Review
, Volume: 13( 2)Photodynamic Therapy: A Versatile Treatment Procedure for Cancer Cure
- *Correspondence:
- Jothi Rajan MA Tamil Nadu State Council for Science and Technology, Chennai, India, Tel: 044 22301428; E-mail: anjellojothi@gmail.com
Received Date: December 26, 2017 Accepted Date: January 4, 2018 Published Date: January 5, 2018
Citation: Jothi Rajan MA, Srinivasan R, Vanitha Kumari G. Photodynamic Therapy: A Versatile Treatment Procedure for Cancer Cure. Phys Chem Ind J. 2018;13(1):117
Abstract
Photodynamic therapy (PDT) is a clinically accepted treatment; it is a minimally invasive treatment. PDT process is toxic activity toward malignant cells. In, PDT process photosensitizer agent absorbs irradiated light and generates singlet oxygen. Singlet oxygen directly reacted with tumor cell and damages the cell membranes. PDT is a selectively treat malignant cell during early stage confirmed by clinical investigations. PDT significantly extend the lifetime of inoperable tumor affected persons. PDT has minimum toxicity, reduced systemic effects, highly reduced long-term morbidity, excellent cosmetic as well as organ function-sparing effects of PDT treatment make it a valuable therapeutic for combination treatments. In future PDT has the potential to become effective method for cancer treatment.
Keywords
Photodynamic therapy; Chemotherapy; Radiotherapy
Introduction
Regardless of advancement in essential research that has given us a superior comprehension of tumor science and prompted mean of new ages of focused medications, late huge clinical trials for growth, with some important exemptions, have been able to see just little contrasts in treatment results [1,2]. Moreover, the numeral of new clinically acknowledged medications is deficiently low [3]. These calming realities call attention to that to develop encourage headway it is important to put an accentuation on other existing yet at the same time overlooked helpful methodologies. Photodynamic treatment (PDT) has the conceivable to assemble numerous right now neglected restorative needs. Despite the fact that as yet developing, it is now an effective and clinically affirmed restorative methodology utilized for the administration of neoplastic and non-harmful illnesses. PDT was the primary medication gadget blend affirmed by the FDA very nearly two decades back, yet even so remains underutilized clinically.
PDT made of three parts photosensitizer, oxygen and light [4,5]. None of these is independently dangerous, yet together they start a photochemical response that comes full circle in the age of a highly reactive item named singlet oxygen. The last can quickly cause critical lethality prompting cell passing through apoptosis or corruption. Antitumor impacts of PDT get from three interrelated components-coordinate cytotoxic consequences for tumor cells, harm to the tumor vasculature and acceptance of a hearty fiery response that can prompt advancement of foundational insusceptibility. The relative commitment of these instruments depends to a huge degree on the sort and measurement of PS utilized, time between PS organization and light presentation, add up to light measurements and its fluence rate, tumor oxygen focus and maybe other still ineffectively perceived factors. In this way, assurance of ideal conditions for utilizing PDT requires a planned interdisciplinary exertion. This audit will address the most essential natural and physico-concoction parts of PDT, condense its clinical status and give a standpoint to its potential future improvement.
Experimental
Basic components of photodynamic therapy
Photodynamic treatment is a two-organize technique. Following organization of a light sensitive PS tumor loci are illuminated with a light of suitable wavelength. The last can be conveyed to essentially any organ in the body by methods for adaptable fiber-optic gadgets. Selectivity is gotten from both, the capacity of helpful photosensitizers to confine in neoplastic injuries and the exact conveyance of light to the treated destinations. Incomprehensibly, the profoundly confined nature of PDT is one of its present impediments, as the treatment is inadequate against metastatic injuries which are the most regular reason for death in disease patients. Progressing research is centered on finding ideal PDT conditions to prompt foundational insusceptibility which may, in any event to some degree, hinder this constraint later on. PDT can be utilized either earlier or after chemotherapy, radiotherapy or surgery without trading off these restorative modalities. None of the clinically affirmed PSs aggregate in cells' cores, restricting DNA harm that could be cancer-causing or prompt advancement of safe clones. Besides, the unfavorable impacts of chemotherapy or radiation are missing. Radio-or chemo-resistance doesn’t influence affectability to PDT.
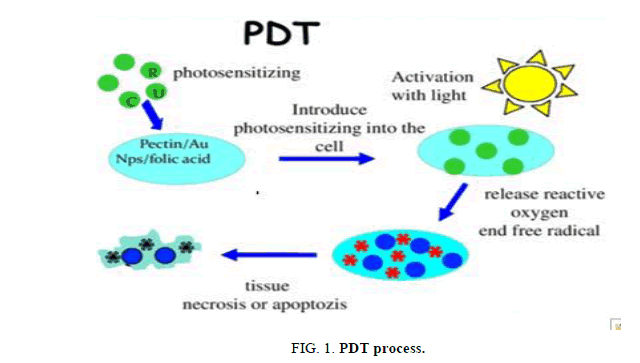
Astounding corrective results make PDT reasonable for patients with skin growths. There are no huge changes in tissue temperature and the protection of connective tissue prompts negligible fibrosis, permitting maintenance of practical life systems and mechanical honesty of empty organs experiencing PDT. Chosen patients with inoperable tumors, who have depleted other treatment choices, can likewise accomplish change in personal satisfaction with PDT. At long last, numerous PDT methodologies can be performed in an out-patient or mobile setting along these lines easing costs, as well as making the treatment understanding cordial. The main unfriendly impacts of PDT identify with torment amid some treatment conventions and a determined skin photosensitization that has been dodged by the more up to date specialists (Figure 1).
