Original Article
, Volume: 12( 5)Organoiodine (III) Mediated One-Pot Synthesis of Several New Coumarinyl-Triazolo-Triazines
- *Correspondence:
- Pundeer R , Department of Chemistry, Kurukshetra University, Kurukshetra, Haryana-136119, India, Tel: 01744-238518; E-Mail: dr.rashmip@gmail.com
Received: September 22, 2016; Accepted: October 14, 2016; Published: October 26, 2016
Citation: Pundeer R, Kiran V. Organoiodine(III) Mediated One-Pot Synthesis of Several New Coumarinyl-Triazolo-Triazines. Org Chem Ind J. 2016;12(5):106.
Abstract
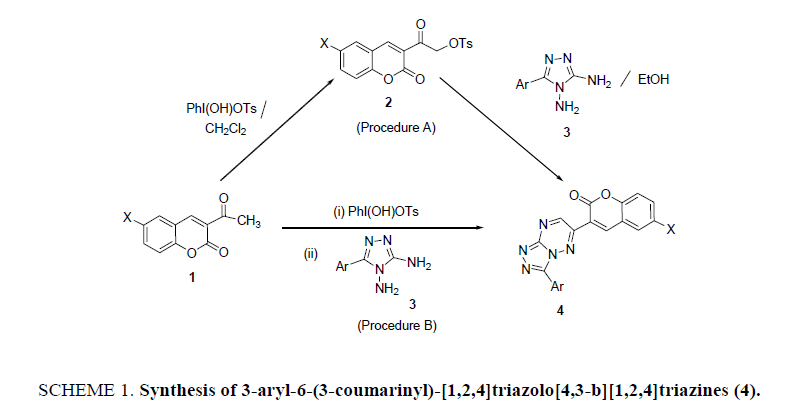
The study foregrounds the efficacy of organoiodine (III) reagent, [hydroxy(tosyloxy)iodo]benzene (PhI(OH)OTs) in the synthesis of new derivatives of coumarinyl containing N-bridgehead heterocyclic compounds namely, 3-aryl-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4] triazines (4) from the reaction of 3-(α-tosyloxyacetyl)coumarins (2) and 5-aryl-3,4-diamino-s-triazoles (3). The umpolung compounds 2 are, in turn, obtained by the α-tosyloxylation of 3-acetylcoumarins 1 with PhI(OH)OTs. The reaction was also carried out in one-pot starting from enolizable ketones 1 by generating the tosyloxyketones 2 in situ. In one-pot procedure, the ketones 1 are first treated with PhI(OH)OTs and then with diaminotriazoles 3 to furnish the expected title compounds 4 in better yields than the stepwise procedure.
Keywords
3-Acetylcoumarins; [hydroxy(tosyloxy)iodo]benzene; One-pot, 3-(α-tosyloxyacetyl)coumarins; 3-aryl-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazines; 5-aryl-3,4-diamino-s-triazoles;
Introduction
Coumarin (2H-chromen-2-one) and its derivatives are considered as privileged compounds in modern heterocyclic era because of their role in synthetic organic chemistry. Coumarins, belonging to a large class of molecules known as benzopyrans, are biodynamic chemotypes showing significant pharmacological and physiological properties [1,2]. In the present study, the 3-acetylcoumarin derivatives 1, possessing the enolizable ketonic group, are employed to prepare the umpolung compounds, 3-(3-(α-tosyloxyacetyl) coumarins 2 on reaction with the organoiodine (III) reagent, [hydroxy(tosyloxy)iodo]benzene (HTIB, Koser’s reagent). HTIB is established to be a versatile reagent for the synthesis of various classes of heterocyclic compounds via α-tosyloxylation of enolizable carbonyl compounds [3-8]. The present work involves the cyclocondensation of 3-(3-(α-tosyloxyacetyl) coumarins and 5-aryl-3,4diamino-1,2,4-triazoles 3 to furnish the title compounds. 1,2,4-Triazole nucleus has been incorporated into a wide range of drugs because of their ability to bind a variety of enzymes and receptors in biological systems via diverse non-covalent interactions [9-11]. Keeping in view the tremendous potential of HTIB in the synthesis of heterocyclic compounds [12-14] and our ongoing research work on the synthesis and biological evaluation of triazole fused heterocycles [15,16], we are reporting here in the synthesis of some new N-containing bridgehead heterocyclic compounds namely, 3-aryl-6-(3-coumarinyl)-[1,2,4] triazolo[4,3-b][1,2,4]triazines 4.
