Original Article
, Volume: 11( 3)Optimization of Carbon and Nitrogen Source in the Biotransformation Media of Baicalin by Aspergillus oryzae
- *Correspondence:
- De-Quan Qiu, Fisheries College, Guangdong Ocean University, Zhanjiang, 524088, Guangdong, China, Tel: 867592383111; E-mail: qmsld@163.com
Received: June 24, 2017; Accepted: June 29, 2017; Published: July 03, 2017
Citation: He M, Chen W, Tian L, et al. Optimization of Carbon and Nitrogen Source in the Biotransformation Media of Baicalin by Aspergillus oryzae. Biochem Ind J. 2017;11(3):117
Abstract
Baicalin was an important effective component in Scutellariae radix, which had extensive pharmacological activities and had been widely applied in most drugs, but it couldn’t be easily absorbed by humans. However, aglycone baicalein has better pharmacological effects and high bioavailability but with lower contents in Scutellariae radix. In this study, Aspergillus oryzae was employed as the biotransformation microorganism. And then the biotransformation of baicalin and the optimization of the carbon source and nitrogen source in the biotransformation media were conducted. Generally, the best biotransformation media of baicalin by Aspergillus oryzae was optimized as the combination of 0.0017% arginine, 0.0017% valine, 0.0017% glutamic acid, 0.0017% alanine, 0.0017% histidine, 0.0017% tyrosine and 0.01% urea, 0.4% KH2PO4, 0.04% CaCl2, and 0.04% MgSO4, pH 6, with a biotransformation efficiency of 88.36%. Compared with using corncob, bran, glucose, dissoluble starch as carbon source, it was significantly better for the biotransformation of baicalin without carbon source except the substrate baicalin. The best nitrogen source was the combination of 0.01% urea and six amino acids. Comprehensively considering biotransformation efficiency and cost, 0.1% urea was also a good inorganic nitrogen source for the biotransformation of baicalin by Aspergillus oryzae.
Keywords
Baicalin; Biotransformation media; Aspergillus aryzae; β-glucosidase
Introduction
As a traditional Chinese medicine, Scutellaria baicalensis has been widely used in China due to the high biological activities in the root. Both of Baicalin and its aglycone baicalein are the major active components in Scutellaria baicalensis, and have extensive pharmacological activities due to their anti-inflammatory, anti-virus, anti-metamorphosis, fever relieving and hepar protecting properties, etc. [1-6]. Comparatively, baicalin is with higher contents in Scutellaria baicalensis but hardly to be absorbed by human digestive tract. However, aglycone baicalein is with lower contents but has better pharmacological effects and high bioavailability [4-9]. So it is necessary to convert baicalin to baicalein in order to improve pharmacological activities and bioavailability of Scutellaria baicalensis.
Baicalin could be transformed into baicalein by biological, chemical and physical methods. Compared with chemical and physical methods, biotransformation is an enzyme-catalyzed process with mild reaction conditions, simple operation, strong effectiveness and selectivity, and environment-friendly. There have been many successful examples of microbial biotransformation. Rutin can be biotransformed by fungi (Streptomyces griseus and Aspergillus niger) with a transformation efficiency of 54% [10,11]. Microbial transformations of Artemisinin were also achieved by algae Cunninghamella echinulata and fungi Aspergillus niger [12]. A number of microorganisms (fungi, bacteria, yeast) can transform limonene to other compounds [13,14]. Both of baicalin and baicalein was obtained from Scutellaria baicalensis and the transformation processes was thought to be accompanied by the reaction of hydrolysis [15]. β-Glucuronidase was considered as the most important enzyme in the microbial biotransformation of baicalin. The activating condition of β-Glucuronidase in the bacteria Lactobacillus delbrueckii Rh2 was optimized, which was used in biotransformation process of baicalin [16]. Moulds were usually used in many biotransformation processes due to their rich enzymes, but there is lack of literatures that reported the biotransformation baicalin to baicalein using moulds.