Photosensitizers
The greater part of the photosensitizers utilized as a part of malignancy treatment depends on a tetrapyrrole structure, like that of the protoporphyrin contained in hemoglobin. A perfect photosensitizing specialist ought to be a solitary unadulterated compound to permit quality control examination with low assembling expenses and great security away. It ought to have a high ingestion top in the vicinity of 600 nm and 800 nm (red to dark red) as assimilation of photons with wavelengths longer than 800 nm does not give enough vitality to energize oxygen to its singlet state, and the limit with regards to shaping a generous yield of receptive oxygen species upon illumination. Since the entrance of light into tissue increments with its wavelength, specialists with solid absorbance in the dark red, for example, chlorins, bacteriochlorins and phthalocyanines offer change in tumor control. It ought to have no dim lethality and moderately quick freedom from ordinary tissues, accordingly limiting phototoxic reactions. Other apropos attractive properties of photosensitizing operators have been outlined elsewhere [6]. While the interim between tranquilize organization and illumination is generally long, with the goal that the sensitizer is given adequate time to diffuse from typical tissues, reports now propose that the tumor reaction might be once in a while better when light is conveyed at a shorter medication light interim when PS is as yet display in the veins, along these lines delivering stamped vascular damage [7]. Some reports recommend that an articulated incendiary reaction and necrotic cell passing after brightening is essential in the invulnerable fortifying capacity of PDT, while others recommend that PSs that create more apoptosis and less irritation are appropriate for applications, for example, mind tumors where swelling is unwanted. Late discoveries demonstrate that specific PDT-instigated apoptotic cell passing instruments are very immunogenic and equipped for driving antitumor invulnerability as well. Finally, the light-interceded demolition of the PS known as photo-bleaching was already thought to be bothersome, yet a few reports recommend that this property may make light dosimetry less basic as finished treatment is dodged when the PS is obliterated amid the illumination [8,9]. The main PS to be clinically utilized for growth treatment was a water-dissolvable blend of porphyrins called hematoporphyrin subsidiary (HPD), a refined type of which later wound up plainly known as Photofrin. In spite of the fact that Photofrin is as yet the most broadly utilized PS, the item has a few disservices including an enduring skin photosensitivity and a generally low absorbance at 630 nm. While a photodynamic impact can be created with Photofrin, adequacy would be enhanced by red-moving the red absorbance band and expanding the absorbance at the longer wavelengths. There has been a noteworthy exertion among restorative scientific experts to find second-age PSs and a few hundred mixes have been proposed as conceivably valuable for anticancer PDT. The revelation that 5-aminolevulanic corrosive (ALA) was a biosynthetic forerunner of the PS protoporphyrin IX [10] has prompted numerous applications in which ALA or ALA-esters can be topically connected, or controlled orally. These are thought to be 'professional medications', waiting be changed over to protoporphyrin to be dynamic photosensitizers. Numerous speculations have been proposed to represent the tumor-limiting properties in PDT [11]. These incorporate the dominance of cracked and convoluted tumor veins because of neovascularization and nonappearance of lymphatic waste known as the improved penetrability and maintenance effect [12]. Some of the best mixes tie specially to low thickness lipoprotein (LDL) recommending that upregulated LDL receptors found on tumor cells could be important [13]. There have been focusing on thinks about in which PSs are covalently connected to different particles that have some partiality for neoplasia or to receptors communicated on particular tumors [14]. The goal is to depend on the capacity of the focusing on vehicle to control confinement factors with the goal that the PS can be picked in light of its photochemical properties. These vehicles incorporate monoclonal antibodies, counter acting agent pieces, peptides, proteins, for example, transferrin, epidermal development factor and insulin, LDL, different sugars, somatostatin, folic corrosive and numerous others.
Light sources
Blue light infiltrates slightest productively through tissue while red and infrared radiations enter all the more profoundly (Figure 1). The locale in the vicinity of 600 nm and 1200 nm is frequently called the optical window of tissue. Notwithstanding, illuminate to just around 800 nm can produce 1O2, since longer wavelengths have inadequate vitality to start a photodynamic reaction [15]. No single light source is perfect for all PDT signs, even with a similar PS. Decision of light source ought to consequently be founded on PS retention (fluorescence excitation and activity spectra), sickness (area, size of sores, availability, and tissue qualities), cost and size. The clinical adequacy of PDT is reliant on complex dosimetry: add up to light measurement, light presentation time, and light conveyance mode (single versus fractionated or even metronomic). The fluence rate additionally influences PDT response [16]. Integrated frameworks that measure the light dispersion and fluence rate either interstitially or on the surface of the tissues being dealt with are so far utilized just in test ponders. The two lasers and glowing light sources have been utilized for PDT and show comparable efficacies [17]. Unlike the vast and wasteful pumped color lasers, diode lasers are little and financially savvy, are easy to introduce, have computerized dosimetry and alignment highlights and a more drawn out operational life. Such lasers are presently being particularly intended for PDT. Light discharging diodes (LEDs) are elective light sources with generally limit otherworldly transfer speeds and high fluence rates [18,19]. Lasers can be coupled into strands with diffusing tips to treat tumors in the urinary bladder and the stomach related tract. Inflatables, secured within with a firmly dissipating material, shaped to fit an organ, are additionally financially available [20]. It is very achievable to embed a light source in strong organs somewhere down in the body under picture direction. The decision of ideal blends of PSs, light sources, and treatment parameters is vital for fruitful PDT [21,22].