Materials and Methods
Melting points were taken in open capillaries in an electrical apparatus and are uncorrected. IR spectra in KBr were recorded on Perkin-Elmer 1800 FT-IR spectrophotometer. The 1H NMR and 13C spectra were recorded on Bruker instrument at 300 MHz and 75 MHz respectively. The chemical shifts were expressed in ppm units downfield from an internal TMS standard. Purity of the compounds was checked by thin layer chromatography (TLC) using silica gel aluminium sheets of Merck and UV lamp was used for the visualization of the compounds. Elemental analyses were carried out in Perkin-Elmer 2400 instrument. All the chemicals were purchased from commercial suppliers and were used without further purification. The acetylcoumarins 1 were prepared by stirring a mixture of corresponding salicylaldehydes and ethyl acetoacetate in the presence of piperidine [17]. α-Tosyloxylation of 1 was then, performed with HTIB in DCM [18]. For the synthesis of triazoles 3, a mixture of thiosemicarbazide and aryl carboxylic acids was refluxed in the presence of concentrated H2SO4 to obtain thiadiazoles; which on treatment with hydrazine hydrate in ethanol gave the required triazoles 3 [19,20].
3-(3-aryl-[1,2,4]triazolo[3,4-b][1,2,4]triazin-6-yl)-2H- chromen-2-ones (4a-i): General Procedure Procedure A: (via isolation of tosyloxyketones)
Step I: 3-(α-Tosyloxyacetyl) coumarins (2): To a stirred solution of 3-acetylcoumarin (1, 10 mmol) in DCM (50 ml) was added HTIB (10 mmol). The resulting mixture was allowed to stir at 40°C to 50°C temperature. HTIB was initially insoluble in DCM, but gradually disappeared as the reaction proceeded. The stirring was allowed to continue for about 4 h. The solvent was evaporated under vacuum and the gummy mass so obtained, was triturated with pet ether (60°C to 80°C) to remove iodobenzene. The resulting colourless solid was thoroughly washed with water. The solid was recrystallized from ethanol to obtain the pure tosylate compound.
Step II: 3-aryl-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazines(4): To a mixture of 3-(α-tosyloxyacetyl)coumarin (2, 10 mmol) and the triazole (3, 10 mmol) in ethanol (20 ml) was added a pinch of anhydrous K2CO3 and the reaction mixture was heated under reflux for about 4 h to 5 h. About half of the solvent was removed under vacuum and the reaction mixture was cooled to room temperature. The solid, separated out, filtered and recrystallized from ethanol to give pure product 4.
Procedure B: One-pot preparation of 4 starting from 3-acetylcoumarin
Typical procedure: To a mixture of 3-acetylcoumarin 1 (10 mmol) in DCM was added HTIB (10 mmol) and the reaction mixture could stir for 4 h to 5 h. The progress of the reaction was checked by TLC. Excess of the solvent was distilled off under reduced pressure. To the residual mixture was added 5-phenyl-3,4-diamino-1,2,4-triazole (10 mmol), ethanol (20 ml) and a pinch of anhydrous K2CO3. The reaction mixture was heated under reflux for 4 h to 5 h. About half of the solvent was removed under vacuum and the reaction mixture was cooled to room temperature. The solid, separated out, filtered and recrystallized from ethanol to obtain the pure product 4.
Characterization Data of 3-aryl-6-(3-Coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazines (4a-4i) 6-(3-coumarinyl)-3-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4a)
IR (νmax, in KBr): 1715 cm-1; 1H NMR (CDCl3, 300 MHz, δ): 7.34-7.43 (m, 3H), 7.50-7.61 (m, 5H), 69-7.71 (m, 1H), 8.51 (s, 1H), 8.93 (s, 1H); 13C NMR (CDCl3, 75.5 MHz δ): 110.20, 116.37, 118.38, 119.64, 120.93, 121.77, 122.09, 123.36, 124.57, 128.29, 129.57, 131.33, 138.88, 144.08, 152.91, 160.73; Elemental analysis: Calculated for C19H11N5O2: C 66.86, H 3.25, N 20.52; Found: C 66.78, H 3.18, N 20.34.
