Original Article
, Volume: 13( 1)Phytochemical and Antifungal Properties of Euphorbia hirta L against Fusarium moliniforme and Phoma sorghina
- *Correspondence:
- Tapsoba I , Laboratory of Environmental and Bio-Organic Analytical Chemistry (LCAE BiO), UFR/SEA, Ouaga I University Joseph, Ouagadougou 03, Burkina Faso, Tel: +22670625536; E-mail: issa.tapsoba@gmail.com
Received: April 13, 2017; Accepted: May 24, 2017; Published: May 29, 2017
Citation: Karanga Y, Ilboudo O, Bonzi S, et al. Phytochemical and Antifungal Properties of Euphorbia hirta L against Fusarium moliniforme and Phoma sorghina. Nat Prod Ind J. 2017;13(1):105.
Abstract
In this study, the whole plant of Euphorbia hirta. L was investigated for phytochemical and antifungal properties. Hydroethanolic extract of E. hirta has been fractioned with different organic solvents and were analysed for chemical screening, antioxidant and antifungal activities against Fusarium moniliforme and Phoma sorghina. The results showed that E. hirta L contains tannins, flavonoids, alkaloids and terpenoids. Ethyl acetate and butanol fractions of E. hirta L present high contents of phenolic compounds with respectively 3.675 and 3.588 mg gallic acid equivalent per gram of extract. The antioxidant activities evaluated by FRAP showed that these two fractions contain 4.147 mg and 3.977 mg Trolox equivalent per gram of extract, respectively. The antifungal activity investigated on Fusarium moniliforme and Phoma sorghina shows that 5 mg/ml of these extracts inhibit the mycelium growth of these fungi at 58.33% and 44.67% respectively and demonstrate that these extracts could be used for cereals protection.
Keywords
Euphorbia hirta L; Chemical screening; Fusarium moniliforme; Phoma sorghina; Antifungal
Introduction
Cereals constitute the most widely consumed food crop in the world and particularly in Burkina Faso where maize, Sorghum and Millet are cultivated. The production of cereals faces phytopathogenic issues causing heavy loss in the production. Farmers usually apply chemical products to control plant diseases. Although the chemical agents are effective, their continuously use led to development of the resistance of various types of micro-organism [1,2]. Therefore, many researchers looked for alternative method in place of chemical pesticide [3-5]. Plants virtually constitute a rich source of bioactive chemicals for the development of biopesticide as safer disease control agents.
Euphorbia hirta L (E. hirta L) has been traditionally used in Burkina Faso and widely for the treatment of many diseases such as diarrhoea, dysentery, bilharzia, asthma, bronchitis, etc. [6,7]. E. hirta L has been studied chemically and found to contain flavonoids belonging to flavonol type such as quercetol, kaempferol, quercetin, and the phenolic acids. Also, many sterols and triterpenes such as the taraxerol, α and the β-amyrine, β-sitosterol, campesterol and stigmasterol [8] were found in E. hirta L. Other pharmacological studies reported the presence of isoquercetrin, luteolin-7-O-glucoside, (-)-epicatechin gallate and (-)-epigallocatechine gallate in E. hirta L extracts [6,9]. It is well-known that the biological properties of plant extracts depend to their phytochemical composition [3,6,7].
In the purpose, we reported herein the phytochemical investigation of Euphorbia hirta L. The antioxidant and antifungal activities of these plants are being studied against Fusarium moliniforme and Phoma sorghina, two-seed fungi.