Photophysics and photochemistry
Most PSs in their ground (i.e. singlet) state have two electrons with inverse twists situated in a vigorously most great atomic orbital. Assimilation of light prompts an exchange of one electron to a higher-vitality orbital. This energized PS is extremely precarious and emanates this overabundance vitality as fluorescence as well as warmth. On the other hand, an energized PS may experience an intersystem crossing to frame a steadier triplet state with modified turn of one electron. The photosensitizer in triplet state can either rot radiation less to the ground state or exchange its vitality to sub-atomic oxygen (O2), which is one of a kind in being a triplet in its ground state. This progression prompts the development of singlet oxygen (1O2), and the response is alluded to as a Type II process [23]. A Type I process can likewise happen whereby the PS responds straightforwardly with a natural particle in a phone microenvironment, securing a hydrogen molecule or electron to shape a radical. Resulting autoxidation of the diminished PS creates a superoxide anion radical (O2 •-). Dismutation or one-electron lessening of O2 •-gives hydrogen peroxide (H2O2), which thus can experience one-electron diminishment to an effective and for all intents and purposes aimless oxidant-hydroxyl radical (HO•). ROS age by means of Type II science is unthinkingly substantially more straightforward than by means of Type I, and most PSs are accepted to work through Type II as opposed to Type I instrument.
Mechanisms of PDT
The lifetime of singlet oxygen (1O2) is short (~10-320 ns), constraining its dissemination to as it were around 10 nm-55 nm in cells [24]. Thus, photodynamic harm will happen near the intracellular area of the PS [25]. Photofrin is a mind-boggling blend of porphyrin ethers with variable confinement designs generally connected with lipid films. Of the other photosensitizing operators in current utilize, the chlorin NPe6 targets lysosomes, the benzoporphyrin subsidiary (BPD) targets mitochondria, m-tetrahydroxyphenylchlorin (mTHPC) has been accounted for to target mitochondria, ER or both, the phthalocyanine Pc 4 has an expansive range of proclivity despite the fact that mitochondria are accounted for to be an essential target [6]. Other specialists that have been produced can have various targets. Particular examples of restriction may fluctuate additionally among various cell sorts. PDT can inspire the three fundamental cell demise pathways: apoptotic, necrotic and autophagy associated cell passing. Apoptosis is a for the most part real cell demise methodology in cells reacting to PDT. Mitochondria external film permeabilization (MOMP), after photodynamic damage is controlled by Bcl-2 relatives and thought to be to a great extent p53-independent [26]. With mitochondria-related PSs, photodamage to layer bound Bcl [27-29] can be a lenient flag for MOMP and the ensuing arrival of caspase activators, for example, cytochrome c and Smac/DIABLO, or other expert apoptotic particles, including apoptosis-prompting factor (AIF) [26]. Lysosomal layer break and spillage of cathepsins from photograph oxidized lysosomes [30,31] actuates Bid cleavage and MOMP [31]. Phototoxicity isn't proliferated just through caspase-flagging however includes other proteases, such as calpains, and in addition non-apoptotic pathways [26]. Typically, restraint or hereditary inadequacy of caspases just defers phototoxicity or movements the phone demise methodology towards necrotic cell death [32]. Recent proof proposes for sure that specific type of corruption can be engendered through flag transduction pathways [33]. The atomic components basic modified rot is as yet subtle, yet certain occasions including initiation of RIP1 (receptor communicating protein 1) kinase, extreme mitochondrial ROS creation, lysosomal harm and intracellular Ca2+-over-burden, are repetitively involved [33,34]. Severe internal mitochondria layer photodamage or intracellular Ca2+-over-burden could advance mitochondrial porousness progress, an occasion that may support necrotic instead of apoptotic phototoxicity [26,35] Photodamage of cells can likewise prompt the incitement of macroautophagy (henceforth alluded to as autophagy) [36,37]. This is a lysosomal pathway for the debasement and reusing of intracellular proteins and organelles. Autophagy can be invigorated by different anxiety signals including oxidative stress [38]. This procedure can have both a cytoprotective and a professional passing part following tumor chemotherapy, including those including ROS as essential harming agents. Recent examinations portray autophagy as an instrument to save cell reasonability following photodynamic injury [37]. PSs that photodamage the lysosomal compartment may trade off consummation of the autophagic procedure, causing deficient leeway of the autophagic payload. Collection of ROS-harmed cytoplasmic parts may then potentiate phototoxicity in apoptosis equipped cells. A superior comprehension of the interaction between autophagy, apoptosis and corruption and how these procedures prompt enhanced tumor reaction will be an essential to devise better helpful methodologies in PDT.