Moulds were usually used for biotransformation processes due to their rich enzymes. Aspergillus oryzae is a strain with complex enzymes including protease, amylase, glucoamylase, cellulase, phytase, etc. which has been safely used in the industry field of food, feed, kojic acid production, wine fermentation for over 1000 years. β-Glucosidase is also an important enzyme in Aspergillus oryzae and played the major role in the microbial biotransformation of baicalin. Wu et al. studied the fermentation conditions of Aspergillus oryzae producing β-glucuronidase, and the media with a good yield was obtained [17]. In this study, we explored the effects of different carbon and nigrogen source in the biotransformation media on the biotransformation of baicalin by Aspergillus oryzae and optimized the carbon and nitrogen source to enhance its biotransformation efficiency.
Material and Method
Microorganisms and chemicals
Aspergillus oryzae was separated, indentified and stored in our laboratory; Scutellariae radix, the root of Scutellaria baicalensis, was purchased from a medical shop in Zhanjiang, China; Baicalin and baicalein standards (purity>98%, HPLC grade) were both purchased from National Engineering Research Center of Chinese medicine solid preparation in NanChang, China; amino acid such as leucine, cysteine, histidine, aminosuccinic acid, glycine, alanine, tyrosine, valine, proline, cystine, lysine, methionine, arginine, glutamic acid and urea were all purchased from Sangon company, Shanghai, China; methanol, H3PO4, KH2PO4, CaCl2, MgSO4, urea and other reagents were all of analytically pure; corncob and soybean powder were bought from a market in Zhanjiang, China.
Media of microorganism
Three media were employed for the activation, intermediate culture of Aspergillus oryzae and the biotransformation of baicalin. According to our previous study [18], integrated potato slant-culture medium and liquid integrated potato medium were separately and prepared for the activation and intermediate culture. The biotransformation media was refer to the media reported by Wu et al. [17].
Extract, purification and identification of baicalin
Baicalin was extracted from Scutellariae radix, and then purified. Then, baicalin was identified by TLC (thin-layer chromatography) analysis, which have been reported in our previous study [18].
The determination of baicalein in the media
Determination of baicalein was conducted by HPLC, as described in our previous study [18]. First, balcalein methonal solution (100 μg/mL) was prepared for stock solution. And then stock solution was diluted to 10 μg/mL, 20 μg/mL, 30 μg/mL, 40 μg/mL and 50 μg/mL respectively. Each diluted solution (20 μL) of balcalein was measured by HPLC. According to the peak area of each concentrations of balcalein and its corresponding concentration, the standard curve was drawn and regression equation was obtained.
The activation of Aspergillus oryzae and biotransformation of baicalin
Aspergillus oryzae was activated in integrated potato slant-culture medium and then transfer to liquid integrated potato medium for intermediate culture. After that, 1 mL liquid integrated potato medium containing cultured Aspergillus oryzae was added into 250 mL of the biotransformation media. And then 0.2 g balcalin is added into the biotransformation media for 3 days in a shaker. Biotransformation media (5 mL) was extracted with ether (2 mL × 4) and the organic layers were combined. The solution was evaporated to dryness to afford yellow solid. The solid was dissolved in methanol (10 mL) and determined by HPLC contrasted to baicalein standard.
Conversion rate of balcalin was calculated as the following formula:
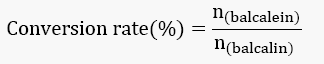
Optimization of the carbon and nitrogen sources in the biotransformation media of baicalin
The optimization of the carbon and nitrogen sources in the biotransformation media was conducted based on the basic media of 0.4% KH2PO4, 0.04% CaCl2, and 0.04% MgSO4.
0.1% peptone was employed as nitrogen source of the biotransformation media for optimizing the carbon source. Corncob, bran, glucose, dissoluble starch was used as the carbon sources of biotransformation media. The effect of carbon sources was not only investigated through changing the concentration of corncob, but also the carbon species such as 2% bran, 1% bran and 1% corncob, 2% glucose, 2% dissoluble starch, and even without carbon source.
The carbon source with the highest biotransformation efficiency was employed for the nitrogen optimization of the biotransformation media. First, the impact of nitrogen sources (peptone, yeast extract, soybean powder, urea, NH4NO3, (NH4)2SO4 fixed with 0.1% and without nitrogen sources were evaluated. The best nitrogen source was obtained and applied to figure out the best concentration for the biotransformation. Second, the effects of single amino acid (leucine, histidine, glycine, tyrosine, proline, cysteine, lysine and arginine, etc.) with concentration of 0.01% were explored. Third, the influence of the combination with different levels of the amino acid mentioned above and the combination of amino acid mixtures with different concentrations of urea on the biotransformation of baicalin were also determined.