Materials and Methods
Plant material and preparation of extracts
Euphorbia hirta L was collected during dried season, at mid-February in a field at Ouagadougou, Sample has been identified by the professor Jeanne Millogo, botanist at the Laboratory of Biology and Vegetable Ecology (LABEV) of the University of Ouaga I Pr. Joseph KI-ZERBO. The whole plant was washed carefully with water milli Q, dried at room temperature in laboratory (35°C) during 96 hours and powdered. One hundred grams of powder was then defatted by hexane (3 × 250 ml). The plant material has been separated by filtration and dried. Fifty gram of this powder have been macerated in 500 ml of a mixture of ethanol-water mixture in the proportion of 80/20 v/v during 20 hours. After filtration with paper Wattman No1, the obtained filtrate was concentrated on rotary evaporator at 50°C until a volume of 150 ml. The remaining aqueous phase is then freeze-dried to obtain 4 g of crude extract names EB. The extract was dissolved in 150 ml of water milliQ and extract successively with solvents by increasing polarity. The extraction was done with (3 × 50 ml) of dichloromethane (DCM), followed by (3 × 50 ml) of ethyl acetate (AcOEt) and finally by (3 × 50 ml) of n-butanol (BuOH). The obtained fractions were evaporated to dryness and then kept in fridge for further studies. For each fraction, a stock solution of 5 mg/ml is prepared before each experiment.
Biological material
The antifungal tests were carried out on Fusarium moliniforme and Phoma sorghina, two fungi that infecting considerably major species of cereal such as maize, sorghum and millet cultivated in tropical areas. Both are mould agents usually well-known to be develop on cereals cultivated around the world and are identified in Burkina Faso. These fungi were isolated in the laboratory of phytopathology of the university of Bobo Dioulasso, Burkina Faso from sorghum samples collected in the region in 2007 and they were cultivated in potato dextrose agar medium (PDA) [10]. P. sorghina was codified according the following reference 32So-06 and Fusarium moliniforme with 1341So07-Fm as reference code. Using a 5-day old colony, four mycelium explants of identical size (5 mm) were placed in the centre of Petri dishes and incubated in the heat chamber at 28°C and keep in the dark for 10 days.
Phytochemical screening
The phytochemical screening has been carried out on the different extracts of E. Hirta to identify qualitatively the presence or absence of various active constituents. So, the presence or absence of these constituents have been investigated following standard phytochemical tests procedures such as Ferric chloride test for tannins and flavonoids, Froth test for saponins, Libermann-Burchard’s for test terpenoids, Dragendroff’s test for alkaloids and NaOH test for quinones.
Total phenolic determination
Total phenolic content was evaluated according to the Folin-Ciocalteu method [5]. This method is based on the properties of phenolic compounds to reduce the phosphomolybdotungstic reagent or reactive of Folin-Ciocalteu [5]. The amount of total phenolic in each sample is determined by extrapolation of the standard curves obtained starting from a stock solution of 500 μg/ml of Gallic acid by dilution in water milliQ until the final concentration of 1/128. Indeed, in each test tube, were added, 25 μL of each sample of stock solution and 125 μl of reagent of Folin-Ciocalteu (RFC) (0.2 N in distilled water milli Q). After 5 min, 100 μL sodium carbonate μL (75 g/l) were added and the mixture shaken. The solutions were then incubated in shelters of light during two (02) hours. Each concentration was triplicate and the absorbance was read three times at 760 nm compare to a blank made up of a mixture of 125 μl of RFC; 25μl of methanol and 100 μl of sodium carbonate.
Antioxidant activity determination
The antioxidant activity was carried out according to FRAP method: This method is based on the capacity of the compounds to reduce the ferric ion [11]. Thus, 500 μl of each stock solution of extract is mixed with 1.25 ml of plug phosphates (0.2 M pH = 6) and 1.25 ml of a solution of potassium hexacyanoferrate [K3Fe (CN) (1%). After 30 min of incubation at 50°C, 1.25 ml of acid trichloroacetic (10%) is added and the mixture is centrifuged during 10 min. After centrifugation 125 μl of the supernatant are also taken and mixed with 125 distilled water milli Q and 25 μl of a solution of FeCl3 (1%) aqueous. The absorbance is read at 700 nm and compare to a blank. The white contains 500 μl methanol in the place of the extract, the other reagents remaining unchanged. A calibration curve is carried out with the Trolox (0 mg/l to 200 mg/l).
