Original Article
, Volume: 15( 1)Phytochemical Investigation of Caralluma lasiantha: Isolation of Stigmasterol, an Active Immunomodulatory Agent
- *Correspondence:
- Suresh Babu K , Department of Chemistry, Mallareddy Engineering College, Hyderabad, Telangana, India, Tel: +919849180377; E-mail: babuiict@gmail.com
Received: December 02, 2016; Accepted: February 27, 2017; Published: March 03, 2017
Citation: Malladi S, Ratnakaram VN, Suresh Babu K, et al. Phytochemical Investigation of Caralluma lasiantha: Isolation of Stigmasterol,an Active Immunomodulatory Agent. Int J Chem Sci 2017;15(1):102.
Abstract
Stigmasterol, a phytosteroid was isolated for the first time from C. lasiantha using n-hexane as a solvent. Stigmasterol was characterized on the basis of physical, chemical and spectral data (IR, 1H NMR, 13C NMR, 1HNMR, DEPT-45, 90 & 135, and MS) analysis as well as by comparing them to their literature data. A sequence of steps was adopted like saponification, fractional crystallization and gradient elution column chromatography to isolate stigmasterol because some phytosterols possess identical physical properties which makes it difficult to isolate the constituents.
Keywords
Caralluma lasianth; Indian traditional medicine; stigmasterol isolation; phytochemical screening; structure elucidation
Introduction
Asclepiadaceae family consists of 2500 species falling under 200 genera. Caralluma is one of the genus of Asclepiadaceae family and grows up generally in dry areas [1]. Caralluma umbellata extracts are reported with superior antibacterial activity especially against bacteria causing stomach disorder and pain [2]. Caralluma lasiantha (syn. Boucerosia lasiantha) is very well known species of the genus. It is called with different local names like Sirumankeerai in Tamil and Kundeti Kommulu in Telugu [3]. It is a famous indoor ornamental plant and succulent in habit [4]. It is found in dry regions of Andhra Pradesh, India and especially in Anantapur and Chittoor districts. It is used as traditional medicine to reduce body heat [5]. Methanolic extracts of Boucerosia lasiantha (Caralluma lasiantha) have shown good antioxidant activity [6], anti-proliferative property [7] and antihyperglycemic/hypoglycemic activity [8]. In our studies, we have reported the antibacterial activity of C.lasiantha against pathogenic Gram (-) and Gram (+) bacteria [9]. Similarly, antifungal activity of extracts of Caralluma lasiantha against storage fungi also reported by us recently [10].