Mechanisms of cytoprotective
Various distributions have detailed cytoprotective instruments that tumor cells adventure to stay away from cytotoxic impact of PDT [26]. The main system recognized depended on the vast variety saw in the level of cell reinforcement atoms communicated in growth cells [39]. Both water-dissolvable cancer prevention agents, e.g., some amino-acids, glutathione (GSH) or vitamin C and lipid-solvent cell reinforcements, e.g., vitamin E are available at variable levels in numerous disease cell sorts clarifying the huge variety in PDT sensitivity [40]. A moment component is related with articulation in malignancy cells of chemicals that can detoxify ROS. Despite the fact that there is no particular cell protein that can specifically detoxify 1O2, chemicals engaged with different ROS digestion can impact the cytotoxic impact of PDT. For instance, superoxide dismutase (SOD) over-articulation or treatment with SOD mimetics have been appeared to balance the cytotoxic impact of PDT [41]. An expansion of the SOD action has been likewise seen in different disease cell sorts following PDT, and this is related with a decline in glutathione peroxidase and catalase activities [42]. The third cytoprotective component includes proteins whose encoding qualities are themselves instigated by PDT. Numerous classifications can be determined however the majority of them are a piece of flagging pathways that can manage PDT induced apoptosis [43] for a review or take part in the repair of sores prompted by oxidative anxiety. NF-κB hindrance by finished articulation of the IκBα super-repressor or by the utilization of pharmacological inhibitors firmly sharpens disease cells to apoptosis initiated by PDT [44]. Other anxiety related translation factors prompted by PDT incorporate AP-1, hypoxia inducible factor (HIF) or Nrf2. PDT was appeared to up-direct heme oxygenase-1 (HO-1) articulation and the instrument is reliant on Nrf2 atomic amassing and on p38MAPK what's more, PI-3K exercises. As a result of the cancer prevention agent action of HO-1, it can be imagined that Nrf2-subordinate flag transduction can control cell insurance against PDT-interceded cytotoxic impact. PDT was found to instigate articulation of different HSPs for which a defensive part in PDT has been depicted. For instance, transfection of tumor cells with HSP27 quality expanded survival of tumor cells after PDT [45]. Similarly, expanded HSP60 and HSP70 levels are contrarily connected with affectability to the photodynamic treatment [46,47]. The least difficult clarification for these perceptions is the capacity of HSPs to tie to oxidatively harmed proteins. Also, intracellular capacity of HSPs isn't just confined to protein refolding. Numerous HSPs "customer" proteins assume a basic part in the control of pro-survival pathways. PDT additionally prompts expanded ubiquitination of carbonylated proteins subsequently labeling them for corruption in proteasomes, which counteracts development of poisonous protein aggregates [48].
PDT for antivascular effects
Photodynamic bother of tissue microcirculation was first announced in 1963 [49]. An examination by Star et al. [50] used a window chamber to mention coordinate objective facts of embedded mammary tumor and in nearby ordinary tissue microcirculation in rats some time recently, amid, and at different circumstances after PDT sharpened with HPD. An underlying whitening and vasoconstriction of the tumor vessels was trailed by heterogeneous reactions including possible finish blood stream stasis, drain, and in some bigger vessels, the development of platelet totals. Perceptions performed on extracted tissues from murine models, exhibited an extensive variety of vascular reactions including disturbance of blood stream to subcutaneous urothelial tumors and to ordinary rodent jejunum, breakdown of the blood cerebrum hindrance in the typical mind of mice, and endothelial cell and organelle harm in subcutaneous tumors and typical tissue [51,52]. Other investigations showed that tumor cells treated with a possibly healing photodynamic measurement in vivo were clonogenic if expelled quickly from the host [53,54]. Progressive misfortune in clonogenicity was seen when tumors were left in the host for expanding lengths; this related to movement of PDT-actuated hypoxia as decided radio-organically. Hypoxia adequate to block coordinate tumor cell slaughtering was recognized at sub-corrective PDT measurements. These examinations proposed a focal part for vascular harm in representing the tumor reaction to PDT in mouse models. Many reports referred to above specifically involve the endothelium as an essential focus for PDT in vivo; this fortified research into the relative affectability of endothelial cells to PDT and the reactions of endothelial cells that could start the different marvels at the vessel level. Gomer et al. [55] demonstrated that cow-like endothelial cells were fundamentally more delicate to Photofrin-PDT than smooth muscle cells or fibroblasts from similar species. This expanded affectability, surveyed by clonogenic examine, was not an aftereffect of expanded Photofrin amassing. Affectability to HPD-interceded PDT of ox-like aorta endothelial cells and human colon adenocarcinoma cells was examined by West et al. [56]. Exponentially developing endothelial cells were altogether more delicate than also multiplying tumor cells, and the distinction in affectability was joined by more prominent PS collection in the endothelial cells. Endothelial cell reactions to sub-deadly measurements of PDT may likewise add to vascular changes saw in tissue. Expanded vessel porousness to egg whites in the rodent cremaster muscle amid and after Photofrin-PDT was accounted for by Fingar et al. [57] More as of late, intravital fluorescence imaging has been utilized to exhibit treatment-incited increments in tumor vessel penetrability for verteporfin-and NPe6-PDT [58,59]. In a spearheading study, Synder et al. [60] demonstrated that HPPH-PDT enlistment of expanded tumor vascular penetrability brought about upgraded gathering of Doxil, a liposome-exemplified plan of doxorubicin. At the point when Doxil was managed instantly after PDT, tumor control and selectivity were potentiated essentially in respect to either methodology alone. In an investigation roused by the need to convey chemotherapeutic operators to the cerebrum adjoining a tumor, ALA-PDT was utilized effectively to temporarily disturb the blood mind obstruction in typical rodent cerebrum in vivo [61] These and different parts of vascular-focused on PDT speak to critical ebb and flow look into headings.