Antifungal activity determination
The antifungal activity of the various fractions was carried out in vitro on the inhibition of the mycelial growth of Phoma sorghina and Fusarium moniliforme. Each fraction was dissolved in dimethyl sulfoxide (DMSO) (1%), and tested at three concentrations 1, 3 and 5 mg/ml. The culture medium used is the potato dextrose agar (PDA). The mixture is sterilized in an autoclave for 30 min at 120°C. After cooling, a volume of 25 ml of each medium was poured in Petri dishes of 9 cm of diameter. In order to compare the obtained results, control solution was prepared with 1% of DMSO in distilled water used as medium. Mycelium explantat or sterile disc of 5 mm in diameter of Phoma sorghina and Fusarium moniliforme collected from microbial culture collection unit (MCCU) was placed in the center of Petri dish containing medium with or without adding tested products. The Petri dishes were incubated at 28°C during 10 days and the mycelial growth was measured every two days up to 10 days. Evaluating radial growth involved tracing two perpendicular lines on the Petri dish lid, which pass through the centre of the explant. The diameters of the mycelium colonies (in cm) were measured at 10 DAI (days after incubation). An average diameter was calculated for each of the three Petri dishes. A variance analysis was then conducted using SAS INC software and the average diameters for each fungus were compared, using the student Newman and Keuls multiple comparison test based on a 5% threshold. The experiments were repeated three times and the percentage of inhibition of the mycelium growth on each medium is calculated following the relation:
% inhibition = 100[(A-B)/A]
A: size of the mycelium in control
B: size of the mycelium in the treatment
Results and Discussion
Chemical screening
In this part, we investigate chemical screening to determine qualitatively the different chemical groups present in crude extract and different fractions of Euphorbia hirta L. as obtained according the process detailed in section relative to phytochemical screening.
The results in Table 1 show that, dichloromethane, ethyl acetate, butanol fractions and crude extract of E. hirta L contain tannins, flavonoids and terpenoïds. This result agrees with previous works [6,9] which demonstrated that E. hirta L contains a lot of flavonol derivatives. However, saponins [12] and quinones were not detected in any fractions of E. hirta L. The alkaloids are absent in dichloromethane fraction. These results are in agreement with the work reported by Parekh and Chanda [13] and Prashant et al. [14] which showed that E. hirta L. contains alkaloids, flavonoids, tannins and phenolic derivatives.
| Want chemical groups | Fractions | |||
|---|---|---|---|---|
| EB | DCM | AcOEt | BuOH | |
| Tannins | + | + | + | + |
| Saponins | - | - | - | - |
| Flavonoids | + | + | + | + |
| Quinones | - | - | - | - |
| Terpenoids | + | + | + | + |
| Alkaloids | + | - | + | + |
(+): positive test; (-): negative test
Table 1: Results of the screening of chemical groups in E. hirta.
Total phenolic compounds (TP)
The phenolic compounds have demonstrated their potential target in many studies for their antioxidant and antifungal activities [15]. In order to establish the relationship between biological activities and phenolic compounds, the amount of phenolic compounds have been evaluated by the Folin-Ciocalteu [5] method and the results were shown in Table 2.
| Extract | Concentration (mg EAG/g extract) |
|---|---|
| EB | 2.599 ± 0.017 |
| DCM | 1.215 ± 0.095 |
| AcOEt | 3.675 ± 0.101 |
| BuOH | 3.588 ± 0.022 |
Table 2: The amount of phenolic compounds expressed in milligrams Gallic acid equivalent per gram of extract.
The obtained results showed that ethyl acetate and butanol fractions contain the highest quantity of phenolic compounds estimated to 3.675 mg and 3.588 mg EAG/g c.a. of extract respectively. This high concentration of phenolic compounds in the extracts could be explained by the presence of tannins and flavonoids, which constitutes most phenolic compounds. The phenolic compounds were collected by ethyl acetate and butanol solvents which are well-known to extract flavonoids [16,17]. One can observe in Table 2, a small amount of phenolic compound obtained in dichloromethane fraction. This could be explained by the weak polarity of dichloromethane leading to a remove of phenolic compounds.