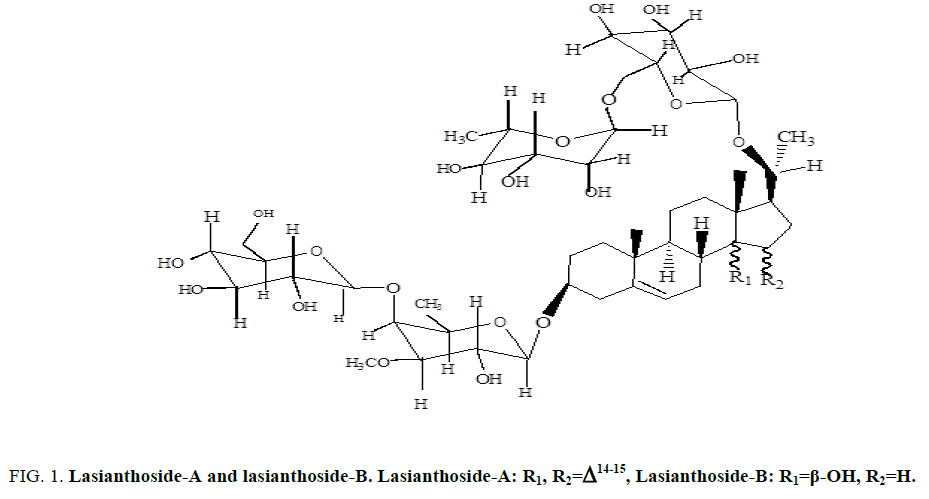
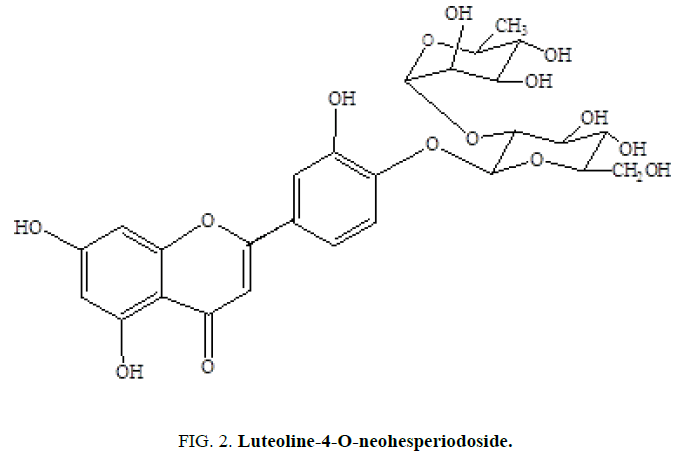
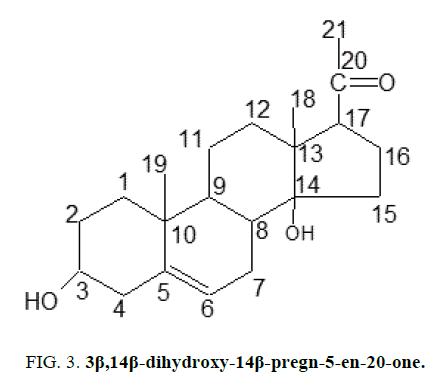
Two C-21 steroidal glycosides (lasianthoside-A and lasianthoside-B) [11] (Figure. 1) and a known flavone glycoside (Luteoline-4-O-neohesperiodoside) [12] (Figure. 2) were extracted from C. lasiantha by using polar solvents like alcohols. In our previous communication, we have reported the isolation of one C21 pregnane steroid, 3β, 14β-dihydroxy-14β-pregn-5-en-20-one (Figure. 3) from C. lasiantha [13]. Thorough literature shows that no extractions from C. lasiantha were carried out using solvents having low polarity. Hence, the present study is intended for phytochemical screening of n-hexane extracts of C. lasiantha which is least explored species.
Figure 1: Lasianthoside-A and lasianthoside-B. Lasianthoside-A: R1, R2=Δ14-15, Lasianthoside-B: R1=β-OH, R2=H.
Materials and Methods
All chemicals were purified by following regular procedures [14] and all chemicals used Analytical Reagent grade, Merck India Co. Ltd. Stems and roots of Caralluma lasiantha (Asclepiadaceae) were collected from Gooty, Anantapur District, Andhra Pradesh, India in February 2012. A voucher specimen of it was deposited in Herbarium, Department of Botany, Sri Krishna Devaraya University, Anantapur
Isolation and identification
Shade dried stems and roots were powdered and sieved (sieve No. 14). Afterwards the powder was stored in air tight container. The powder was weighed and extracted with soxhlet extractor by using solvents of polarity from non-polar end to polar end (Hexane, Chloroform, and Methanol) with successive solvent extraction. To concentrate the extracts and removal of final traces of solvent, rotavapor (Buchi Labotec rotavapor model R-205) was used [15,16]. Then, recrystallization was done to purify the crude extracts. Melting point was taken by using Fisher-John apparatus. IFS-120H spectrometer was used to record the IR. The 1H NMR and 13C NMR spectra were taken on Bruker 75 MHz and 300 MHz, spectrometer, using an internal standard like TMS. Mass spectra were recorded by using ZAB-HS mass spectrometer.
Test for alcohol
Small amount of crude extract was dissolved in 0.5 ml of dioxane. The obtained solution was added to 0.5 ml of ceric ammonium nitrate reagent. Then about one ml of dioxane was added and shaken. Yellow to red color formation indicates the existence of an alcoholic hydroxyl group [17].