Results and Discussion
Measurement of baicalein
Baicalein was measured by HPLC. The results showed that there was a good linear relationship between the peak area and the concentration of baicalein within the concentration from 10 μg/mL to 50 μg/mL. Regression equation was y=58.68 × -23.77, and the R2 was 0.99977.
The optimization of carbon sources in the biotransformation media
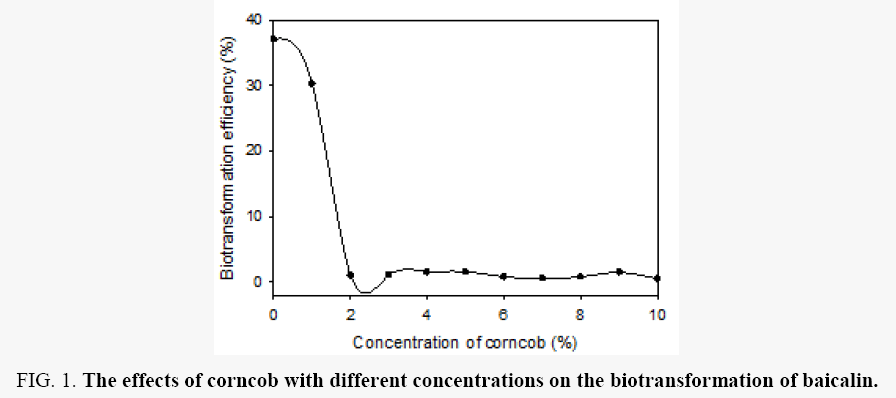
Effects of corncob: Corncob was considered as the best carbon source in biotransformation of isoflavone in Aspergillus oryzae [17]. Accordingly, the effects of different concentration (0%-10% respectively, w: v) of corncob on the biotransformation of balcalin were studied. The results showed corncob was not good carbon resource in biotransformation of baicalin in Aspergillus oryzae, which is different from biotransformation process of commonly isoflavones. The highest biotransformation efficiency was obtained when the biotransformation media had no corncob, with a biotransformation efficiency of 37.1% (Figure 1). In order to improve the biotransformation efficiency, other types of carbon resources was applied as following.
Figure 1: The effects of corncob with different concentrations on the biotransformation of baicalin.
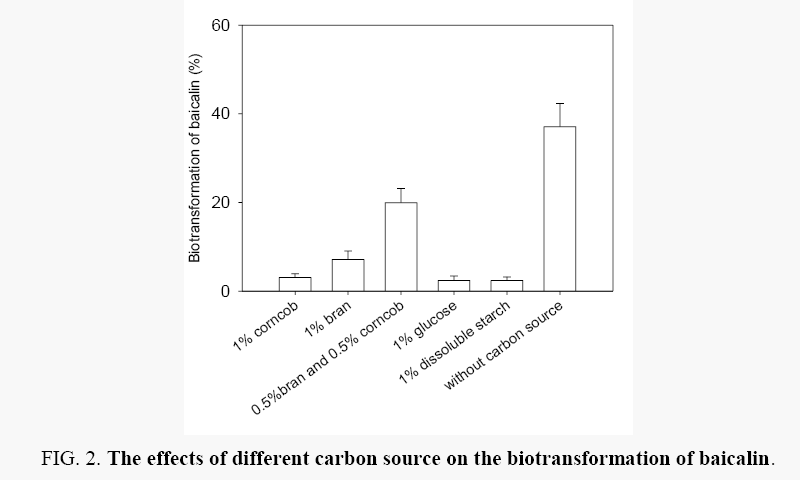
Effects of carbon sources: The effects of 2% (W:V) carbon sources including bran, combination of equal bran and corncob, glucose, dissoluble starch and even without carbon source on the biotransformation of baicalin by Aspergillus oryzae was explored. Generally, the biotransformation efficiency of baicalin was ranged from 0.4% to 37.1% (Figure 2). As shown from the Figure 2, when the media had no carbon source, the biotransformation efficiency is also highest. The results showed that the best carbon resource is baicalin itself, not external carbon resource. The media had no carbon source, which was used in following optimization process.