Results and Discussion
The immune response of PDT
PDT regularly incites a solid intense fiery response saw as limited edema at the focused on site. 4. This response is a result of PDT-initiated oxidative anxiety. Along these lines, PDT can be positioned among disease treatments (counting cryotherapy, hyperthermia and centered ultrasound removal) delivering compound/physical affront in tumor tissue apparent by the host as limited intense injury. This prompts the host to dispatch defensive activities advanced for managing danger to tissue respectability and homeostasis at the influenced site [62] The intense fiery reaction is the main defensive effector process occupied with this unique circumstance. Its fundamental errand is containing the disturbance of homeostasis, guarantee expulsion of harmed cells, and afterward advance nearby mending with rebuilding of ordinary tissue work. The aggravation evoked by PDT is a tumor antigen non-particular process arranged by the natural insusceptible system [63]. The acknowledgment arm of this framework, specifically pattern recognition receptors is in charge of distinguishing the nearness of PDT inflicted tumor-limited affront uncovered to its sensors as the presence of "adjusted self". PDT shows up especially powerful in creating quickly a plenitude of alert/threat signals, likewise called harm related sub-atomic examples (DAMPs,) or cell demise related sub-atomic examples (CDAMPs), at the treated site that can be recognized by the inborn resistance caution elements. The beginning of PDT-actuated aggravation is set apart by sensational changes in the tumor vasculature that winds up noticeably penetrable for blood proteins and pro-adhesive for incendiary cells [62]. This happens even with those PSs that for the most part target tumor as opposed to vascular cells, where the fiery procedure is overwhelmingly started by signals starting from photooxidative harm delivered in perivascular locales with chemotactic inclinations achieving the vascular endothelium. The incendiary cells, drove by neutrophils and took after by pole cells and monocytes/macrophages, quickly and hugely attack tumors experiencing PDT [4,63]. Their essential errand is to kill the wellspring of DAMPs/CDAMPs by taking out garbage containing traded off tissue components including harmed and dead cells.
Harm and brokenness of photodynamically-treated tumor vasculature oftentimes winds up with vascular impediment that serves to "divider off" the harmed tumor tissue until the point that it is evacuated by phagocytosis subsequently keeping the spreading of the disturbed homeostasis.62 Depletion of these incendiary cells or restraint of their movement after PDT was appeared to reduce restorative effect [64-67]. Among cytokines associated with the direction of the provocative procedure, the most basic part in tumor PDT reaction is played by IL-1β and IL-6 [68,69]. Blocking the capacity of different attachment atoms was demonstrated additionally hindering to PDT response. On the other hand, blocking mitigating cytokines, for example, IL-10 and TGF-β can particularly enhance the cure rates after PDT [62].
Various pre-clinical and clinical examinations have shown that PDT can impact the versatile safe reaction in dissimilar ways; a few regimens result in potentiation of versatile insusceptibility, while others prompt immunosuppression. The exact component prompting potentiation versus concealment is vague; be that as it may it seems like the impact of PDT on the invulnerable framework is needy upon the treatment regimen, the zone treated and the photosensitizer type [66,70]. PDT initiated safe concealment is to a great extent bound to cutaneous and transdermal PDT regimens including expansive surface areas [70,71]. PDT viability has all the earmarks of being reliant upon the enlistment of hostile to tumor invulnerability. Longterm tumor reaction is reduced or missing in immunocompromized mice [64,72]. Reconstitution of these creatures with bone marrow or T cells from immunocompetent mice brings about expanded PDT viability. Clinical PDT viability likewise seems to rely upon hostile to tumor resistance. Patients with vulval intraepithelial neoplasia (VIN) who did not react to ALA-PDT will probably have tumors that needed significant histocompatibility complex class I atoms (MHC-I) than patients who reacted to ALA-PDT [73]. MHC-I acknowledgment is basic for initiation of CD8+ T cells and tumors that need MHC-I are impervious to cell-interceded hostile to tumor insusceptible reactions [74]. VIN patients who reacted to PDT had expanded CD8+ T cell penetration into the regarded tumors when contrasted with non-responders. Immunosuppressed and immunocompetent actinic keratoses and Bowen's ailment patients had comparable starting reaction rates to PDT; however, immunosuppressed patients displayed more prominent constancy of sickness or appearance of new lesions [75] Canti et al. [76] were the first to indicate PDT-actuated safe potentiation, showing that cells confined from tumor-depleting lymph hubs of PDT-treated mice could give tumor protection from innocent mice. Ensuing examinations exhibited that PDT coordinated against murine tumors brought about the age of resistant memory [77]. Recent reports have demonstrated that clinical hostile to tumor PDT additionally builds hostile to tumor invulnerability. PDT of multifocal angiosarcoma of the head and neck brought about expanded resistant cell invasion into far off untreated tumors that was joined by tumor regression [78]. PDT of basal cell carcinoma (BCC) expanded safe cell reactivity against a BCC-related antigen [79]. The system whereby PDT improves hostile to tumor insusceptibility has been inspected for as long as quite a few years. PDT actuates both humoral and cell-intervened hostile to tumor invulnerability, despite the fact that the significance of the humoral reaction is hazy. PDT adequacy in mice and people is diminished without CD8+ T cell initiation as well as tumor infiltration [64,73,80]. Therefore, most robotic investigations have concentrated on the methods by which PDT potentiates CD8+ T cell enactment. Unmistakably acceptance of against tumor insusceptibility following PDT is endless supply of inflammation [81] PDT-instigated intense neighborhood and fundamental irritation is proposed to finish in the development and actuation of dendritic cells (DCs). Develop DCs are basic for actuation of tumor particular CD8+ T cells and acceptance of hostile to