Libermann-Burchard test
Few drops of acetic anhydride were added to the extract and boiled. Concentrated sulphuric acid was added to the above cooled solution. Presence of sterols is confirmed from formation of a brown ring at the junction of two layers and green color in upper layer [18].
Results and Discussion
Polarity of solvents influences the nature of extractable phytochemical. n-Hexane extracts were taken in the present study. Presence of sterols in the crude extract is indicated with positive results of alcohol test [17] and Libermann-Burchard test [18]. A thorough literature shows that separation of stigmasterol in its pure form is difficult [19,20] as the presence of double bond (C22=C23) is only the difference from that of β-sitosterol. As a result, they possess identical physical properties to make the separation very complex. Moreover, same Rf value was reported to both stigmasterol and β-sitosterol inspite of usage of many solvent systems [21]. In addition, literature shows that it is difficult to obtain sitosterol in pure state [22-24]. Consequently, many researchers reported the isolation of a mixture of β-sitosterol and stigmasterol [19,21,25]. Therefore, in the present study in order to separate stigmasterol from β-sitosterol and other phytosterols, a sequence of steps was adopted like saponification, fractional crystallization and gradient elution column chromatography.
Saponification
The crude extract (dark greenish brown oily mass) was air dried at room temperature. It was extracted successively into solvents with an increase in polarity. The extracts were concentrated under vacuum and then labelled. In order to remove fatty material, the petroleum ether extracts were saponified with alcoholic KOH (1M). Unsaponified matter was extracted in petroleum ether and removed the solvent. A small amount of unsaponified extract was dissolved in suitable solvent (chloroform) and tested with TLC by spotting on silica gel 60 F254 precoated on aluminium. After a number of pilot tests, n-hexane and ethyl acetate mixture (8:2) was found to be the suitable eluent. Presence of six different molecules with steroidal nucleus was confirmed by spraying the chromogenic agent (5% H2SO4 in methanol) and development of chromatograms in iodine chamber. Developed chromatogram in iodine chamber yielded five to six un-detached and continuous spot pattern in the Rf range (0.4 to 0.8). Less number of TLC spots for unsaponified matter shows the presence of fewer compounds in it compared to that of above combined petroleum extracts.
Fractional crystallization for separation of stigmasterol from phytosterol mixture
n-Pentanol and cyclohexanone were proved to be effective solvents for separation and purification of stigmasterol and β-sitosterol, based on the solubility difference and variation of solubility of these phytosterols with temperature [26,27]. Considering the advantage of these points, a fractional crystallization procedure was proposed by Xu et al. [28] to obtain 92% pure stigmasterol after five stages. The same procedure was adopted in this study due to various other advantages like simple operational procedure, easy recovery of solvent and low pollution. 25 g of phytosterol mixture was dissolved in 75 ml cyclohexanone by stirring for an hour at 60°C. Crystalline stigmasterol was precipitated by cooling the solution to room temperature. The filtered mass was dried in vacuum drying oven at 60°C. This fractional crystallization operation was repeated five times to obtain relatively high purity stigmasterol which is evident from reasonably separated spots on TLC plates.
Column chromatographic separation
The so obtained compound was further purified by column chromatography. It was conducted by packing silica gel (Mesh 60-120) using hexane for making wet packing method. Gradient elution technique was followed in which the polarity of solvent mixture was increased by addition of Et OAC (0%-100%) to hexane. The eluates were monitoring using TLC as mentioned above. Out of the collected 172 eluates, similar fractions were pooled together. Preparative TLC was used for auxiliary purification. Spots with same Rf values were scraped and extracted into petroleum ether as a solvent. Presence of sole phytochemical was confirmed from single spot even after subjecting TLC using different solvent mixtures like chloroform: ethanol (9.5:0.5), ethyl acetate: ethanol (9.5: 0.5), chloroform: ethyl acetate (4:1).