Antioxidant activity (AAO) by FRAP method
The antioxidant activity of different extracts was determined using the equation of the calibration curve of Trolox as follow (y=28.672x + 0.0668; R2=0.9996). The obtained values are shown in the Figure. 1.
One can observe on the Figure. 1 that all fractions of the extract of E. hirta L exhibit antioxidant activity and the best ones corresponding to 4.147 ET/g and 3.977 mg ET/g c.a of equivalent of Trolox were obtained on ethyl acetate and butanol fractions respectively. These results are correlated to the high content of phenolic compounds in these fractions as reported in Table 2 [18]. The correlation coefficient between phenolic compounds and antioxidant activities of E. hirta L extracts is highly significant (R2=0.9791), indicating that 97% of the antioxidant capacity of extracts, is due to the contribution of phenolic compounds derivatives. These results agree with previous works indicating the correlation between total phenolic content and antioxidant activities [18-20].
Antifungal activity
The evaluation of the antifungal activity of the extracts of E. hirta L against Fusarium moniliforme and Phoma sorghina was done using the mycelial growth inhibition test. The investigation is done by following the effect of E. hirta L extracts concentrations on the inhibition percentage of the mycelial growth of the two fungi.
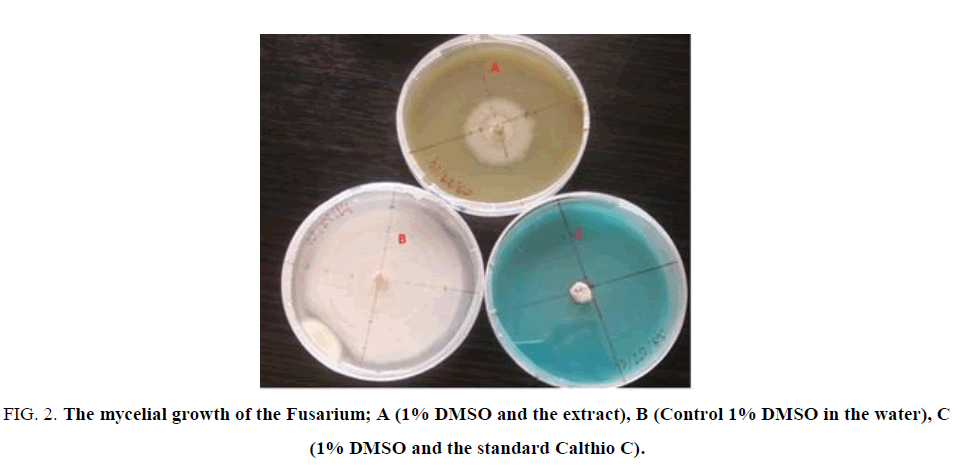
Figure. 2 illustrated the effects of the 5 mg/L of E. hirta L. extract on the mycelium growth of Fusarium moniliforme in comparison with the blank (control) and the standard fungicide Calthio C. One can see that the extract inhibits the growth of the Fusarium moniliforme.
Figure 2: The mycelial growth of the Fusarium; A (1% DMSO and the extract), B (Control 1% DMSO in the water), C (1% DMSO and the standard Calthio C).
The exploitation of the mycelium growth measurement led to the determination of the inhibition percentage and the obtained results are summarizing in Table 3.