3-(4-chlorophenyl)-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4b)
IR (νmax, in KBr): 1705 cm-1; 1H NMR (CDCl3, 300 MHz, δ): 7.39-7.43 (m, 3H), 7.47-7.59(m, 3H), 7.98-8.02 (d, 2H), 8.51 (s, 1H), 8.89 (s, 1H); 13C NMR (CDCl3, 75.5 MHz δ): 110.23, 117.25, 119.23, 119.61, 121.86, 124.58, 128.29, 130.25, 131.32, 133.39, 137.61, 138.81, 143.99, 152.75, 159.71, 164.65; Elemental analysis: Calculated for C19H10ClN5O2: C 60.73, H 2.68, N 18.64; Found: C 60.87, H 2.77, N 18.48.
6-(3-coumarinyl)-3-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4c)
IR (νmax, in KBr): 1713cm-1; 1H NMR (DMSO-d6, 300 MHz, δ): 7.35-7.38 (m, 2H), 7.48-7.63 (m, 4H), 7.66-7.69 (m, 2H), 8.55 (s,1H), 9.35 (s, 1H); 13C NMR (DMSO-d6, 75.5 MHz δ): 110.11, 116.13, 116.38, 119.57, 120.90, 121.01, 124.61, 128.32, 131.41, 138.91, 143.99, 152.91, 156.05, 159.68, 161.36, 164.37; Elemental analysis: Calculated for C19H10N6O4: C 59.07, H 2.61, N 21.75; Found: C 58.95, H 2.53, N 21.94.
6-(3-(6-chlorocoumarinyl))-3-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4d)
IR (νmax, in KBr): 1705 cm-1; 1H NMR (CDCl3, 300 MHz, δ): 7.10-7.14(m, 1H), 7.40-7.43 (m, 4H), 7.59-7.61 (m, 1H), 7.61-7.73 (m, 2H), 8.51 (s, 1H), 8.98 (s, 1H); 13C NMR (CDCl3, 75.5 MHz, δ): 116.44, 119.59, 119.87, 121.02, 124.67, 126.75, 128.48, 129.04, 130.39, 131.59, 133.32, 139.75, 148.65, 153.13, 159.87, 167.39; Elemental analysis: Calculated for C19H10ClN5O2: C 60.73, H 2.68, N 18.64; Found: C 60.65, H 2.62, N 18.75.
6-(3-(6-chlorocoumarinyl))-3-(4-chlorophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4e)
IR (νmax, in KBr): 1713 cm-1; 1H NMR (CDCl3, 300 MHz, δ): 7.23 (d, 2H, J=7.8 Hz), 7.31-7.40 (m, 3H), 7.53-7.64 (m, 2H), 8.60 (s, 1H), 8.83 (s, 1H); 13C NMR (CDCl3, 75.5 MHz, δ): 109.91, 116.35, 119.19, 119.59, 120.86, 124.55, 128.28, 130.10, 131.29, 133.38, 137.59, 138.80, 143.97, 152.83, 159.70, 164.63; Elemental analysis: Calculated for C19H9Cl2N5O2: C 55.63, H 2.21, N 17.07; Found: C 55.50, H 2.12, N 17.25.
6-(3-(6-chlorocoumarinyl))-3-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4f)
IR (νmax, in KBr): 1720 cm-1; 1H NMR (DMSO-d6, 300 MHz, δ): 7.40-7.61 (m, 3H,), 7.79-7.96 (m, 4H), 8.61 (s, 1H), 9.12 (s, 1H); 13C NMR (CDCl3, 75.5 MHz, δ): 116.37, 119.53, 119.96, 121.12, 124.69, 126.84, 128.57, 129.14, 130.44, 131.67, 133.37, 139.85, 148.78, 153.25, 159.96, 167.48; Elemental analysis: Calculated for C19H9ClN6O4: C 54.24, H 2.16, N 19.97; Found: C 54.39, H 2.04, N 19.78.
