH: Methanol
Table 1. Chemical groups in different tissues of C. giganteus extracts.
| Extracts | Total phenolic content (µg GAE/mg of extract) | |||
|---|---|---|---|---|
| Flowers | Leaves | Stems | Roots | |
| DCM | 34.44 ± 1.21 | 32.63 ± 0.33 | 14.94 ± 0.32 | 17.52 ± 0.34 |
| Eth. Ac. | 50.17 ± 0.60 | 44.57 ± 0.24 | 20.95 ± 1.56 | 30.17 ± 0.52 |
| MeOH | 171.17 ± 1.42 | 132.95 ± 2.20 | 98.55 ± 2.14 | 51.93 ± 0.17 |
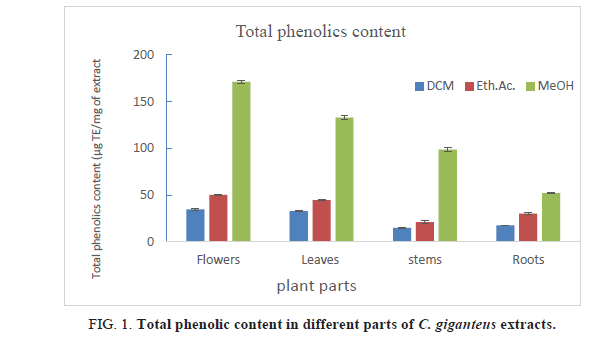
Table 2. Total phenolic content in different parts of C. giganteus extracts.
Flower extracts, whatever the solvents used, did not contain saponins. Tannins and anthracenosides were rather present in methanol extract. However, flavonoids, alkaloids and phenolic acids were all present in hexane, ethyl acetate and methanol extracts. Thus, except sponins, C. giganteus flower extracts contain most of the chemical groups screened.
Results from ethyl acetate leaves extracts did not show the presence of any chemical groups while most of the chemical groups were found in methanol extracts. Saponins were found only in DCM extract. Methanol seems to be the suitable solvent to obtain these chemical from C. giganteus leaves.
Methanol stems extracts are rich in tannins, flavonoids, anthracenosides and phenolic acids but did not contain alkaloids and saponins. No chemical group could be found in hexane and DCM stems extracts, except alkaloids and saponins.
Anthracenosides, alkaloids and saponins were highly present in roots extracts. Tannins and flavonoids could be found only in methanol roots extracts. C. giganteus roots extracts did not contain phenolic acids.
So many chemicals could be found in C. giganteus different parts, depending on the type of solvent used for the extraction. This plant might certainly have a broad use against many diseases.
TPC in different parts of C. giganteus varied from 14.94 μg GAE/mg of dried extract for DCM stems extract to up to 171.17 μg GAE/mg of dried extract for Methanol flowers extract. Flowers possessed highest TPC compared to others different parts. Total phenolics contents in flowers are 34.44 μg GAE/mg, 50.17 μg GAE/mg and 171.17 μg GAE/mg of extract respectively in DCM, ethyl acetate and methanol extracts. On the other hand, methanol extracts had highest TPC (51.93 to 171.17 μg GAE/mg of extract), followed ethyl acetate extracts (20.95 μg GAE/mg to 50.17 μg GAE/mg of extract) and DCM extract (14.94 to 34.44), as it’s clearly shown in Figure 1. Methanol, as well as ethanol, is suitable solvents to extract phenolic compounds but methanol might extract many other compounds that are not phenolics. These results are matched with phytochemical results which showed that methanol extract contained many chemical groups.
These TPC are largely higher than those reported in Lippia mulflora leaves extracts according to our previou s works [9]. Furthermore, the methanol extracts of C. giganteus flowers and leaves contained higher TPC than those reported by Kouassi et al [18] in C. citratus methanol leaves extracts from Ivory Coast (118.14 mg GAE/g of extract). DCM extracts showed the lowest TPC but these values are higher compared to TPC reported by Karanga et al. [19] in different extracts of Euphorbia hirta from Burkina Faso.
C. giganteus different parts extracts, whatever the solvent used, contained strong phenolic contents, so this plant could be used as natural source of phenolic compounds.