tumor immunity [82] DCs are initiated in light of PDT [69] and relocate to tumor depleting lymph hubs where they are thought to animate T cell activation [69,83]. Generation of CD8+ effector and memory T cells is every now and again, however not generally subordinate upon the nearness and enactment of CD4+ T cells [84]. PDT instigated against tumor insusceptibility may [64] or may not rely upon CD4+ T cells80 and might be expanded by common executioner (NK) cells [80]. PDT-interceded improvement of hostile to tumor resistance is accepted to be expected, at any rate to some degree, to incitement of DCs by dead and passing on tumor cells, recommending that in vitro PDT-regarded tumor cells may go about as powerful hostile to tumor vaccines [85]. This theory has been demonstrated by a few examinations utilizing a wide assortment of photosensitizers and tumor models in both protection and remedial settings [67,85-87]. Mechanistic investigations demonstrated that hatching of youthful DCs with PDT-treated tumor cells prompts upgraded DC development, initiation and expanded capacity to fortify T cells [85,88]. PDT of tumor cells causes both cell demise and cell stress [4,89,90] and it is conjectured that the enactment of DCs by PDT-treated cells is the aftereffect of acknowledgment of DAMPs/CDAMPs discharged/emitted/uncovered by PDT from biting the dust cells [91-93]. The level of articulation of HSP70 in PDT-treated tumor cells seems to correspond with a capacity to fortify DC maturation [96] and start of inflammation [92-97]. Furthermore, opsonization of photodynamically-treated tumor cells by supplement proteins expands the viability of PDT-created vaccines [86]. PDT in this way initiates numerous risk signals equipped for activating antigen-showing cell actuation and against tumor insusceptibility. The ramifications of PDT-prompted against tumor resistance and strong PDT-produced immunizations are huge and give an energizing probability to utilizing PDT in the treatment of metastatic malady and as an adjuvant in mix with other growth modalities. A few pre-clinical examinations exhibited that PDT can control the development of tumors display outside the treatment field [80,98] in spite of the fact that others have neglected to exhibit control of far off sickness following PDT [99] PDT was likewise appeared to be in a compelling surgical adjuvant in non-little cell lung growth patients with pleural spread [100]. The clinical utilization of PDT for growth dates to the late 1970s when there was an examination on the impacts of HPD + light in five patients with bladder cancer. In 1978, Dougherty revealed the main vast arrangement of patients effectively treated with PDT with HPD. Since this early work, there have been more than 200 clinical trials for PDT. Latest efficient reviews revealed that PDT can be viewed as a sensible alternative, in the treatment of dangerous and pre-harmful non-melanoma skin injuries. It is likewise useful in the treatment of Barrett's throat and unrespectable cholangiocarcinoma. Be that as it may, its adequacy in the administration of different sorts of tumors has not yet been unequivocally demonstrated. The significant purpose behind this is just few satisfactorily controlled randomized controlled trials were performed up until this point. Orderly investigation of the writing is restricted because of absence of ideal PDT parameters (light conditions or PS measurements) that could be tantamount among these examinations. PDT delivers for the most part shallow impacts. Because of a constrained light entrance through tissues the profundity of tumor decimation ranges from couple of millimeters up to one centimeter. This clear inconvenience can be positively misused in the treatment of shallow ailments, for example, premalignant conditions (mucous dysplasia, actinic keratosis), carcinoma in situ or shallow tumors, (for example, dangerous pleural mesothelioma or intraperitoneal scattered carcinomatosis. In addition, PDT can be utilized supplemental to surgery, to light tumor quaint little in the likelihood of long haul nearby infection control.
Conclusion
PDT is as yet thought to be another and promising antitumor technique. Its maximum capacity has however to be appeared and its scope of uses alone or in mix with other affirmed or test remedial methodologies is certainly not depleted. The upsides of PDT contrasted, and surgery, chemotherapy or radiotherapy are lessened long haul dismalness and the way that PDT does not bargain future treatment choices for leftover or intermittent illness. Because of an absence of regular components of 1O2 end and a one of a kind instrument of cytotoxicity transformations that present protection from radiotherapy or chemotherapy don't bargain antitumor adequacy. In addition, PDT can be rehashed without trading off its adequacy. These are noteworthy restricting elements for chemotherapeutics and radiotherapy. At last, numerous traditional antitumor medicines convey danger of prompting immunosuppression. PDT-actuated immunogenic cell demise related with acceptance of an intense nearby fiery response offers the likelihood to prosper into a remedial methodology with phenomenal neighborhood antitumor movement and equipped for boosting the safe reaction for powerful pulverization of metastases. Interdisciplinary uniqueness of PDT moves masters in material science, science, science and drug and its further advancement and novel applications must be restricted by their gigantic creative ability.
References
- Bergh J. Quo vadis with targeted drugs in the 21st century. J Clin Oncol. 2009;27(1):2–5.
- Fojo T, Grady C. How much is life worth: Cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:(15)1044–8.
- Hampton T. Targeted cancer therapies lagging: Better trial design could boost success rate. JAMA.2006;296(16):1951–2.
- Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387.
- Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn Ther. 2010;7(2):61–75.
- Chen B, Roskams T, de Witte PA. Antivascular tumor eradication by hypericin-mediated photodynamic therapy. Photochem Photobiol. 2002;76(5):509–513.
- Garg AD, Nowis D, Golab J, et al. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim Biophys Acta. 2010; 1805(1):53–71.
- Ascencio M, Collinet P, Farine MO, et al. Protoporphyrin IX fluorescence photobleaching is a useful tool to predict the response of rat ovarian cancer following hexaminolevulinate photodynamic therapy. Lasers Surg Med. 2008;40(5):332–341.