6-(3-(6-bromocoumarinyl))-3-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4g)
IR (νmax, in KBr): 1705 cm-1; 1H NMR (DMSO-d6, 300 MHz, δ): 7.44-7.48 (m, 3H), 7.62-7.67 (m, 2H), 7.78-7.89 (m, 3H), 8.63 (s, 1H), 9.36 (s, 1H); 13C NMR (CDCl3, 75.5 MHz, δ): 114.45, 118.89, 119.67, 121.27, 124.61, 127.05, 128.63, 129.28, 130.42, 131.66, 133.58, 139.81, 148.73, 153.84, 160.04, 167.53; Elemental analysis: Calculated for C19H10BrN5O2: C 54.31, H 2.40, N 16.67; Found: C 54.18, H 2.31, N 16.83.
6-(3-(6-bromocoumarinyl))-3-(4-chlorophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4h)
IR (νmax, in KBr): 1713 cm-1; 1H NMR (DMSO-d6, 300 MHz, δ): 7.34-7.43(m, 3H), 7.50-7.72 (m, 2H), 8.08 (d, 2H, J=4.5 Hz), 8.51 (s, 1H), 8.93 (s, 1H); 13C NMR (DMSO-d6, 75.5 MHz, δ): 112.44, 119.45 119.64, 121.22, 124.67, 126.45, 128.65, 129.14, 130.46, 131.72, 133.52, 138.75, 147.65, 154.13, 159.07, 164.34. Elemental analysis: Calculated for C19H9BrClN5O2: C 50.19, H 2.00, N 15.40; Found: C 50.07, H 1.93, N 15.26.
6-(3-(6-bromocoumarinyl))-3-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4i)
IR (νmax, in KBr): 1705 cm-1; 1H NMR (DMSO-d6, 300 MHz, δ): 7.34-7.43 (m, 2H), 7.48 (d, 2H, J=8.7 Hz), 7.56-7.71 (m, 1H), 8.01 (d, 2H, J=8.7 Hz), 8.51 (s, 1H), 8.89 (s, 1H); 13C NMR (CDCl3, 75.5 MHz, δ): 112.38, 118.19, 119.87, 121.36, 124.84, 126.84, 128.69, 129.37, 130.45, 131.59, 133.48, 139.75, 148.60, 153.23, 159.63, 167.57; Elemental analysis: Calculated for C18H11NO2S: C 49.05, H 1.95, N 18.06; Found: C 48.91, H 1.84, N 18.23.
Results and Discussion
Initially the reaction of the tosylate compound, 3-(α-tosyloxyacetyl)coumarin (2a) and the s-triazole, 3,4-diamino-5-phenyl- 1,2,4-triazole (3a) was examined. Taking clues from the literature reports and after making several attempts under different conditions, the reaction proceeded successfully in ethanol under reflux conditions in the presence of catalytic amount of K2CO3 . The progress of the reaction was monitored through thin layer chromatography and a single product was obtained in very good yield (85%) which was identified as the cycloadduct, 6-(3-coumarinyl)-3-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazine (4a). Encouraged by the fruitful outcome of the attempt, it was considered worthwhile to extrapolate the reaction for the synthesis of variously substituted new derivatives of 3-aryl-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazines by taking different 6- halosubstituted 3-(α-tosyloxyacetyl)coumarins and 5-aryl-3,4-diamino-1,2,4 triazoles. Fortunately, in all the examples, the expected products 4a-4i were obtained, in 78% to 85% yield (Scheme 1, Table 1). Therefore, the HTIB mediated reaction evidenced to be an efficient method for the synthesis of several new coumarinyl-triazolo-triazines.
| Compounds | X | Ar | M.P. (°C) | Yielda (%) | |
|---|---|---|---|---|---|
| Stepwise | One-pot | ||||
| 4a | H | C6H5 | 186-187 | 85 | 94 |
| 4b | H | 4-ClC6H4 | 226-228 | 78 | 90 |
| 4c | H | 4-NO2C6H4 | 284-286 | 83 | 92 |
| 4d | Cl | C6H5 | 235-236 | 81 | 92 |
| 4e | Cl | 4-ClC6H4 | 218-219 | 80 | 93 |
| 4f | Cl | 4-NO2C6H4 | 290-291 | 82 | 95 |
| 4g | Br | C6H5 | 241-242 | 79 | 91 |
| 4h | Br | 4-ClC6H4 | 184-186 | 81 | 94 |
| 4i | Br | 4-NO2C6H4 | 308-309 | 82 | 90 |
aYield (%) of isolated pure product 4 were calculated w.r.t. 1.