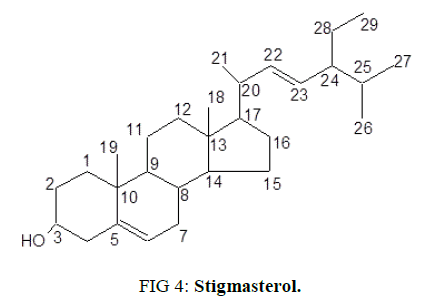
After recrystallizing in acetone, a white crystalline powder was obtained with a melting point of 171-172°C and Rf value of 0.7 (hexane: ethyl acetate=8:2 v/v) [29]. By using Mass (ESI-MS), IR, 1H-NMR and C13-NMR techniques, structure was elucidated as stigmasterol (Figure. 4).
Mass (ES-IMS) (m/z): 413.3 (M+H)+
Molecular ion peak in mass spectrum at m/z 413.3 (M+H)+ corresponds to molecular formula C29H48O which is equivalent to the formula of stigmasterol, a well known phytosterol.
I.R. : vmax 3419, 3025, 2935, 2866, 1567, 1021 cm-1
The IR signal absorption band observed at 3419 cm-1 is characteristic of O-H stretching. Absorption at 3025 cm-1 is typical olefinic=CH- stretching while the absorption at frequencies such as 1567 cm-1 and 1021 cm-1 are as a result of C=C and C-O absorption respectively.
1H NMR (CDCl3)
δ 5.35 (1H, d, J=4.62 Hz, H-22), 5.15 (1H, q, J=7.86 Hz, H-23), 5.01 (1H, q, J=7.85 Hz H-6), 3.52 (1H, m, J=5.09 Hz H-3), 2.26 (2H, t, J=8.13 Hz, H-4α, 4β), 2.03 (3H, m, J=8.07 Hz, 3-OH, H-20, H-24), 1.85 (2H, d, J=10.05 Hz, H7α, 7β), 1.67 (3H, d, J=10.35 Hz H-15α, H-17, H-25), 1.46 (8H, m, J=5.91 Hz H-2α, H-2β, H-8, H-9, H-11α, H-15β, H-16α, H-16β), 1.18 (6H, m, J=6.95 Hz 11β, H-12α, H-12β, H-14, H-15β, Me-19α, H-28α, H-28β,), 1.02 (8H, d, J=6.57 Hz , H-1α, 1β, Me-18, Me-19β, Me-19γ, Me-21α), 0.93 (1H, t, J=5.40 Hz Me-21β), 0.82 (10H, q, J=7.19 Hz, Me-21γ, Me-26, Me-27, Me-29).
1H NMR spectrum consists of peaks primarily in the up field region. However, two signals corresponding to olefinic region were observed with high chemical shifts values. A multiplet at δ 3.52 (1 H, m, H-3α) is characteristic to a carbinylic proton of sterol moiety. Peaks in low absorption field i.e., at δ δ 5.35 (1H, d, J=4.62 Hz, H-22), 5.15 (1H, q, J=7.86 Hz, H-23) and 5.01 (1H, q, J=7.85 Hz H-6) correspond to two and one ethylene protons respectively present on C22=C23 and C6. High intensity Peaks at δ 1.02 and 0.82 are corresponding to methyl groups (Me-19, Me-18, Me-26, Me-27 and Me-29).
13C NMR (CDCl3):
δ 140.86 (C-5), 138.44 (C-23), 129.38 (C-22), 121.8 (C-6), 71.88 (C-3), 56.98 (C-17), 56.06 (C-24), 51.36 (C-14), 50.27 (C-4), 42.39 (C-9), 42.32 (C-13), 40.62 (C-10), 39.8 (C-2), 37.38 (C-20), 36.62 (C-1), 32.01 (C-12), 32.01 (C-25), 32.01 (C-7), 31.74 (C-8), 29.04 (C-28), 25.53 (C-15), 24.48 (C-16), 21.35 (C-11), 21.21 (C-18), 21.21 (C-19), 19.52 (C-26), 19.11 (C-27), 12.37 (C-21), 12.16 (C-29).
