Research
, Volume: 15( 3) DOI: 10.37532/0974-7435.2019.15(3).190Optimization of β-Galactosidase Production by Trametes versicolor J5 in Whey
- *Correspondence:
- Leandro de Souza Lopes, Universidade Federal de Viçosa, Departamento de Microbiologia Agrícola, Viçosa, MG, Brasil, E-Mail: souzalopesleandro@gmail.com
Received: April 22, 2019; Accepted: May 10, 2019; Published: May 17, 2019
Citation: Elias LF, Pravato VT, Lopes LS, et al. Optimization of β-galactosidase production by Trametes versicolor J5 in whey. Biotechnol Ind J. 2019;15(3):190.
Abstract
In this work the fungus Trametes versicolor J5 was grown in whey with the objective of producing the enzyme β-galactosidase that has a number of applications, mainly in the food industry. In fermentation for 48 hours, we have pH 8 and the temperature close to 30°C, as optimal points. In fermentations for 96 and 120 hours, we have little temperature interference for maximum β-galactosidase production. In relation to pH, we have values close to 8 as ideal for the 96 and 120 hours of fermentation times. In this way, the Trametes versicolor J5 fungus was able to grow in the whey with β-galactosidase production, thus reducing the costs of the process, as well as solving the problems associated with its elimination.
Keywords
Whey; trametes versicolor; fungi; residue; optimization
Introduction
The optimization through factorial planning and response surface analysis is a common practice in the field of biotechnology. In order to determine optimum values for the processing parameters, such as pH, temperature, and aeration [1-4], Response Surface Methodology (RSM) can be understood as a combination of experiment planning techniques, regression analysis, and optimization methods [5]. They are very useful during the stage of product or process development and also in the improvement of existing products. For the biotechnological products, there is a great need to study techniques that can be carried out to increase the scale of production with minimization of process costs, without loss of yield [6,7].
The biotechnology has shown numerous possibilities for large-scale production of various proteins/enzymes that are important in research related to pharmaceutical, food and industrial applications. β-galactosidase has potential applications in the food processing industry [8]. This enzyme is used to obtain lactose hydrolysate from milk and whey for use in bakery products, feed and as a source of sugar for various fermentation products. β-galactosidase is used to prevent the crystallization of lactose in ice cream and dairy products. The syrup produced by the conversion of lactose is used in the dairy and soft drinks industries. In the pharmaceutical industry, it is used to produce lactose-free milk for lactose intolerant consumers who are unable to digest milk lactose [9,10].
There is a demand for the production of biotechnological products with the use of residues or by-products of the industry to minimize the costs, allowing the production of more accessible enzymes [11-13]. A number of studies have been carried out, replacing carbon and nitrogen sources with agroindustrial residues that provide the necessary amount of these nutrients for the growth of the microorganism and for the production of β-galactosidase. The milk whey is used as a substrate for the generation of biotechnological products. Whey represents 80% to 90% of the total volume of the milk used during cheese production and contains approximately 55% of milk nutrients: soluble proteins, lactose, vitamins, minerals and a minimum amount of fat [14,15], being a rich medium for the growth of microorganisms, and is still a by-product of dairy products, where it often becomes an environmental problem, since many industries pour whey into rivers [16].
The use of the experimental design methodology, followed by the application of the central rotational compound design to investigate the best conditions of β-galactosidase production in the presence of cheese serum, has been applied by some authors [17-19]. Thus, the objective of this work was to evaluate the fermentation of whey reconstituted by a basidiomycete fungus to produce the β-galactosidase enzyme, by means of experimental design of the central rotational compound design (EDCRC) and evaluation of the results by means of RSM.
Materials and Methods
This work was conducted at the Research Laboratory of Ifes-Campus Venda Nova do Imigrante-ES, Brasil.
Inoculum
The Trametes versicolor isolate J5 is of fungal collection of the Laboratory of Mycorrhizal Association. The strain was grown for 15 days at 25 ± 1°C in Petri dishes containing 20 mL of Potato-Dextrose Agar medium (PDA).
Production of the enzyme via reconstituted serum fermentation
For the production of the enzymes, we selected some parameters to be evaluated during the process, such as temperature, wort pH and protein concentration in the wort, which was bound to the serum concentration in the wort, that is, reconstitution of whey powder to achieve a certain concentration of protein.