Table 1: Physical data of the products 4 prepared according to Scheme 1.
All the newly synthesized coumarin substituted triazolotriazines 4a-4i are new compounds and their structure has been confirmed through appropriate analysis of the spectral and elemental data. The C=O stretch originally present around 1684 cm-1 in the IR spectra of tosylates2 was disappeared in the IR of 4. Also, the singlets positioned around δ 2.47 and 5.40 due to CH3 and CH2 in the 1H NMR spectra of 2, were absent in 4 as expected. Moreover, the 1H NMR spectra of the title products (4a-4i) displayed a characteristic singlet in the region δ 8.8-9.3 due to C7–H. All other protons appeared in the aromatic region.
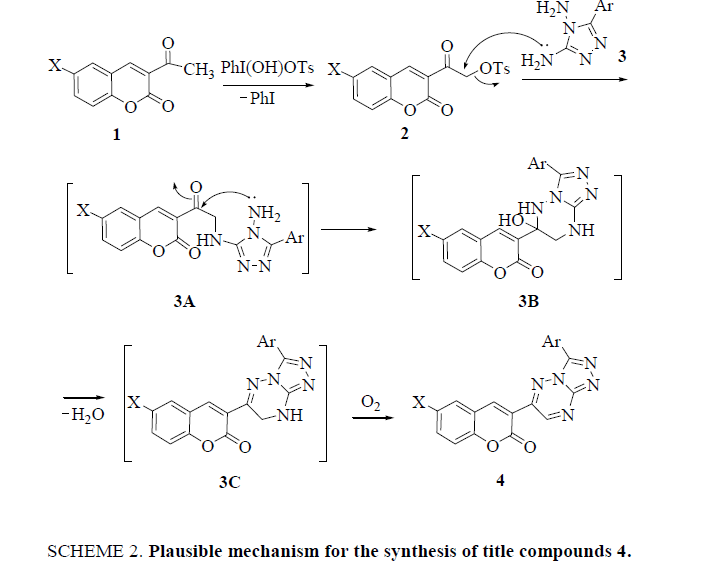
It is well documented that the advantage of tosyloxyketones (TK) mediated syntheses is that it is possible to carry out the reaction in one-pot starting from ketones, by generating the TK in situ, without its isolation. Hence, to further extend the utility of the present synthetic approach, the conversion 1 → 3 was performed in one-pot without isolating the intermediate 3-(α-tosyloxyacetyl)coumarins 2 (SCHEME 1). The title compounds 4 were obtained in all the cases in overall yields (90% to 94%) better than the stepwise procedure. The physical data of the compounds 4 is summarized in Table 1. The probable mechanism of the synthetic reaction is outlined in Scheme 2. HTIB being moderately electrophilic at iodine attacks 3- acetylcoumarins (1) to give the tosylate derivatives 2 with the reductive loss of iodobenzene. 3-(α-Tosyloxyacetyl)coumarins (2) undergo nucleophilic substitution by the attack of amino group of the triazole 3 to give an intermediate 3A. The intermediate 3A, cyclizes to furnish the triazolotriazines 4 by subsequent loss of water molecule and aerial oxidation.
Conclusion
The present study provides organoiodine (III) mediated simplistic synthesis of new derivatives of 3-aryl-6-(3-coumarinyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazines (4) starting from the reactants 3-acetylcoumarins 1 and triazoles 3 through the intermediacy of tosylates 2. The efficacy of the reaction was further extended by adopting the single vessel procedural development without isolating the intermediate TK. The one reactor procedure was proved to be superior in terms of experimentation, time duration and yield.