13C NMR spectrum shows the presence of 6 methyl, 9 methylene, 11 methine and 3 quaternary carbons. Signals at δ140.86 and 121.8 correspond to double bond between carbons C5 and C6 as in the case of Δ5 spirostene [30]. Attachment of β-hydroxyl group to C3 is visible from a peak at δ 71.88 [31]. High intensity peak at δ 21.21 represent angular methyl carbons – C19 and C18. γ-Gauche interaction enhances the screening of C-18 leading to lower chemical shift (δ) as observed for C-1819.
DEPT (CDCl3):
DEPT 45:
C-H Carbons: 138.34, 129.25, 121.70, 71.76, 56.86, 55.93, 51.24, 50.14, 40.53, 31.61, 31.89.
CH2 Carbons: 42.26, 39.68, 37.25, 31.89, 31.89, 28.94, 25.42, 24.37, 21.12.
CH3 Carbons: 21.25, 21.07, 19.41, 19.0, 12.28, 12.06.
DEPT 90:
C-H Carbons: 138.34, 129.25, 121.70, 71.76, 56.86, 55.93, 51.24, 50.14, 40.53, 31.61, 31.89.
DEPT 135:
Up CH3 Carbons: 21.25, 21.07, 19.41, 19.0, 12.28, 12.06.
Up CH Carbons: 138.34, 129.25, 121.70, 71.76, 56.86, 55.93, 51.24, 50.14, 40.53, 31.61, 31.89.
Down CH2 Carbons: 42.26, 39.68, 37.25, 31.89, 31.89, 28.94, 25.42, 24.37, 21.12.
It confirms that the isolated molecule is stigmasterol due to presence of a sterol skeleton with one hydroxyl group (at C-3), two double bonds (at C-5/C-6 and C-20/C-21), six methyl groups [18,22-24]. As the physical, chemical and spectral (I.R., 1H NMR, 13C NMR, DEPT (45, 90 and 135) and ESI-MS) data of the isolated fraction are in consistent with those available in the literature.
Plant sterols are also known as phytosterols which are important structural components of the plasma membrane [32]. Stigmasterol is a well spread phytosterol in fungi, plants and animals. This secondary metabolite is a health promoting constituent [33] and exhibits antibacterial [34], antioxidant, hypoglycemic, thyroid inhibiting properties [35]. β-sitosterol was proved to be responsible for immunomodulatory activity of many plant extracts [30,36]. Large quantity of pure stigmasterol is required for the study of structure-function in different fields like plant biology, nutritional and pharmaceutical.
Literatures survey shows that though stigmasterol was extracted from different species of Asclepiadaceae family, like Calotropis gigantean [24], Calotropis procera [37], Oxystelma esculentum [38], Mondia whytei (Hook. f.) [39], Leptadenia reticulata (Retz) [40], G. sylvestre [41], its isolation for first time from Caralluma genus was reported from Caralluma wissmanni [42]. Whereas, the present study reports first time about the extraction of stigmasterol from C. lasiantha. Various moieties and functional groups attached to synthetic and natural molecules dictate their pharmacological activities [43-46]. Hence, SAR (structure activity relationship) between stigmosterol and such activities can be further studied.
Conclusion
On comparing physical properties as available in literature, analysis of chemical tests and spectroscopic data show that stigmasterol, a well-known sterol was isolated for the first time from C. lasianta using n-hexane as an extracting solvent.
References
- Sireesha M, Suresh babu K, Venkata Nadh R, et al. A review on its vital role in Indian traditional medicine, Res. J Pharm Bio Chem Sci.2017;8:873-9.
- Babu KS, Sireesha M, Venkata Nadh R,et al. Evaluation of In Vitro Antibacterial Activity of Carallumaumbellata Haw Used in Traditional Medicine by Indian Tribes.Annu Res Rev Biol. 2014;4:840-55.
- Arinathan V, Mohan VR, John Debritto A, et al. Wild edibles used by Palliyars of the western Ghats, Tamil Nadu, Indian J Traditional Knowledge. 2007;6163-8.