- De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm Res. 2000;17(12):1447–55.
- Hamblin MR, Newman EL. On the mechanism of the tumour-localising effect in photodynamic therapy. J Photochem Photobiol B. 1994;23(1):3–8.
- Iyer AK, Greish K, Seki T, et al. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J Drug Target. 2007;15(7-8):496–506.
- Kessel D. The role of low-density lipoprotein in the biodistribution of photosensitizing agents. J Photochem Photobiol B. 1992;14(3):261–2.
- Sibani SA, McCarron PA, Woolfson AD, et al. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin Drug Deliv. 2008;5(11):1241–54.
- Juzeniene A, Nielsen KP, Moan J. Biophysical aspects of photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25(1-2):7–28.
- Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38(5):489–93.
- Brancaleon L, Moseley H. Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci. 2002;17(3):173–86.
- Juzeniene A, Juzenas P, Ma LW, et al. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med Sci. 2004;19(3)139–49.
- Szeimies RM, Morton CA, Sidoroff A, et al. Photodynamic therapy for non-melanoma skin cancer. Acta Derm Venereol. 2005;85:483–90.
- Beyer W. Systems for light application and dosimetry in photodynamic therapy. J Photochem Photobiol B. 1996;36(2):153–6.
- Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53(9):61–109.
- Plaetzer K, Krammer B, Berlanda J, et al. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci. 2009;24(2):259–68.
- Foote CS. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162(3857):963–70.
- Dysart JS, Patterson MS. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys Med Biol. 2005;50(11):2597– 616.
- Moan J, Berg K, Kvam E, et al. Intracellular localization of photosensitizers. Ciba Found Symp. 1989;146:95–107.
- Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776(1):86–107.
- Kessel D, Castelli M. Evidence that bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochem Photobiol. 2001;74(2):318–322.
- Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20:3420–7.
- Usuda J, Chiu SM, Murphy ES, et al. Domain-dependent photodamage to Bcl-2. A membrane anchorage region is needed to form the target of phthalocyanine photosensitization. J Biol Chem. 2003;278(3):2021–9.
- Berg K, Moan J. Lysosomes as photochemical targets. Int J Cancer. 1994;59(6):814–822.
- Reiners JJ Jr, Caruso JA, Mathieu P, et al. Release of cytochrome cand activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002;9:934–44.
- Kessel D. Relocalization of cationic porphyrins during photodynamic therapy. Photochem Photobiol Sci. 2002;1(11):837–40.
- Vanlangenakker N, Vanden Berghe T, Krysko DV, et al. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8(11):207–20.
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15.
- Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8.
- Buytaert E, Callewaert G, Vandenheede JR, et al. Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy. 2006;2:238–40.
- Reiners JJ Jr. Agostinis P, Berg K, et al. Assessing autophagy in the context of photodynamic therapy. Autophagy. 2010;6(1):7-18.
- Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6(7):838–54.
- Sattler UG, Mueller-Klieser W. The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol. 2009;85(11):963–71.
- Frank J, Flaccus A, Schwarz C, et al. Ascorbic acid suppresses cell death in rat DS-sarcoma cancer cells induced by 5-aminolevulinic acid-based photodynamic therapy. Free Radic Biol Med. 2006;40(5):827–36.
- Golab J, Nowis D, Skrzycki M, et al. Antitumor effects of photodynamic therapy are potentiated by 2-methoxyestradiol. A superoxide dismutase inhibitor. J Biol Chem. 2003;278(1):407–14.
- Hadjur C, Richard MJ, Parat MO, et al. Photodynamic effects of hypericin on lipid peroxidation and antioxidant status in melanoma cells. Photochem Photobiol. 1996;64(2):375–81.
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: What, where, why and how. Photochem Photobiol Sci. 2002;1:1–21.
- Matroule JY, Bonizzi G, Morliere P, et al. Pyropheophorbide-a methyl ester-mediated photosensitization activates transcription factor NF-kappaB through the interleukin-1 receptordependent signaling pathway. J Biol Chem. 1999;274(5):2988–3000.
- Wang HP, Hanlon JG, Rainbow AJ, et al. Up-regulation of Hsp27 plays a role in the resistance of human colon carcinoma HT29 cells to photooxidative stress. Photochem Photobiol. 2002;76(1):98–104.
- Hanlon JG, Adams K, Rainbow AJ, et al. Induction of Hsp60 by Photofrinmediated photodynamic therapy. J Photochem Photobiol B. 2001;64(1):55–61.
- Nonaka M, Ikeda H, Inokuchi T. Inhibitory effect of heat shock protein 70 on apoptosis induced by photodynamic therapy in vitro. Photochem Photobiol. 2004;79(1):94–8.
- Szokalska A, Makowski M, Nowis D, et al. Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response. Cancer Res. 2009;69(10):4235–43.
- Castellani A, Pace GP, Concioli M. Photodynamic effect of haematoporphyrin on blood microcirculation. J Pathol Bacteriol. 1963;86:99–102.
- Star WM, Marijnissen HP, van den Berg-Blok AE, et al. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986;46(5):2532–40.
- Bhuvaneswari R, Gan YY, Soo KC, et al. The effect of photodynamic therapy on tumor angiogenesis. Cell Mol Life Sci. 2009;66(10):2275–83.
- Tseng MT, Reed MW, Ackermann DM, et al. Photodynamic therapy induced ultrastructural alterations in microvasculature of the rat cremaster muscle. Photochem Photobiol. 1988;48(5):675–81.