Acknowledgements
We thank University Grants Commision (UGC), New Delhi for providing the financial assistance to accomplish the work and to Kurukshetra university, Kurukshetra, Haryana for providing the necessary research facilities.
References
- Govori S. Convenient methods for the synthesis of pentacyclic fused heterocycles with coumarin moiety. Synth Commun. 2016;46(7):569-80.
- Tangeti VS, Kumar RV, Prasad GVS, et al. Synthesis of C3-dihydrofuran substituted coumarins via multicomponent approach. SynthCommun.2016;46(7):613-9.
- Yoshimura A, Zhdankin VV. Advances in synthetic applications of hypervalent iodine compounds. Chem Rev. 2016;116(5):3328-435.
- Koser GF. The Synthesis of Heterocyclic Compounds with hypervalent organoiodine reagents. AdvHeterocycl Chem.2004;86:225-92.
- Koser GF. Aldrichimica Acta. 2001;34:89.
- Moriarty RM, Prakash O. Synthesis of heterocyclic compounds using organohypervalent iodine reagents. Adv Heterocycl Chem.1997;69:1-87.
- Prakash O, Saini N, Sharma PK. Hypervalent iodine reagents in the synthesis of heterocyclic compounds.Synlett. 1994;4:221-7.
- Prakash O, Saini N, Sharma PK. [Hydroxy (tosyloxy) iodo] benzene: a useful hypervalent iodine reagent for the synthesis of hetercocyclic compounds. Heterocycles. 1994;38(2):409-31.
- Zheng H, Wang K, Zhang W, et al.Selenium dioxide?mediated synthesis of fused 1,2,4-Triazoles as cytotoxic agents. Synth Commun.2015;45(24):2849-56.
- Kaur N. Role of Microwaves in the Synthesis of Fused Five-Membered Heterocycles with Three N-Heteroatoms. Synth Commun. 2015;45(4):403-31.
- Kleemann A, Engel J. Pharmaceutical Substances.3rd ed.Stuttgart: Thieme, Germany;1999.
- Pundeer R, Sushma.Indian J Chem. 2015;54B:1275.
- Aggarwal R, Pundeer R, Kumar V, et al.A Facile synthesis of thiazole-2 (3 H)-thionesthrough [Hydroxy (tosyloxy) iodo] benzene. Synth Commun. 2004;34(14):2659-64.
- Prakash O, Pundeer R, Chaudhri V. Organoiodine(III)-mediated one-pot synthesis of N-substituted 2-aminothiazoles. JIndChemSoc. 2004;81:786-8.
- Pundeer R, Vijaykiran, Prakash R, et al.a,a-Dibromoacetophenones mediated synthesis of some new 7H-7-alkoxy-3-alkyl/phenyl-6-aryl-s-triazolo[3,4-b][1,3,4]thiadiazines and their antimicrobial evaluation.Med Chem Res. 2012;21(12):4043-52.
- Pundeer R, Sushma, Sharma C, et al. Dibromoketones in the convenient synthesis of some thiazolo[3,2-B]-1,2,4-Triazoles and their Antimicrobial activity: Isolation of new intemediates. Int J Adv Res PharmaBiosci. 2013;3:102.
- Sahu SK, Mishra A, Behera RK. Synthesis of thiazole, benzothiazole, oxadiazole, thiadiazole, triazole and thiazolidinone incorporated coumarins. IndJHeterocyclChem. 1996;6(2):91-4.
- Kumar M. Organohypervalent Iodine(III) reagents in organic synthesis[dissertation].Kurukshetra: Kurukshetra University, India; 2012.
- Padhy AK, Nag VL, Panda CS. Studies on the synthesis and bioactivity of some thiadiazole derivatives. Ind J Chem. 1999;38(8):998-1001.
- Gupta M, Paul S, Gupta R. Efficient and novel one-pot synthesis of antifungal active 1-substituted-8-aryl-3-alkyl/aryl-4H-pyrazolo[4,5-f][1,2,4]triazolo[4,3-b][1,2,4]triazepines using solid support. Eur J Med Chem. 2011;46(2):631-5.