The parameters and their minimum and maximum values for the tests were determined: wort protein concentration (reconstituted serum), pH and fermentation temperature. As the concentration of proteins is linked to the reconstitution of the serum, and thus, there were limitations in the concentrations, due to the viscosity and solubility of the solution, the increase of the protein content made the wort very viscous and with the formation of lumps and did not allow good growth of the fungus. The limitations of pH and temperature are linked to the characteristics of the Trametes versicolor J5 fungus. The fermentation assay was conducted in a shaker at 50 rpm in 250 mL Erlenmeyer, in a reaction volume of 120 mL.
Experimental planning
The process of optimization of the fermentation for the production of enzymes was performed using an experimental design of the central rotational compound design (DCCR). The experimental design was generated by the Minitab program and the selected parameters were: protein concentration in the wort (performed by reconstituting whey powder with water) (1.0 to 1.5%), pH (4,8) and temperature (25°C to 40°C). The experiment was set up in a completely randomized design in the factorial scheme. A multivariate analysis of variance was performed followed by the Tukey test with 5% significance. The variable response activity of β-galactosidase was monitored during fermentation after 48, 96 and 120 hours of incubation. The levels and values of these variables evaluated were generated by the Minitab program.
Enzymatic activity
The fermented wort was filtered on Whatman #1 filter paper. The filtrate was the crude extract, which was evaluated in the determination of enzyme activity.
The activity of β-galactosidase was performed using Orto-nitrofenil beta-D-galactopiranosídeo (ONPG) as a substrate. The volumes of solutions used in this method were: 2 mL of ONPG in 50 mM Tris-HCl buffer pH 7.5 and 200 μl of crude enzyme extract. The mixture was incubated at 45°C for 15 minutes. The reaction was quenched with the addition of 0.5 mL of 1 M sodium carbonate and the product of the reaction was o-nitrophenol (ONP) chromophore that is yellow in color, measured in a spectrophotometer at 420 nm. A β-galactosidase (U) unit was defined as the amount of enzyme capable of releasing 1 μmol of ONP per minute under the assay conditions.
In this work, the expression of the β-galactosidase production from the cheese serum was expressed in volumetric enzymatic activity.
Results
The activity of β-galactosidase, and optimization of production
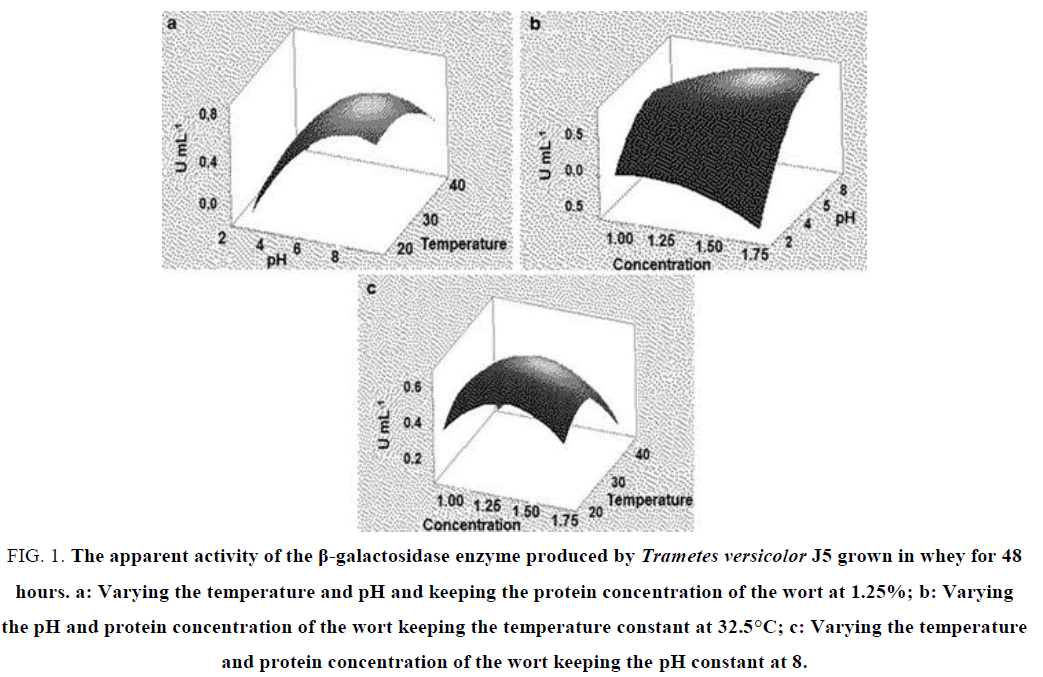
In fermentation for 48 hours (FIG. 1), it is possible to evaluate the points of greatest production of enzymatic activity correlating each parameter analyzed. In relation to pH and temperature, we have pH 8 and the temperature close to 30ºC as optimal points, that is, these points lead to a maximum production of the enzyme of interest (FIG. 1a). We can observe that β-galactosidase activity tends to increase as we increase the pH and temperature to about 8 and 30°C, respectively. This shows a direct relationship between these two analyzed variables. When the variables pH and concentration of the wort were evaluated, we can verify that the activity of the enzyme is maximal in pH, 8 and tends to fall with the increase of the wort concentration (FIG. 1b). There is a positive correlation between the variables wort concentration and temperature. As can be seen in the graph the maximum activity is at the concentration of 1.25% at a temperature of 30 °C (FIG. 1c). This means that it is possible to work with a lower concentration of proteins in the wort and still obtain greater production of the enzyme β-galactosidase.
