Original Article
, Volume: 12( 1)Novel Metal Complexes Derived From 6-(2, 3-Dichlorophenyl)-1, 2, 4-Triazine-3, 5-Diamine (Lamotrigine, L) and their Applications in Biology
- *Correspondence:
- Mohsen M Mostafa , Department of Chemistry, Faculty of Science, Mansoura University, Egypt, Tel: +20 19623; E-mail: amohsenmostafa@yahoo.com
Received: April 03, 2017; Accepted: May 28, 2017; Published: June 13, 2017
Citation: Suddik JK, Mostafa MM. Novel Metal Complexes Derived From 6-(2, 3-Dichlorophenyl)-1, 2, 4-Triazine-3, 5-Diamine (Lamotrigine, L) and their Applications in Biology. Inorg Chem Ind J. 2017;12(1):112.
Abstract
The reaction of lamotrigine (L) with Cu2+, Co2+, Cr3+ and Fe3+ chlorides and Ni2+ and Hg2+ acetates affords new metal complexes. The isolated solid complexes were synthesized and characterized by chemical, spectral, thermal and magnetic methods. IR spectral data of the isolated complexes suggest that L acts in a monodentate manner towards the metal ions. The general formulae of the chloride and acetate complexes are [Cu2(L)2Cl4].½H2O, [Co(L)2Cl2].½EtOH, [Fe(L)2Cl3].H2O, [Cr(L)3Cl3], [Ni2(L)(Ac)4].6H2O and [Hg(L-H)Ac].5H2O, respectively. The results of electronic spectra and magnetic measurements for Cu2+ complex suggest a distorted-octahedral, tetrahedral for Ni2+ and Co2+ and octahedral structures around the Cr3+ and the Fe3+ ions. TGA data suggest the mechanism of decomposition for the metal complexes. Biological activity of the isolated metal complexes derived from lamotrigine against human tumor cell lines of the types MCF-7 and Hela normal cell line was investigated.
Keywords
Lamotrigine complexes; Biological activity; Spectroscopic studies; Triazine derivatives
Introduction
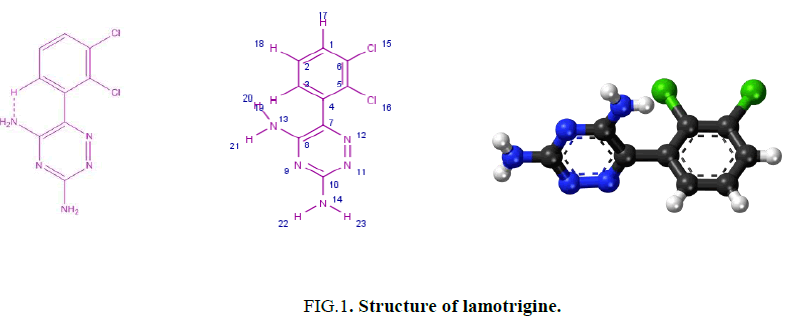
Lamotrigine (Figure. 1) is used for treatment of epilepsy [1-5] and bipolar disorder [5-7]. Also, it is used to treat partial, primary and secondary tonic-clonic seizures and also seizures associated with lennox-gastaut syndrome besides acting as a mood stabilizer. Moreover, it used for treating certain types of seizures. Lamotrigine may be used alone or with other medicines. It also used to delay the occurrence of mood problems in certain patients with bipolar disorder [8]. No work has been reported in the last four decades including the synthesis and characterization of the metal complexes derived from lamotrigine. One of our goals in this paper is to investigate the biological activity of the isolated metal complexes derived from lamotrigine. The antitumor activity is carried out in vitro on human mammary gland (breast) MCF-7 and cervical cancer cell-HeLa. The results suggest that Fe3+ is more promising than the rest of complexes. Hence the aim of our work is to synthesize new transition metal complexes derived from lamotrigine and characterizing the isolated complexes using chemical, spectral and magnetic measurements. Also, one of our aims is to follow the role and the importance of triazine compounds. Finally, the shortage of any information for these complexes gives us the push to throw more light on the chemistry and importance of lamotrigine complexes.
6-(2, 3-Dichlorophenyl)-1, 2, 4-triazine-3, 5-diamine (lamotrigine, L) was supplied from Delta Pharma company and used without purification. The salts used in this investigation [anhydrous FeCl3, CuCl2.2H2O, CoCl2.6H2O, CrCl3.6H2O, Ni (Ac)2.4H2O and Hg(Ac)2] were obtained from Fluka and Aldrich. Ethanol was purchased from Fisher chemicals. Lamotrigine is soluble in most organic solvents and easily soluble in DMF and DMSO.