The optimization of nitrogen sources in the biotransformation media
The biotransformation media for the optimization of the nitrogen sources was on the basis of the result, with 0.4% KH2PO4, 0.04% CaCl2, 0.04% MgSO4, without external carbon source, and with 0.1% nitrogen sources.
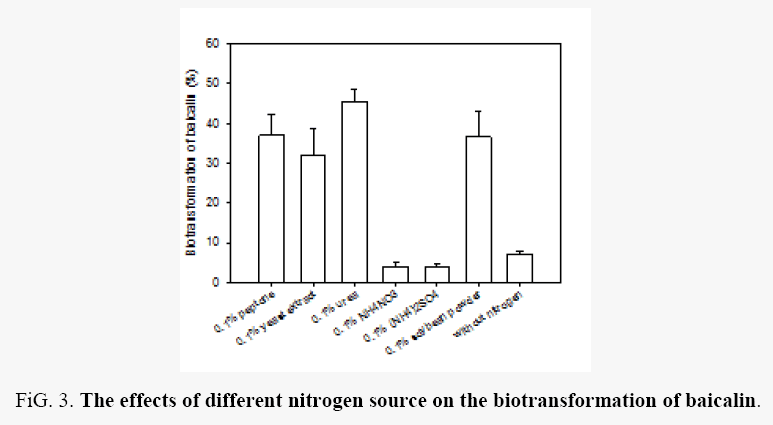
Effects of inorganic nitrogen sources: The effects of 0.1% peptone, yeast extract, soybean powder, urea, NH4NO3, (NH4)2SO4, and even without nitrogen on the biotransformation of baicalin by Aspergillus oryzae were evaluated. As shown in Figure 3, the biotransformation efficiency of baicalin was ranged from 3.71% to 45.49%. Peptone, yeast extract, urea and soybean powder were good nitrogen source for the biotransformation of baicalin by Aspergillus oryzae, with a biotransformation efficiency from 31.87% to 45.49%, and urea was the best.
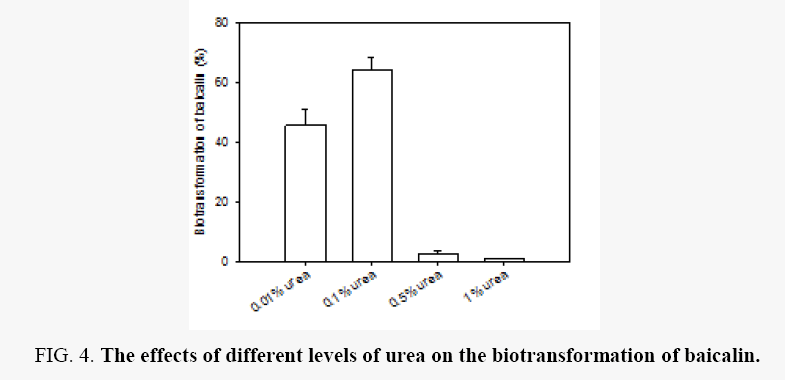
Effects of different levels of urea: Among the nitrogen sources studied above, urea was the best nitrogen resource for the biotransformation of baicalin. Accordingly, the impact of urea with four different levels (0.01%, 0.1%, 0.5% and 1%) on the biotransformation of balcalin was determined (Figure 4). With increasing the concentration of urea, biotransformation efficiency is first increased and then decreased. HPLC determined that balcalin was almost completely bio transformed when the concentration of urea is more than 0.5%, but less balcalein was determined with the concentration of urea increasing. From the literatures, balcalein was easily decomposed and oxidized. The result showed that high concentration of urea accelerates the decomposition and oxidation of balcalein. In biotransformation process, in order to improve the yield of balcalein, convertion efficiency of balcalin and protection of balcalein were considered simultaneously. Thus, urea with the concentration of 0.1% and 0.01% was selected as the nitrogen resource, which was used in following optimization process.