| Extracts | Extract concentration (mg/ml) | |||||
|---|---|---|---|---|---|---|
| F. moniliforme | P. sorghina | |||||
| 1 | 3 | 5 | 1 | 3 | 5 | |
| EB | 36.00f ± 2.74 | 40.33ef ± 1.70 | 47.00d ± 1.79 | 12.67f ± 1.40 | 15.67f ± 3.57 | 22.00e ± 0.32 |
| AcOEt | 44.00de ± 1.70 | 57.67c ± 1.70 | 58.33c ± 1.92 | 24.33e ± 1.70 | 36.33d ± 1.28 | 44.67c ± 1.70 |
| BuOH | 35.33f ± 2.57 | 49.67d ± 3.33 | 57.00c ± 3.57 | 12.67f ± 1.70 | 23.00e ± 1.11 | 35.33d ± 1.70 |
| Calthio. C | 83.00b ± 8.39 | 100a ± 0.00 | 100a ± 0.00 | 81.33b ± 6.09 | 100a ± 00 | 100a ± 00 |
Table 3: Effect of the concentration of the crude extract (EB), the ethyl acetate fraction (AcOEt), the butanol fraction (buoh) E. hirta L and Calthio C on the inhibition percentage of the mycelial growth of F. moniliforme and P. sorghina.
As shown in Table 3, it appears clearly that the crude extract and the different fractions of E. hirta L inhibit the mycelial growth of the two fungi. The percentage of the inhibition increases slightly with the concentration of crude extract and different fractions. This effect is more efficient on F. moniliforme than on P. sorghina whereas for Calthio C, a recommended chemical fungicide in seed treatment, the inhibition of the mycelial growth is total for the two fungi.
Among the different fractions of E. hirta L, the ethyl acetate fraction reduces the mycelium growth of F. moniliforme at least 40% for a concentration of 1 mg/ml. This inhibition increases with the concentration of extract to reach 58.33% ca. for a concentration of 5 mg/ml. However, on P. sorghina this effect is less pronounced. Indeed, it reduces the mycelium growth of P. sorghina from 24.33% to 44.67% for a concentration 1 mg/ml to 5 mg/ml of ethyl acetate fraction respectively. The rate of inhibition of the ethyl acetate fraction is in agreement with the work reported by Gayathri et al. which showed that ethyl acetate extract of E. hirta possesses an antifungal activity against Aspergillus flavus [21]. The important quantity of phenolic compounds in the ethyl acetate extract could explain its strong antifungal activities as demonstrated in many studies [22-24]. This result shows that F. moniliforme is more sensitive to the ethyl acetate fraction than P. sorghina and was already observed previously by Ilboudo et al. [25] which demonstrated the antifungal effect of flavonoid diglycosides isolated from Mentha piperita against these two cereals fungi with the same extracts. The difference observed between the two fungi towards E. hirta L extracts could be due to the capacity by a variation in the concentration of the active substance [25,26].
One can observe through these results that the antifungal activity of the E. hirta extracts is correlated to the phenolic compounds. More, the amount of phenolic compounds is high and more the antifungal activity is important. While the extraction of E. hirta is made following the polarity of the solvent, the phytometabolites present in butanol extract are different from the acetate one. These two extracts are demonstrated some antifungal properties against F. moniliforme and P. sorghina. This result is in agreement with several works of the literature which showed the antifungal activity of the phenolic compounds particularly flavonoids on the fungi [27-29].
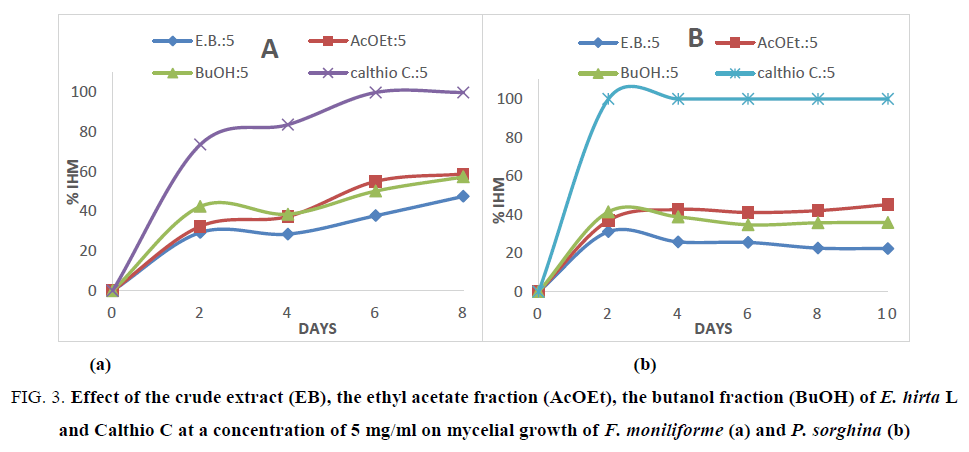
In addition, the effects on mycelial growth of both fungi have been followed every two days using different fractions and Calthio C. as standard at 5 mg/ml during 10 days. The obtained results are shown in Figure. 3a and 3b.