- Reddy SR, ReddyAM,Yasodamma N. Exploration of wild ornamental flora of YSR district, Andhra Pradesh, Indian J FundamAppl Life Sci. 2012;2:192-9.
- Vikneshwaran D, Viji M, Rajalakshmi K. Ethnomedicinal plants survey and documentation related to Paliyar community, Ethnobotanical Leaflets.2008;12: 1108-15.
- Madhuri V, Siva Rama Krishna C. Evaluation of cellular antioxidant activity of selected species of Caralluma and Boucerosia on cell lines. Int J Appl Sci Biotechnol. 2014;2:83-7.
- MadhuriV, MurthyKSR, AmruthaAV. Evaluation of anti proliferative properties of selected species of Caralluma and Boucerosia on skin cancer cell lines. Eur J Exp Biol. 2014;4:160-7.
- Harsha Kumar VS. Anti-hyperglycemic effect of Carallumalasiantha extract on hyperglycemia induced by cafeteria-diet in experimental model. Int J Pharm Sci Res. 2016;7:2525-30.
- Sireesha M, Suresh babu K, Venkata Nadh R, et al. Antibacterial Activity of Carallumalasiantha.
- Sireesha M, Suresh babu K, Venkata Nadh R, et al.Pullaiah, Antifungal Activity of Carallumalasiantha.
- Qiu SX, CordellGA, Ravi KumarB, et al.Bisdesmosidicpregnane glycosides from Carallumalasiantha.Phytochemistry.1999;50:485-91.
- Ramesh M, Rao YN, KumarMR, et al. Flavone glycoside from three Caralluma species. BiochemSyst Ecol. 1999;27:85-6.
- Sireesha M, Suresh babu K, Venkata Nadh R, et al. C21 pregnane steroid in Carallumalasiantha.
- Vogel AI. A Text Book of Practical Organic Chemistry.3rd edn, ELBS, London 1971.
- TreaseGE, Evans WC.Pharmacognosy, Saunders. Elsevier, Amsterdam, The Netherlands 2002;36:p.51.
- Gupta AK.Introduction to Pharmaceutics-1. CBS publication, New Delhi 2004.
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. 3rd edn, Chapman and Hall, London 1998;p.302.
- Edeoga HO, OkwuDE,Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol2005;4:685-8.
- Anjoo K,Saluja AK. Isolation of stigmasterol and ß-sitosterol from petroleum ether extract of aerial parts ofAgeratum conyzoides (Asteraceae). Int J PharmPharm Sci 2011;3:94-6.
- PatehUU, Haruna AK, Garba M, et al. Isolation of stigmasterol, ß-sitosterol and 2-Hydroxyhexadecanoic acid methyl ester from the Rhizomes ofStylochitonlancifoliuspyer and Kotchy (Araceae). Niger J Pharm Sci 2008;7:19-25.
- Pierre LL, MosesMN. Isolation and Characterisation of stigmasterol and ß-sitosterol from Odontonemastrictum (Acanthaceae). J Innovations Pharma Biol Sci. 2015;2:88-96.
- Pateh UU, HarunaAK, Garba M, et al. Ambi, Isolation of stigmasterol, ß-sitosterol and 2-hydroxyhexadecanoic acid methyl ester from the rhizomes of StylochitonlancifoliusPyer and Kotchy (Araceae), Niger. J Pharm Sci. 2009;7:19-25.
- JamalAK, AhmadWYW, Din L. A chemical study on Phyllanthuscolumnaris. Eur J Sci Res.2009;28:76-81.
- Habib M, Nikkon F, Rahman M, et al. Isolation of stigmasterol and ß-sitosterol from methanolic extract of root, Pak. J Biol Sci. 2007;10:4174-6.
- Gololo SS, ShaiLJ, SethogaL, et al. Isolation of a mixture of phytosterol compounds from the n-Hexane extract of Jatropha lagarinthoides (Sond) collected from Zebediela sub-region in Limpopo province, South Africa. J Chem Pharma Sci. 2016;9:3084-7.