Total flavonoids content (TFC)
Total flavonoids contents (TFC), expressed as μg of Quercetin equivalent/mg of extract, are gathered in Table 3. Results showed that:
| Extracts | Total flavonoids content (µg QE)/mg of extract) | |||
|---|---|---|---|---|
| Flowers | Leaves | Stems | Roots | |
| DCM | 93.91 ± 2.83 | 147.55 ± 23.24 | 207.20 ± 4.60 | 348.34 ± 12.48 |
| Eth. Ac | 132.49 ± 4.701 | 270.41 ± 32.20 | 189.38 ± 11.62 | 323.86 ± 31.55 |
| MeOH | 250.12 ± 14.94 | 134.23 ± 4.051 | 58.31 ± 8.01 | 159.02 ± 28.41 |
QE: Quercetine Equivalent
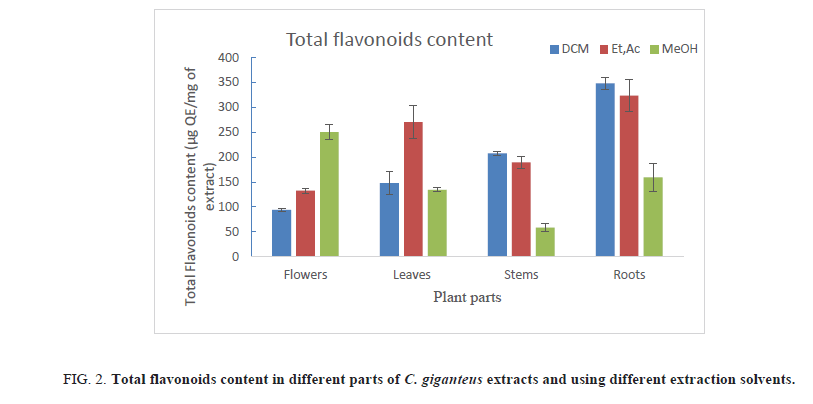
Table 3. Total flavonoids content of different parts of C. giganteus extracts.
1. Using DCM as extraction solvent, roots had the highest TFC, followed by stems, leaves and flowers. TFC values in these different plant parts are respectively 348.34 ; 207.2, 147.55 and 93.91 μg QE/mg of extract;
2. In ethyl acetate extracts, roots presented the highest TFC (323.86 μg QE/mg of extract) compared to leaves (270.4 μg QE/mg of extract);
3. In methanol extracts, the highest TFC were found in flowers (250.12 μg QE/mg of extract) and the lowest were noted in stems extracts.
Results also showed that the type of solvent used to extract phenolics and flavonoids plays an important role. Indeed, phenolic compounds were easily extracted by methanol whereas ethyl acetate and sometimes, DCM are appropriate to extract flavonoids (Figure 2). Although C. giganteus flowers are rich in phenolic compound, its roots remain the plant part with highest TFC, whatever the solvent used for extraction (Table 3).
Figure 2: Total flavonoids content in different parts of C. giganteus extracts and using different extraction solvents.
Total antioxidant content (TAC) of different parts of C. gyganteus extracts
TAC were measured using two different methods: DPPH radical scavenging test and Ferric Reducing Antioxidant Power (FRAP) assay. Results are presented in Table 4. We note that C. giganteus different parts possessed significant TAC. Using DPPH test, the highest TAC could be found in flowers (12.52 μg of TE/mg to 100.40 μg of TE/mg of extract), then in leaves (17.63 μg of TE/mg to 57.15 μg of TE/mg of extract) and stems (16.74 μg of TE/mg to 39.21 μg of TE/mg of extract) and at last, in roots (9.33 μg of TE/mg to 38.68 μg of TE/mg of extract). Results also showed that for each plant part, methanol extracts exhibited the highest TAC compared to ethyl acetate and DCM extracts. For flowers extracts for example, 100 μg of TE/mg of extract were found in methanol extract, about three times the TAC in ethyl acetate extract and ten times in DCM extract. Results obtained by FRAP method were similar to those found with DPPH test, even if sometimes, FRAP assay seems to overestimate TAC in extracts.