- Henderson BW, Waldow SM, Mang TS, et al. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985;45(2):572–6.
- Henderson BW, Fingar VH. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem Photobiol. 1989;49(3):299–304.
- Gomer CJ, Rucker N, Murphree AL. Differential cell photosensitivity following porphyrin photodynamic therapy. Cancer Res. 1988;48(16):4539–42.
- West CM, West DC, Kumar S, et al. A comparison of the sensitivity to photodynamic treatment of endothelial and tumour cells in different proliferative states. Int J Radiat Biol. 1990;58(1):145–56.
- Fingar VH, Wieman TJ, Wiehle SA, et al. The role of microvascular damage in photodynamic therapy: the effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992;52:4914–21.
- Chen B, Pogue BW, Luna JM, et al. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin Cancer Res. 2006;12(3):917–23.
- Mitra S, Foster TH. In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-L-aspartylchlorin-e6. Neoplasia. 2008;10(5):429–38.
- Snyder JW, Greco WR, Bellnier DA, et al. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res. 2003;63(23):8126–31.
- Hirschberg H, Uzal FA, Chighvinadze D, et al. Disruption of the bloodbrain barrier following ALA-mediated photodynamic therapy. Lasers Surg Med. 2008;40(8):535– 42.
- Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–8.
- Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995;71:549–55.
- Korbelik M, Cecic I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999;137(1):91–8.
- de Vree WJ, Essers MC, Koster JF, et al. Role of interleukin 1 and granulocyte colony stimulating factor in photofrin-based photodynamic therapy of rat rhabdomyosarcoma tumors. Cancer Res. 1997;57(13):2555–8.
- Kousis PC, Henderson BW, Maier PG, et al. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67(21):10501–10.
- Korbelik M, Cecic I. Mechanism of tumor destruction by photodynamic therapy. In: Nalwa HS, editor. Handbook of Photochemistry and Photobiology. American Scientific Publishers, Stevenson Ranch, CA, USA. 2003;39-77.
- Sun J, Cecic I, Parkins CS, et al. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem Photobiol Sci. 2002;1(9):690– 5.
- Gollnick SO, Evans SS, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–9.
- Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998;7(1):57–64.
- Yusuf N, Katiyar SK, Elmets CA. The immunosuppressive effects of phthalocyanine photodynamic therapy in mice are mediated by CD4+ and CD8+ T cells and can be adoptively transferred to naive recipients. Photochem Photobiol. 2008;84(2):366–70.
- Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–52.
- Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61(1):192–6.
- Maeurer MJ, Gollin SM, Storkus WJ, et al. Tumor escape from immune recognition: loss of HLAA2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6. Clin Cancer Res. 1996;2(4):641–52.
- Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation. 2004;77(1):115–21.
- Canti GL, Lattuada D, Nicolin A, et al. Immunopharmacology studies on photosensitizers used in photodynamic therapy. Proc SPIE. 1994;2078:268–75.
- Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59(8):1941–6.
- Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8(10):950–2.
- Kabingu E, Oseroff AR, Wilding GE, et al. Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res. 2009;15(1):4460–6.
- Kabingu E, Vaughan L, Owczarczak B, et al. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96:1839–48.
- Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64(6):2120–6.
- Reis e Sousa C. Activation of dendritic cells: Translating innate into adaptive immunity. Curr Opin Immunol. 2004;16(1):21–5.
- Sur BW, Nguyen P, Sun CH, et al. Immunophototherapy using PDT combined with rapid intratumoral dendritic cell injection. Photochem Photobiol. 2008;84:1257–64.
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu Rev Immunol. 2006;24:519–40.
- Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62(6):1604–8.
- Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol Immunother. 2006;55(8):900–9.
- Korbelik M, Merchant S, Huang N. Exploitation of immune response-eliciting properties of hypocrellin photosensitizer SL052-based photodynamic therapy for eradication of malignant tumors. Photochem Photobiol. 2009;85(6):1418–24.
- Jalili A, Makowski M, Switaj T, et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res. 2004;10(13):4498–508.
- Henderson BW, Gollnick SO. Mechanistic principles of photodynamic therapy. In: Vo-Dinh T, editor. Biomedical Photonics Handbook. CRC Press, Boca Raton. 2003;36.31-27.
- Oleinick NL, Evans HH. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat Res. 1998;150(5):S146–56.
- Gollnick SO, Owczarczak B, Maier P. Photodynamic therapy and anti-tumor immunity. Lasers Surg Med. 2006;38(5):509–15.
- Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65(3):1018–26.
- Korbelik M, Stott B, Sun J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br J Cancer. 2007;97:1381–7.
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–84.
- Gomer CJ, Ryter SW, Ferrario A, et al. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56(10):2355–60.
- Gollnick SO, Kabingu E, Kousis PC, et al. Stimulation of the host immune response by photodynamic therapy (PDT). Proc SPIE. 2004;5319:60–70.
- Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother. 2007;56(5):649–58.
- Gomer CJ, Ferrario A, Murphree AL. The effect of localized porphyrin photodynamic therapy on the induction of tumour metastasis. Br J Cancer. 1987;56:27–32.
- van Duijnhoven FH, Aalbers RI, Rovers JP, et al. Immunological aspects of photodynamic therapy of liver tumors in a rat model for colorectal cancer. Photochem Photobiol. 2003;78(3):235–40.
- Thong PS, Olivo M, Kho KW, et al. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J Environ Pathol Toxicol Oncol. 2008;27(1):35–42.