- XuWL, WangYQ, HuangYB. Studies on the selection of solvents for the recrystallization and purification of phytosterol, J Yangzhou Univ. 2002;5:58-61.
- Xu WL, HuangYB, QianJH, et al. Studies on the solubility of stigmasterol and beta-sitosterol in cyclohexanone and n-pentanol. J ChemEng Chin.2003;17:707-10.
- XuWL, HuangYB, QianJH, et al. Separation and purification of stigmasterol and ß-sitosterol from phytosterol mixtures by solvent crystallization method. Sep Purify Technol. 2005;41:173-8.
- Govindarajan P,SaradaDVL. Isolation and characterization of stigmasterol and b-sitosterol from Acacia nilotica (l.) Delilessp Indica (benth.) Brenan. J Pharm Res. 2011;4:3601-2.
- Khanam S, Sultana R. Isolation of ß-sitosterol&stigmasterol as active immunomodulatory constituents from Fruits ofSolanum xanthocarpum (Solanaceae). Int J Pharm Sci Res. 2012;3:1057-60.
- Pretsch E, Buhlmann P. 1H NMR Spectroscopy. In:Structure determination of organic compounds. Springer, Berlin Heidelberg 200:p.161-243.
- Gendron JM, WangZY. Multiple mechanisms modulate brassinosteroid signaling.CurrOpin Plant Biol.2007;10:436-41.
- EFSA. Plant sterols and blood cholesterol. EFSA J. 2008;781:1-12.
- Sileshi W, Adane L, Tariku Y, et al.Evaluation of antibacterial activities of compounds Isolated From Sidarhombifolia Linn. (Malvaceae). Nat ProdChem Res. 2012;1:2-8.
- Panda S, Jafri M, Kar A, et al. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma, Fitoterapia.2009;80:123-6.
- Anton R, Jiang Y, Weniger B, et al. Rivier. Pharmacognosy of Mimosa tenuiflora (Willd.) poiret. JEthnopharmacol. 1993;38:145-52.
- Dhananjay D, Monika S, AnilKG, et al. Isolation of Stigmasterol from Alcoholic Extract of Bark of Calotropisprocera. J ChemCheml Sci. 2013;3:277-9.
- Pandya DJ, AnandIS. Isolation and HPTLC estimation of Stigmasterol from Oxystelmaesculentum. Asian J Biochem Pharm Res. 2011;1:17-29.
- Githinji CG, MbuguaPM, KanuiTI, et al. Antinociceptive effects of stigmasterol and 9-hexacosene isolated from Mondiawhytei (Hook. F.) root. Int J Phytopharmacol. 2011;2:70-5.
- Subramanian PS,LakshmananAJ. Constituents of leptadenia-reticulate wight and arn-occurrence of simiarenol. Indian J Chem. 1977;15:180-2.
- KokateCK, PurohitAP,GokhaleSB, et al.Pharmacognosy. 36, Nirali Prakashan, Pune. 2006;p.252.
- Abdelaziz MD, AhmedYM, MamdouhAM, et al. Phytochemical investigation of Carallumawissmannii O. Schwart. Res J Pharm BiolChem Sci. 2012;3:884-92.
- SudhirMS, RatnakaramVN. Antibacterial activity of some newer 1,2,3-benzotriazole derivatives synthesized by ultrasonication in solvent-free conditions.BulgChemCommun. 2014;46:25-30.
- Sudhir MS,RatnakaramVN, Radhika S. Antifungal activities of novel 1,2,3-benzotriazole derivatives synthesized by ultrasonic and solvent free conditions.Drug Invention Today. 2013;5:126-32.
- Sudhir MS,RatnakaramVN. Evaluation of in vitro anthelmintic activities of novel 1,2,3-benzotriazole derivatives synthesized in ultrasonic and solvent free conditions. J Pharm Res. 2013;7:47-52.
- Suresh G, Ratnakaram VN, Srinivasu N, et al. Novel coumarin isoxazoline derivatives: synthesis and study of antibacterial activities. Synth Comm. 2016;46:1972-80.