| Total Antioxidant content (µg TE/mg of extract) | |||
|---|---|---|---|
| Flowers | Extracts | DPPH | FRAP |
| DCM | 12.52 ± 0.3 | 14.89 ± 1.47 | |
| Eth. Ac | 37.96 ± 4.02 | 38.2 ± 3.2 | |
| MeOH | 100.40 ± 8.41 | 112.94 ± 2.34 | |
| Leaves | DCM | 20.14 ± 0.05 | 20.30 ± 1.50 |
| Eth. Ac | 17.63 ± 1.67 | 19.67 ± 4.08 | |
| MeOH | 57.15 ± 6.47 | 68.56 ± 7.53 | |
| Stems | DCM | 16.74 ± 0.00 | 18.16 ± 2.38 |
| Eth. Ac | 27.47 ± 2.55 | 36.73 ± 0.91 | |
| MeOH | 39.21 ± 2.34 | 31.46 ± 6.52 | |
| Roots | DCM | 9.33 ± 1.79 | 7.60 ± 8.45 |
| Eth. Ac | 16.17 ± 3.27 | 13.77 ± 2.44 | |
| MeOH | 38.68 ± 2.63 | 45.17 ± 4.28 | |
TE: Trolox Equivalent
Table 4. Total antioxidant content (TAC) C. in giganteus different parts extracts.
It is well known that extracts with strong TPC and TFC generally present higher TAC [20]. In this work, methanolic extracts of flowers, leaves, stems and roots of C. giganteus were found to possess, in most of cases, highest TPC, TFC and TAC. So methanol extracts of different parts of this plant were tested for their antifungal activity.
Antifungal activity
The percentage of inhibition of each methanol extract, tested at 10 mg/mL on the four fungi, was calculated every two days up to ten days; results are gathered in Table 5.
| % of inhibition of methanol extracts | |||||
|---|---|---|---|---|---|
| Plant parts | Duration | Fusarium moniliforme | Phoma sorghina | Aspergilus Flavus | Bypolaris orezae |
| Flowers | 2nd day | 22.31 ± 5.36a | -2.39 ± 15.55a | 10.56 ± 6.9a | -34.49 ± 11.72c |
| 4th day | 16.23 ± 4.17a | -9.13 ± 13.95a | 8.33 ± 8.3a | -85.18 ± 11.95b | |
| 6th day | 13.66 ± 12.80a | -26.47 ± 18.6a | 2.36 ± 0.01a | -114.05 ± 15.6b | |
| 8th day | 25.87 ± 5.60a | -3.47 ± 0.6a | 1.27 ± 0.54a | -168.35 ± 21.04a | |
| 10 th day | 19.00 ± 6.39a | ND | 13.04 ± 2.4a | -182.4 ± 16.75a | |
| Leaves | 2nd day | 18.160 ± 4.77b | -11.81 ± 15.77a | ND | ND |
| 4th day | 3.865 ± 4.64ab | -38.27 ± 21.4a | ND | ND | |
| 6th day | 1.610 ± 6.70ab | -55.43 ± 18.8a | ND | ND | |
| 8th day | 2.197 ± 6.29ab | -25.00 ± 0a | ND | ND | |
| 10 th day | -1.839 ± 3.18 a | ND | ND | ND | |
| Stems | 2nd day | 9.857 ± 6.72a | -3.99 ± 2.55a | -9.86 ± 6.3a | -55.8 ± 9.0c |
| 4th day | 1.018 ± 11.24a | -23.72 ± 3.22a | -1.81 ± 0.8a | -122.5 ± 19.01bc | |
| 6th day | 4.659 ± 4.20a | -33.61 ± 1.18a | -3.53 ± 5.18a | -144.0 ± 35.03ab | |
| 8th day | 5.814 ± 5.89a | -12.96 ± 9.4a | -6.98 ± 1.4a | -202.7 ± 35.76a | |
| 10 th day | 10.321 ± 5.58a | ND | 2.56 ± 5.7a | -186.1 ± 25.7ab | |
| Roots | 2nd day | 20.16 ± 4.49a | 21.11 ± 4.32a | 8.9a ± 2.76 | -45.00 ± 18.02b |
| 4th day | 17.30 ± 4.4a | 20.81 ± 4.23a | 9.09 ± 2.14 | -64.46 ± 24.32ab | |
| 6th day | 9.17 ± 8.05a | 16.17 ± 6.77a | 12.45 ± 5.9 | -81.17 ± 10.08ab | |
| 8th day | 15.20 ± 3.46a | 19.68 ± 4.62a | 10.15 ± 0.5 | -102.74 ± 22.64a | |
| 10th day | 19.00 ± 8.27a | 18.01 ± 1.32 | 13.4 ± 5.6 | -93.85 ± 33.36ab | |
ND: Not Determined
Table 5. Percentage of inhibition of methanol extracts of C. giganteus different parts on Fusarium moniliforme, Phoma sorghina, Aspergilus Flavus and Bypolaris orezae.