Figure 3: Effect of the crude extract (EB), the ethyl acetate fraction (AcOEt), the butanol fraction (BuOH) of E. hirta L and Calthio C at a concentration of 5 mg/ml on mycelial growth of F. moniliforme (a) and P. sorghina (b)
In Figure. 3, a slow toxic activity is noted during 8 days for the different extracts on F. moniliforme. The inhibition reaches 58.33%in 8 days in presence of ethyl acetate extract. In the case of P. sorghina, a slight inhibition is observed with ethyl acetate extract. Indeed, after 2 days of incubation a rate inhibition of 36.95% was observed whereas during 8 days of incubation, the inhibition is 45.18%. This means that the toxicity of E. hirta extract is covered by a nutritional effect on fungi. This kind of reversed effect was observed with the macerated extracts of B. aegyptiaca which significantly reduce radial growth of C. graminicola, but have no effect on mycelium growth of P. sorghina. This difference can be explained by the different activities presented by the various compounds in each extract. The fractions are rich in flavonoids but each type of flavonoids glycosylated does not present specific activity on mycelium growth towards fungi spores. In addition, as shown in the Figure. 1 and Table 3, one can note that the high antioxidant fraction of E. hirta L exhibited the best antifungal activities. It is well known that the antioxidant activities are due to the radical consumption by phenolic compounds [28]. This result could be lead to the conclusion that the antifungal activities are correlated to the scavenging of hydroxyl radical generated by the fungi as reported by Mihara et al. [28].
Conclusion
The chemical screening reported in this study showed that the hydroethanolic extract of the whole plant of E. hirta L contains phenolic compounds such as tannins, flavonoids, saponins, alkaloids. Ethyl acetate and butanol fractions are more rich in flavonoids derivatives confirmed by the total phenolic compounds determination. A good correlation between the amount of phenolic compounds and antioxidant activities of ethyl acetate and butanol fractions of E. hirta L because 97% of the antioxidant capacity of extracts is due to the contribution of phenolic compounds derivatives. In addition, our screening experiments showed that ethyl acetate and butanol fractions of E. hirta L demonstrated antifungal activities against Fusarium moniliforme and Phoma sorghina. The results showed that the active constituents present in this plant must be isolated for further chemical characterization.
Acknowledgment
The authors acknowledge the International Science Program (ISP) for their financial support on antifungal natural products investigation through the research project BUF 01.
Conflict of Interest
The authors declare no conflict of interest.
References
- Hamdache A, Lamarti A, Badoc A. In vitro resistance of Botrytis cinerea to three fungicides. Belletinde, Bordeaux Pharmaceuticals Society. 2010;149:103-14.
- Katan T. Resistance to 3,5-dichlorophenyl-N-cyclicimide (dicarboximide) fungicides in the gray mold pathogen Botrytis cinerea on protected crops. Plant Pathol. 1982;31:133-41.
- Lefeuvre G. Use of medicinal plants in Senegal.
- Shih MF, Cheng YD, Shen CR, et al. A molecular pharmacology study in the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J Nat Med. 2010;64:330-5.
- Singleton VL, Orthofer R, and Lamuela-Raventos RM. Analysis of total phenols and other oxidant substrates and antioxidants by means of Folin-Coicalteu reagent. Methods Enzymol. 1999;299:152-78.