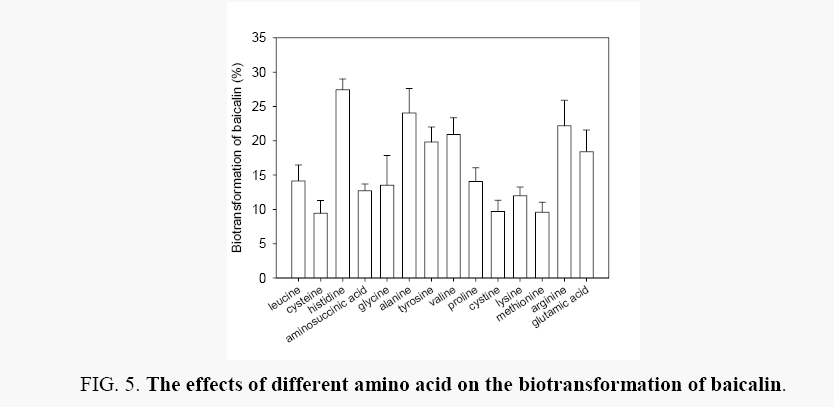
Effects of organic nitrogen source-amino acid: The effects of 14 species of amino acid were investigated including leucine, cysteine, histidine, aminosuccinic acid, glycine, alanine, tyrosine, valine, proline, cystine, lysine, methionine, arginine, glutamic acid, on the biotransformation of baicalin (Figure 5). When individual amino acid was used as nitrogen source, the biotransformation efficiency of balcalin was ranged from 9.43% to 27.43%, and their efficiency was lower than the effect of 0.01% urea (with the value 45.77%, Figure 4). Histidine, alanine, tyrosine, valine, arginine, glutamic acid was shown better effects in biotransformation of baicalin. And these amine acid and urea were combined, in order to obtain the best nitrogen resource.
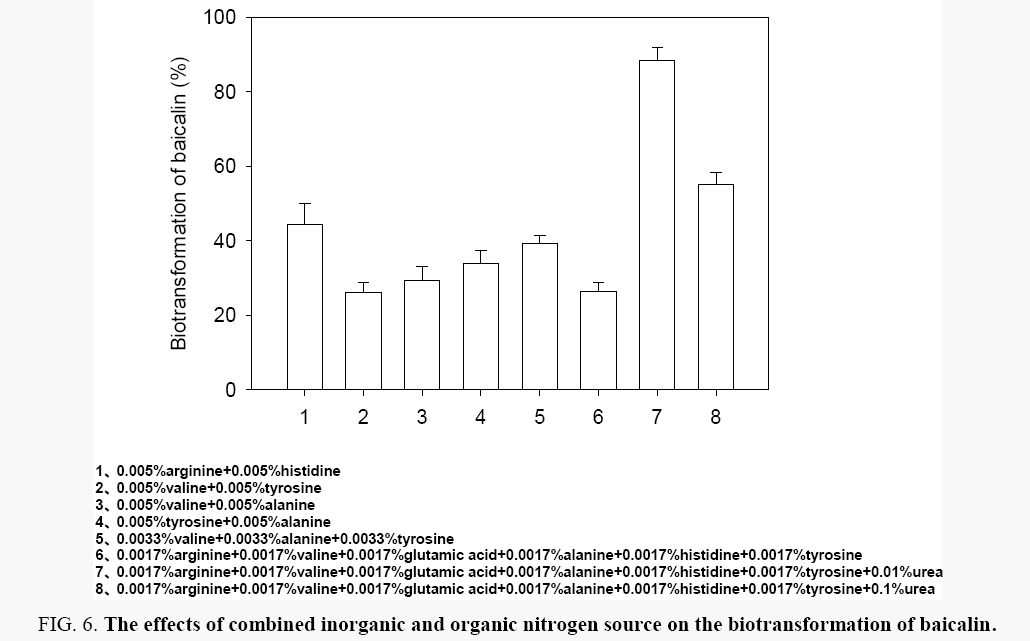
Effects of different combinations of urea and amino acid: In this part, the better nitrogen sources for the biotransformation of baicalin were combined. Keeping the amino acids with the total concentration of 0.01%, 0.1% urea, 0.01% urea and the six good amino acids (histidine, alanine, tyrosine, valine, arginine, and glutamic acid) were separately combined and their effects on the biotransformation of baicalin were studied. As shown in Figure 6, the biotransformation of balcalin was range from 26.11% to 88.36% under these biotransformation conditions, and the best nitrogen source was combined of 0.0017% arginine, 0.0017% valine, 0.0017% glutamic acid, 0.0017% alanine, 0.0017% histidine, 0.0017% tyrosine and 0.1% urea.