FIG. 1. The apparent activity of the β-galactosidase enzyme produced by Trametes versicolor J5 grown in whey for 48 hours. a: Varying the temperature and pH and keeping the protein concentration of the wort at 1.25%; b: Varying the pH and protein concentration of the wort keeping the temperature constant at 32.5°C; c: Varying the temperature and protein concentration of the wort keeping the pH constant at 8.
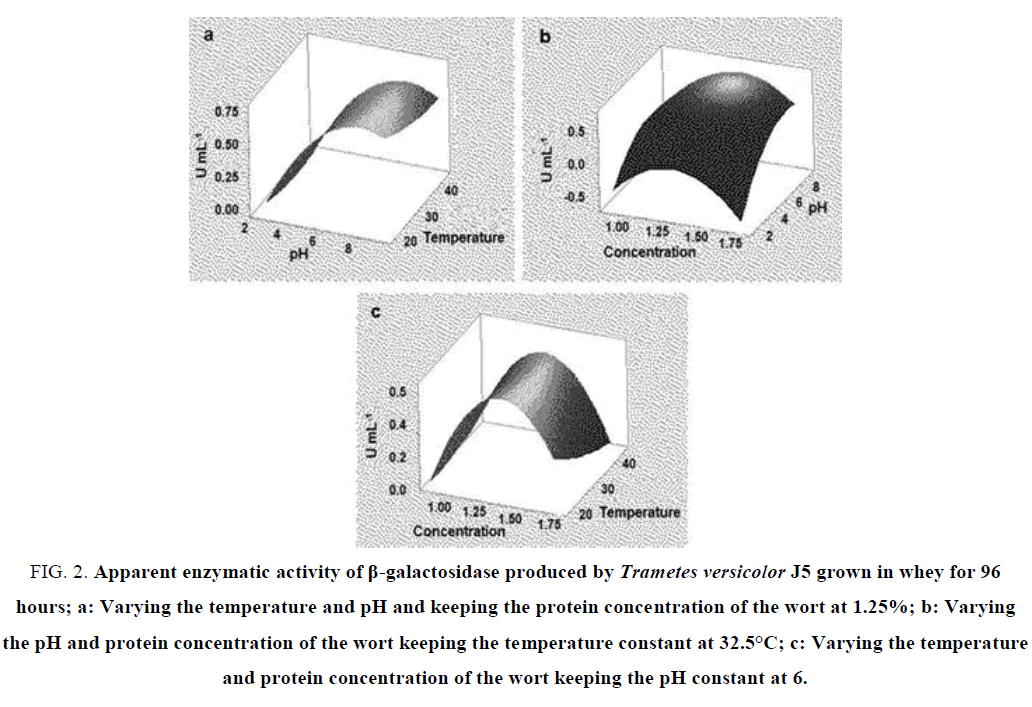
In the fermentation that occurred for 96 hours when we analyzed the variables pH and temperature together, we verified that there is an increase in the activity of β-galactosidase as the pH is increased up to 8 and little interference of the temperatures analyzed in the activity of this enzyme (FIG. 2a). As in the fermentation for 20 hours, we also observed an increase in the activity of the enzyme as the pH increases, with maximum activity at pH 8 and concentration of the mostro at 1.25% (FIG. 2b). In the analysis of the variables concentration of the wort and temperature there is a maximum activity of the enzyme in the substrate concentration around 1.25 to 1.50% and at a temperature of 20ºC to 30ºC. The β-galactosidase activity was minimal at concentrations of 1 and 1.75% (FIG. 2c).