- Basma AA, Zakaria Z, Latha LC, et al. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac J Trop Med. 2011;4:386-90.
- Nacoulma-Ouédraogo OG. Medicinal plants and traditional medical practices in Burkina Faso: Case of the central plateau. University of Ouagadougou. 1966;p 285.
- Holmes EM. Euphorbia pilulifera. J pharma pharmacol. 1923;10:162-163.
- Pioro-Jabrucka E, Pawelczak A, Przyby JL, et al. Accumulation of phenolic and sterolcompounds in E.hirta L. 2011;57:30-7.
- Bonzi S, Somda S, Zida EP, et al. In vitroantifungal activity of various local plant extracts in the control of Phomasorghina (Sacc.) Boerema et al. and Colletotrichum graminicola (Ces.) Wilson, as Sorghum seed mold pathogen in Burkina Faso. Tropicultura. 2012;30:103-6.
- Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307-15
- Sidambaram RR, Dinesh MG, Jayalakshmi ET. An in vitro study of cytotoxic activity of Euphorbia hirta on HEP-2 cells of human epithelioma of Larynx. Int J Pharm Pharm Sci. 2011;3:101-3.
- Parekh J, Chanda S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk J Biol. 2007;31:53-8.
- Yadav P, Kumar A, Mahour K, et al.Phytochemical analysis of some indiegenous plants potent against endoparasite. J Adv Lab Res Biol. 2010;1:72-8.
- Lamien-Meda A, LamienCE, Compaoré MMY, et al. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13:581-94.
- Jenifer S, Laveena DK, Priya S. Antimicrobial screening of Euphorbia hirta L. and Pedalium murex L: A comparative study. World J Pharm Pharma Sci. 2014;3:1221-6.
- Kumar S, Malhotra R, and Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 2010;4:58-61.
- Athamena S. Antioxidant and antimicrobial activity of extracts of Cuminumcyminum L. Lebanese SciJ. 2010;11:69-91.
- Turkmen N, Velioglu YS, Sari F, et al. Effect of extraction conditions on total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484-96.
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940-9.
- Ramesh KV, Gayathri A. Antifungal activity of Euphorbia hirta L. inflorescence extract against Aspergillus flavus a mode of action study. IntJCurrMicrobiolAppSci. 2013;2:31-4.
- Kosar M, Dorman HJD, Baser KHC. Screening of free radical scavenging compounds in water extracts of mentha simples using a postcolumn derivatization method. J Agric Food Chem. 2004;52:5004-10.
- Inoue T, Sugimoto Y, Masuda H, et al. Antiallergic effect of flavonoid glycosides obtained from Menthapiperita L. Biol Pharm Bull. 2002;25:256-9.
- Sroka Z, Fecka I, Cisowski W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. naturforsch. C Bio Sci. 2005;60:826-32.
- Ilboudo O, Bonzi S, Tapsoba I, et al. In vitro antifungal activity of flavonoid diglycosides of Menthapiperitaand their oxime derivatives against two cereals fungi. C R Chim. 2016;19:857-62.
- Kanoun K. Study of the effectiveness of the ethanol extract of Punica granatum Linn bark on two phytopathogenic strains: Ascocyhtarabiei (PASS) LABR. and Fusarium oxysporum F. SP. Radicis-lycopersici. Eur Sci J. 2014:10.
- Lopesa NP, Kato MJ, Yoshida M. Antifungal constituents from roots of Virolasurinamensis. Phytochemistry. 1999;51:29-33.
- Mihara R, Barry KM, Mohammed CL, et al. Comparison of antifungal and antioxidant activities of Acacia mangium and A. auriculiformis heartwood extracts. J Chem Ecol. 2005;31:789-804.
- McNulty J, Nair JJ, Bollareddy E, et al. Isolation of flavonoids from the heartwood and resin of Prunus avium and some preliminary biological investigations. Phytochemistry. 2009;70:2040-6.