Positive values mean that extract has inhibitory effect on fungi; negative values mean that either extract has no inhibitory effect or extract stimulates fungus radial growth. Means (positive) values of inhibition percentage are presented in Table 6.
| Percentage of inhibition of extracts (%) | |||||
|---|---|---|---|---|---|
| Fungi | Flowers | Leaves | Stems | Roots | Calcio C* |
| Fusarium monififorme | 19.41 | 6.45 | 6.33 | 16.16 | 100 |
| Phoma sorghina | NIE | NIE | NIE | 19.15 | 100 |
| Aspergilus Flavus | 7.11 | NIE | NIE | 10.79 | 100 |
| Bypolaris orezae | NIE | NIE | NIE | NIE | 85.02 |
NIE: No inhibitory effect; Calcio C* was tested at 1 mg/mL
Table 6. Mean values of percentage of inhibition of each extract on Fusarium moniliforme, Phoma sorghina, Aspergilus Flavus and Bypolaris orezae.
Results showed that all the extracts inhibited the radial growth of F. moniliforme. Methanol flowers extract had the highest antifungal activity (19.41%), followed by roots (16.16%), leaves (6.45%) and stems extract (6.33%) (Table 6). These values are weak, compared to those reported by Karanga et al. [19]. Indeed, these authors found that F. moniliforme was sensitive to ethanol-water crude extracts, ethyl acetate and butanol extracts of E. hirta L. At a concentration of 5 mg/mL, they recorded percentages of inhibition of 47.00%; 58.33% and 57.00% respectively.
In contrario, none of extracts exhibited an inhibitory effect on Bypolaris orezae. Indeed this fungus seems to be hardly controlled by natural extract; even calcio C, a potent antifungal product, did not fully control its radial growth. For Phoma sorghina, only roots extracts had an inhibitory effect on its radial growth. This inhibitory effect was low (19.15%) compared to the values reported by Karanga et al. [19] who found that E. hirta extracts, at 5 mg/mL, showed percentages of inhibition on this fungus ranged from 22.00% to 44.46%.
Aspergilus flavus was weakly sensitive to flowers extract (7.11%) and roots extracts (10.79%) but no inhibitory effect was observed for leaves and stems extracts. Pane et al. [21] also tested Hyptis spicigera, C. giganteus and C. citratus extracts on several strains of Aspergilus. They found that at concentration of 10 mg/mL, C. giganteus essential oil showed a slight inhibitory effect on Aspergillus strains but no inhibitory could be observed for Hyptis spicigera essential oil and organic extracts. To our knowledge, antifungal activity of the organic extracts of C. giganteus plant parts has been carried out for the first time. Roots and flowers of this plant might contain bioactive compound with broad action on several fungi at once.
Phoma sorghina as well as Aspergillus flavus are generally less sensitive to several plant extracts as reported in many previous works [1,21,22]. Certainly, plant extracts might behave like nutritive substances for these kind of fungi; they enrich the culture medium and produce greater mycelium growth than the control. On the other hand, the weak inhibitory effect of our extracts on fungi could be explained by their poor diffusion in the culture medium. Indeed, phytochemical results showed that methanol extracts contained many chemical groups which can interfere, resulting in a weak diffusion. So further fractionation of the extracts might reduce their complexity and each fraction could be tested again for its antifungal activity. Further studies could be focused on C. giganteus roots and flowers extracts regarding their high TPC, TFC and antifungal activity.
Conclusion
The results obtained from this work showed that C. giganteus flowers, leaves, stems and roots contained several phytochemicals, particularly phenolic and flavonoids. All the different plant parts were more or less rich in phenolic and flavonoids compounds but flowers and roots were found to exhibit highest total phenolic, flavonoids and total antioxidant contents. Furthermore, methanolic extracts of flowers and roots both had an inhibitory effect on mycelium growth of Fusarium moniliforme and Aspergillus flavus but roots extracts also inhibited Phoma sorghina mycelium growth, a dangerous fungus which is generally hardly controlled by natural antifungal. C. giganteus roots and flowers extracts could be a natural source of antifungal as well as antioxidants. Further studies are need to determine the chemical compounds that are responsible for interested activities.