Figure 6: The effects of combined inorganic and organic nitrogen source on the biotransformation of baicalin.
Conclusions
β-Glucosidase is also an important enzyme in Aspergillus oryzae and played the major role in the microbial biotransformation of baicalin. After the optimization of the carbon and nitrogen source in the biotransformation media, the biotransformation efficiency of baicalin was enhanced to 88.36%. The best biotransformation media was optimized as 0.0017% arginine, 0.0017% valine, 0.0017% glutamic acid, 0.0017% alanine, 0.0017% histidine, 0.0017% tyrosine, 0.01% urea, 0.4% KH2PO4, 0.04% CaCl2, and 0.04% MgSO4, without external carbon source.
Thus it can be seen that, the combination of inorganic and organic nitrogen was benefit for the improvement of the biotransformation of baicalin, and inorganic nitrogen had a greater function in the biotransformation than organic amino acid. Comprehensively considering both of the biotransformation efficiency and the cost, 0.1% urea is also a good nitrogen source for the biotransformation of baicalin by Aspergillus oryzae because of the high-cost amino acid.
Acknowledgments
We thank the anonymous reviewers for their helpful comments on this work. This study was supported by PetroChina Innovation Foundation (2015D-5006-0210) and the National Natural Science Foundation of China (NO. 41472124).
References
- Li BQ, Fu T, Dongyan Y, et al. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. BiochemBiophys ResCommun. 2000;276(2):534-8.
- Tiwari RK, Trivedi M, Guang ZC, et al. Agrobacterium rhizogenes mediated transformation of Scutellariabaicalensis and production of flavonoids in hairy roots. Biol Plant. 2008;52(1):26-35.
- Chou TC, Chang LP, Li CY, et al. The anti-inflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesthesia & Analgesia. 2003;97(6):1724-9.
- Shen YC, Chiou WF, Chou YC, et al. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465(1):171-81.
- Motoo Y, Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86(1):91-5.
- Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20(5A):2861-5.
- Chou CC, Pan SL, Teng CM, et al. Pharmacological evaluation of several major ingredients of Chinese herbal medicines in human hepatoma Hep3B cells. Eur J Pharm Sci. 2003;19(5):403-12.
- Liu JJ, Huang TS, Cheng WF, et al. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer. 2003;106(4):559-65.
- Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002;68(02):128-32.
- Wang Y. Biotransformation of rutin by streptomycesgriseus and antioxidation of metabolites. Chinese Journal of Natural Medicines. 2006;4(1):66-9.
- Chen QY, Zhang YQ, Gan X, et al. Study on biotransformation of Rutin by Aspergillusniger (As3. 4309) formation [J]. Biotechnol. 2009;2:035.
- Zhan JX, Zhang YX, Guo HZ, et al. Microbial metabolism of artemisinin by mucorpolymorphosporusand Aspergillusniger. J Nat Prod. 2002;65(11):1693-5.
- Duetz WA, Bouwmeester H, Van Beilen JB, et al. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. ApplMicrobiolBiotechnol. 2003;61(4):269-77.
- Trytek M, Fiedurek J. A novel psychrotrophic fungus, Mortierellaminutissima, for D-limonene biotransformation. BiotechnolLett. 2005;27(3):149-53.
- Kostrzewa-Sus?ow E, Dmochowska-G?adysz J, Oszmia?ski J. Microbial transformation of baicalin and baicalein. J MolCatal B: Enzym. 2007;49(1):113-7.
- Ku S, Zheng H, Park MS, et al. Optimization of β-glucuronidase activity from Lactobacillus delbrueckii Rh2 and and its use for biotransformation of baicalin and wogonoside. J Korean SocApplBiol Chem. 2011;54(2):275-80.
- WuXG, Zeng Y, Zhou LM, et al. Study on fermentation conditions of Aspergillusoryzae producing β-glucosidase [J]. J Food Sci Technol. 2005;6:004.
- He M, Qiu DQ, Bo SJ. Biotransformation of Baicalin by Aspergillusoryzaeactivated twice and purification and determination of transformation product [J]. Journal of Guangdong Ocean University. 2007;6:008.