FIG. 2. Apparent enzymatic activity of β-galactosidase produced by Trametes versicolor J5 grown in whey for 96 hours; a: Varying the temperature and pH and keeping the protein concentration of the wort at 1.25%; b: Varying the pH and protein concentration of the wort keeping the temperature constant at 32.5°C; c: Varying the temperature and protein concentration of the wort keeping the pH constant at 6.
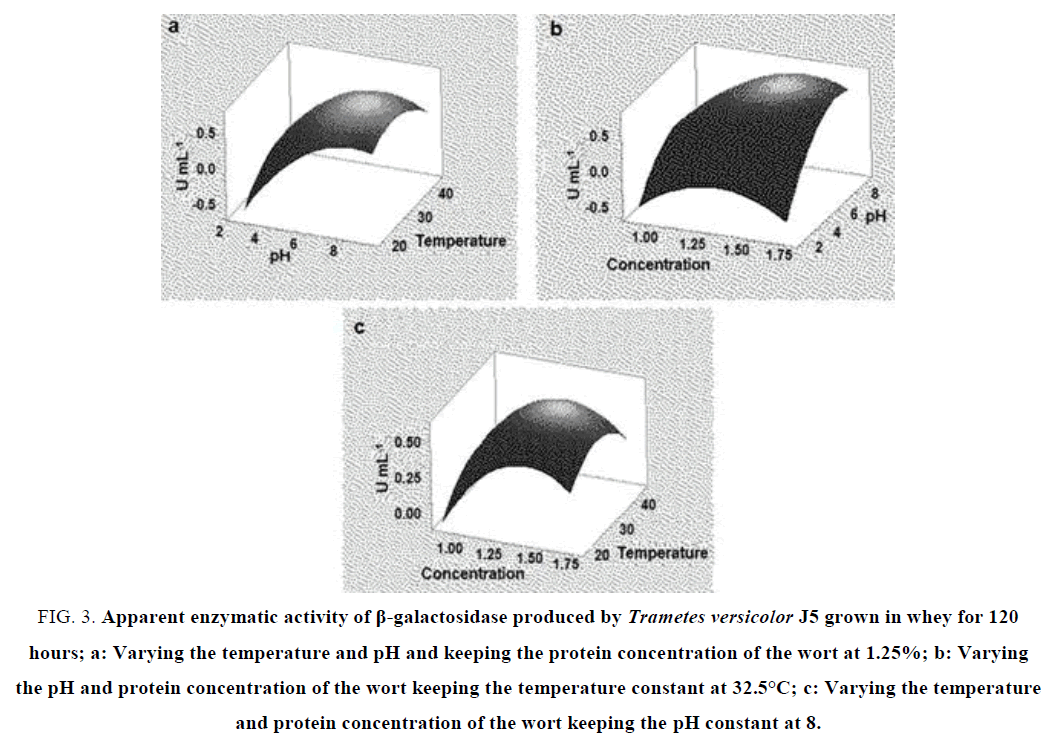
When analyzing the pH and temperature variables in the fermentation for 120 hours, the maximum enzyme activity is reached at pH 6 at a temperature of 20°C to 40°C. In this case, this variable had no significant impact on enzyme activity in the range studied (FIG. 3a). This thermostability of the enzyme is an interesting feature in the industrial application. As for the variables and substrate concentration and pH, the peak β-galactosidase activity was again at pH 8 at the concentration of 1.25% to 1.50% of the wort (FIG. 3b). For the concentration and temperature variables, the peak of enzymatic activity was also in the concentrations of 1.25% to 1.50% of the mostro at the temperature close to 30°C (FIG. 3c). This pattern of enzymatic synthesis demonstrated by Trametes versicolor J5 shows that this fungal isolate can be a good source for the industrial production of thermostable β-galactosidase.
FIG. 3. Apparent enzymatic activity of β-galactosidase produced by Trametes versicolor J5 grown in whey for 120 hours; a: Varying the temperature and pH and keeping the protein concentration of the wort at 1.25%; b: Varying the pH and protein concentration of the wort keeping the temperature constant at 32.5°C; c: Varying the temperature and protein concentration of the wort keeping the pH constant at 8.
The Trametes versicolor grown in whey had a maximum production of β-galactosidase (1248 μmol L-1) after 20 days of incubation, at 37°C and pH ranging from 3 to 4 [20]. This value is well above that found in our work, but besides the whey used in the culture medium for the fermentation, other sources of nutrients were added to the same, such as peptone, yeast extract and some minerals that may aid in the development of the fungus and thus greater production in a shorter period of time.