References
- Bonzi S, Somda I, Zida EP, et al. In vitro antifungal activity of various local plants extracts in the control of Phoma sorghina (Sacc.) Boerema and Collectotrichum graminicola (Ces) Wilson, as Sorghum seed mold pathogen in Burkina Faso. Tropicultura. 2012;30:103-6.
- Harris CA, Renfrew MJ, Woolridge MW. Assessing the risk of pesticide residues to consumers: Recent and future developments. Food Addit Contam. 2001;18:1174-78.
- Lacey J. The microbiology of cereal grains from areas of Iran with a high incidence of oesophageal cancer. J Stored Prod Res. 1988;24:39-50.
- Desjardins AE, Manandhar G, Plattner RD, et al. Occurrence of Fusarium species and mycotoxins in Nepalese Maize and Wheat and the effect traditional processing method on mycotoxin levels. J Agric Food Chem. 2000;48:1377-83.
- Majumder UK, Gupta M, Mukhopadhyay DK. Effect of mycotoxins isolated from Penicillium nigricans on glucose-6-phosphate dehydrogenase. Indian J Exp Biol. 1997;35:1233-36.
- Okigbo RN, Ogbonnaya UO. Antifungal effects of two tropical plant leaf extracts (Ocimum gratissimum and Aframomum melegueta) on post-harvest yam (Dioscorea spp.) rot. Afr J Biotechnol. 2006;5:727-31.
- Mohana DC, Raveesha KA. Anti-bacterial activity of Caesalpinia coriaria (Jacq.) Willd against plant pathogenic Xanthomonas pathovars: An eco-friendlyapproach. Journal of Agricultural Technology. 2006;2: 317-27.
- Ranaware A, Singh V, Nimbkar N. In vitro antifungal study of the efficacy of some plant extracts for inhibition of Alternaria carthami fungus. Indian J Nat Prod Resour. 2010;1:384-6.
- Dabiré CM, Bationo KR, Nebié HA, et al. Total phenolics content, flavonoids profiling and antioxidant activity of Lippia multiflora leaves extracts from Burkina Faso. Asian J Plant Sci Res 2015;5:28-33.
- Mattar MADR, Casali YA, Graciela S, et al. Antifungal activity of plant extracts used in folk medicine in Argentina. Rev Peru Biol. 2007;14:247-51.
- Menut C, Bessiére JM, Samaté D, et al. Aromatic plants of tropical West Africa. XI. Chemical composition, antioxidant and antiradical properties of the essential oils of three Cymbopogon species from Burkina Faso. J Essent Oil Res. 2000;12:207-12.
- Popielas L, Moulis C, Keita A, et al. The Essential Oil ofCymbopogon giganteus. Planta Med. 1991;57;586-87.
- Wagner H, Bladt S. Plant Drug Analysis. 996, 2nd ed. Munich.
- Nihal TY, Sedat V, Ferda S, et al. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484-96.
- Zhishen J, Mengscheng T, Jianming W. Food Chem. 1999;64:555-59.
- Lamien-Meda A, Lamien CE, Compaoré MMY, et al. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13:581-94.
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396-3402.
- Kouassi EK, Coulibaly I, Rodica P, et al. HPLC phenolic compounds analysis and antifungal activity of extract’s from Cymbopogon citratus (DC) Stapf against Fusarium graminearum and Fusarium oxysporum sp tulipae. Journal of Scientific Research and Reports. 2017;15:1-11.
- Karanga Y, Ilboudo O, Bonzi S, et al. Phytochemical and antifungal properties of Euphorbia hirta L. against Fusarium moniliforme and Phoma sorghina. Nat Prod Ind J. 2017;13:1-10.
- Ehsan Karimi, Hawa ZEJ, Sahida A. Phenolics and flavonoids profiling and antioxidant activity of three varieties of Malaysian indigenous medicinal herb Labisia pumila Benth. J Med Plants Res. 2011;5:1200-6.
- Ouattara-Sourabie PB, Nikiema PA, Traore A. Antifungal activity of Hyptis spicigera (Lamiaceae) extracts and essential oils of Cymbopogon citratus (Poaceae) and Cymbopogon giganteus against the growth of Aspergillus strains isolated in Burkina Faso. J Pharm Pharmacol. 2017;7:17-27.
- Satish S, Mohana DC, Raghavendra MP, et al. Antifungal activity of some extracts against important seed borne pathogens of Aspergillus sp. Journal of Agricultural Technology. 2007;3:109-19.