The maximum enzyme activity for a yeast isolate was in the range of pH 5.3-5.5 and incubation temperature of 29°C to 31°C [21], values very close to those found in this work for Trametes versicolor J5, although pH 8 is the one with the highest activity, at pH 6 we achieved high values of enzymatic production using less substrate when compared to pH 8.
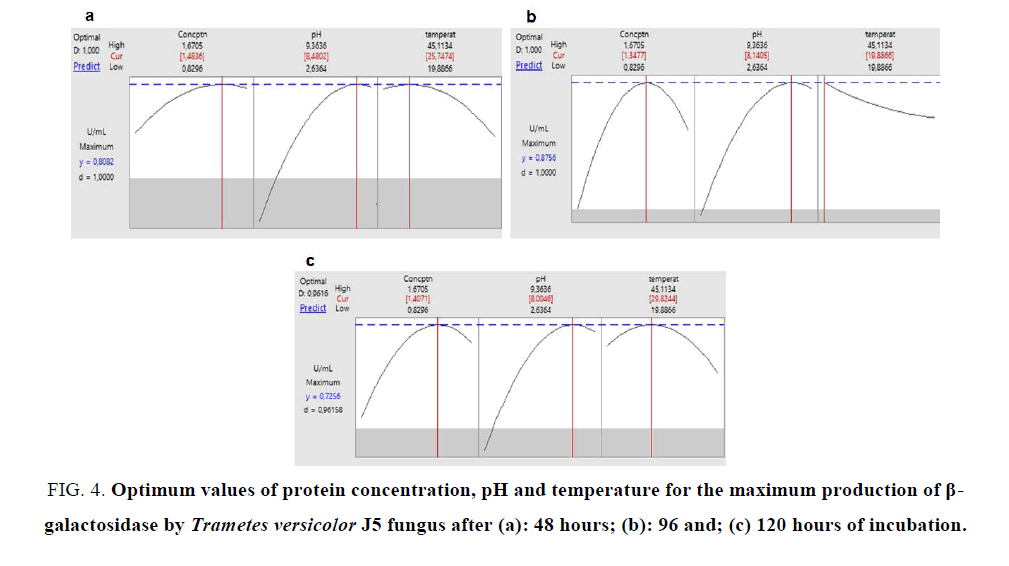
Using the Minitab program, we performed a predictive analysis from the experimental results. Observing the values of enzymatic activities found for the 3 fermentation periods, it was verified that the highest activity occurred in the 96 hour period with a value of 0.8795 U mL-1 and for the other times (48 and 120 hours) the maximum values of β-galactosidase activity were 0.80 and 0.75 respectively (FIG. 4). The obtained results indicate that in 96 hours of fermentation occurs greater β-galactosidase production. In a work with K. marxianus grown on whey, optimal β-galactosidase enzyme production was achieved after 40 to 50 hour of incubation [22]. In further work with this yeast, optimized β-galactosidase production was achieved after 27 hours of incubation, yielding 1.73 IU mg-1, not increasing after this period [23], reaching maximum production of this enzyme in a shorter period of time than Trametes versicolor J5.
FIG. 4. Optimum values of protein concentration, pH and temperature for the maximum production of β-galactosidase by Trametes versicolor J5 fungus after (a): 48 hours; (b): 96 and; (c) 120 hours of incubation.
Aspergillus nidulans had the production of optimized β-galactosidase obtaining maximum production value of 60 U mg-1 after 10 days of incubation at 35°C and pH 3 [9]. However, the optimization of the parameters was done separately, without taking into account the interaction between them, as was done in this work. An isolated β-galactosidase from Aspergillus oryzae has maximum activity at 59°C and remained stable at that temperature for up to 50 min, decreasing after that period [23].
Discussion and Conclusion
Response surface methodology proved to be a useful tool in the development of optimum conditions for the production of β-galactosidase from whey. The best parameters for the production of this enzyme by Trametes versicolor J5 (0.8795 U mL-1) were at pH 6, at 32.5°C, with the highest concentration of 1.25% for 96 hours. The use of whey to produce β-galactosidase can not only reduce process costs but can also solve the problems associated with its elimination.
References
- Batista E, Rodrigues MI, Meirelles AJ. Optimization of a secondary reflux and vaporization (SRV) distillation process using surface response analysis. Comput Chem Eng. 1998;22:737-40.
- Rodrigues L, Teixeira J, Oliveira R, et al. Response surface optimization of the medium components for the production of biosurfactants by probiotic bacteria. Process Biochem. 2006;4:1-10.
- Iyer R, Tomar SK, Singh AK. Response surface optimization of the cultivation conditions and medium components for the production of folate by Streptococcus thermophilus. J Dairy Res. 2010;77:350-56.
- Yin H, Fan G, Gu Z. Optimization of culture parameters of selenium-enriched yeast (Saccharomyces cerevisiae) by response surface methodology (RSM). LWT-Food Sci Technol. 2010;43:666-69.
- Novaes CG, Yamaki RT, de Paula VF, et al. Otimização de Métodos Analíticos Usando Metodologia de Superfícies de Resposta-Parte II: Variáveis de Mistura. Rev Virtual Quim. 2018;10:1-28.
- Barros Neto B, Scarminio IS, Bruns RE. Como fazer experimentos: pesquisa e desenvolvimento na ciência e na indústria. Campinas, SP: Editora da UNICAMP. 2007;3:480.
- Rodrigues MI, Iemma AF. Planejamento de experimentos e otimização de processos. Campinas, SP: Cárita Editora. 2009;2:231-46.
- Husain Q. β-Galactosidases and their potential applications: A review. Crit Rev Biotechnol. 2010;30:41-62.
- Kamran A, Bibi Z, Aman A, et al. Hyper production of β‐galactosidase from newly isolated strain of Aspergillus nidulans. J Food Process Eng. 2017;40:12452.
- Saqib S, Akram A, Halim SA, et al. Sources of β-galactosidase and its applications in food industry. Biotech. 2017;7:7-79.
- Damaso MCT, Passianoto MA, Freitas SCD, et al. Utilization of agroindustrial residues for lipase production by solid- state fermentation. Braz J Microbiol. 2008;39:676.
- Da Silva GA, Almeida WE, Martins ES, et al. Produção e caracterização de protease obtida por Gliocladium verticilloides através da fermentação em estado sólido de subprodutos agroindustriais. Braz J Agroindustrial Technol. 2009;3:28-41.
- Ernandes FMPG, Boscolo M, Cruz CHG. Influência da composição do meio para a produção de Zimomonas mobilis. Acta Scientiarum Technol. 2010;2:21-26.
- Oliveira DF, Bravo CEC, Tonial IB. Soro de leite: um subproduto valioso. Revista do Instituto de Laticínios Cândido Tostes. 2012;67:64-71.
- Alves MP, Moreira RO, Rodrigues Júnior PH, et al. Soro de leite: Tecnologia para o processamento de coprodutos. J Dairy Inst Cândido Tostes. 2014;69:212-26.
- Perini BLB, Souza HCM, Kelbert M, et al. Production of β-galactosidase from cheese whey using Kluyveromyces marxianus CBS 6556. Chem Eng Trans. 2013;32:991-96.
- Manera AP, Ores JC, Ribeiro VA, et al. Use of agroindustrial residues in biotechnological process by beta-galactosidase production from Kluyveromyces marxianus CCT 7082. Acta Scientiarium Technol. 2011;33:155-61.
- Rashmi R, Siddalingamurthy KR. Optimization of β-galactosidase production by response surface methodology. Int J Biosci. 2011;1:119-27.
- Al-Jazairi M, Abou-Ghorra S, Bakri Y, et al. Optimization of (Beta)-galactosidase production by response surface methodology using locally isolated Kluyveromyces marxianus. Int Food Res J. 2015;22:1361.
- Silvério SC, Macedo EA, Teixeira JA, et al. New β-galactosidase producers with potential for prebiotic synthesis. Bioresource Technol. 2018;250:131-39.
- Kumari S, Panesar PS, Kaur R, et al. Statistical modeling of β-galactosidase production from novel yeast isolate using cheese whey. J Sci Ind Res. 2019;78:81-5.
- Rajoka MI, Samia K, Riaz S. Kinetics and regulation studies of the production of β-galactosidase from Kluyveromyces marxianus grown on different substrates. Food Technol Biotechnol. 2003;41:315-20.
- Klein MP, Sant’Ana V, Hertz PF, et al. Kinetics and thermodynamics of thermal inactivation of β-galactosidase from Aspergillus oryzae. Braz Arch Biol Technol. 2018;61:1-18.