Experimental Methods
Preparation of metal complexes
The complexes derived from Cu2+, Co2+, Cr3+ and Fe3+ chlorides with the general formulae, [Cu2(L)2Cl4].½H2O, [Co(L)2Cl2].1½EtOH, [Cr(L)3Cl3] and [Fe(L)2Cl3H2O] were synthesized by the direct reaction of CuCl2.2H2O (0.57 g, 0.01 mol), CoCl2.6H2O (0.79 g, 0.01 mol), CrCl3.6H2O (0.89 g, 0.01 mol) and/or anhydrous FeCl3 (0.82 g, 0.01 mol), dissolved in EtOH (50 ml) with lamotrigine (0.84 g, 0.01 mol) dissolved in absolute EtOH (25 ml). Also, the complexes derived from Ni2+ and Hg2+ acetates with the general formulae, [Ni2(L) (Ac)4(H2O)2].4H2O and [Hg3(L)(Ac)6], were obtained by the reaction of Ni(Ac)2.4H2O (0.83 g, 0.01 mol) and Hg(Ac)2 (1.86 g, 0.01 mol) with lamotrigine (0.84 g, 0.01 mol) dissolved in absolute EtOH (25 ml). The pH of the reaction mixtures were adjusted in the 6-7 range using sodium acetate followed by refluxing on a water bath for ~ 3 h. The products were filtered off, washed several times with absolute EtOH followed by dry diethyl ether and finally dried in a vacuum desiccator over anhydrous CaCl2. The results of elemental analyses and some physical properties are shown in Table1.
| No. | Compound; Empirical formula |
(M. Wt) | Color | M.P (°C) |
(Calcd.)% found | Spectral bands in DMSO (cm-1 ) |
?m (DMSO) ohm-1 cm2 mol-1 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Ueff (BM). |
||||||||
| 1 | [Cu2(L)2Cl4].½H2O; C18H15Cu2Cl4O½ |
790.102 | Faint green | ?300 | 26.6 (27.4) | 1.9 (1.9) |
17.2 (17.7) |
1.55 | 10870 | 8.5 | |
| 2 | [Co(L)2Cl2].1½EtOH; C21H23Cl2CoN10O1½ |
711.143 | Purple | ?300 | 35.4 (35.5) |
3.3 (3.3) |
19.7 (19.9) |
4.2 | 14900, 16290 | 10 | |
| 3 | [Ni2(L)(Ac)4(H2O)2].4H2O; C17H31N5Ni2O14 |
717801 | Green | ?300 | 28.4 (28.5) |
2.9 (4.4) |
8.9 (9.8) |
3.3 | 15000, 2820, 45045 | 13 | |
| 4 | [Fe(L)2Cl3H2O]; C18Cl5H16Ni2N10O |
692.422 | Golden yellow | > 300 | 31.1 (31.2) |
4.0 (2.3) |
21.4 (20.2) |
1.7 | 17860, 35840, 37740 | 5.6 | |
| 5 | [Cr(L)3Cl3]; C27Cl9CrH21N15 |
926.628 | Pale green | > 300 | 35.4 (35.0) |
1.3 (2.3) |
23.6 (22.7) |
3.6 | 16500, 22222 | 7.0 | |
| 6 | [Hg3(L)(Ac)6]; C21H25Hg3N10 |
1212.147 | Yellow | > 300 | 21.1 (20.8) |
1.9 (2.1) |
Diamag | __ | 1.5 | ||
Table 1: Chemical analyses and some physical data of the solid complexes.
Physical measurements
Elemental analyses contents (C, H and N) were determined at the Microanalytical Unit, Cairo University. Molar conductivities measurements were carried out using Jenco-3173. The IR spectra in the 400-4000 cm-1 range were recorded in KBr using 4100 Jasco Spectrophotometer. The electronic spectra of metal complexes were recorded in DMSO in the range (200-900 nm) using UV-1601 spectrophotometer. 1H-NMR and 13C-NMR spectra of L and some of its metal complexes were recorded on Varian Gemini (200 MHz) Spectrometer in d6-DMSO. Magnetic moments were determined using a Sherwood balance at room temperature (25°C) with Hg[Co(NSC)4] as a calibrate. The diamagnetic corrections for L and the metal atoms were computed using Pascal's constants [9]. Thermal analysis measurements (TGA and DTG) were recorded on a Schimadzu model 50 instrument using 20 mg. The nitrogen flow rate and heating rates were 20 cm3/min and 10°C/min, respectively. The mass spectra were carried out on a Shimadzu of the type (GC/MS-QP5050) at the regional center for mycology and biotechnology, Al-Azhar University, Egypt. The metal contents were determined using the standard methods [10].
Results and Discussion
The chemical analyses and some physical data of the isolated solid complexes are recorded in Table 1. All the isolated metal complexes are colored, stable against light and insoluble in most common organic solvents but easily soluble in DMF and DMSO. The molar conductivities of the complexes in DMSO at 25°C lie in the range 1.5-13 Ω-1 cm2 mol-1 indicating the non-electrolyte nature [11] of the complexes. The high melting points (?300°C) of the isolated complexes suggest the stability of these compounds as indicated also from the results of TGA.
IR spectra
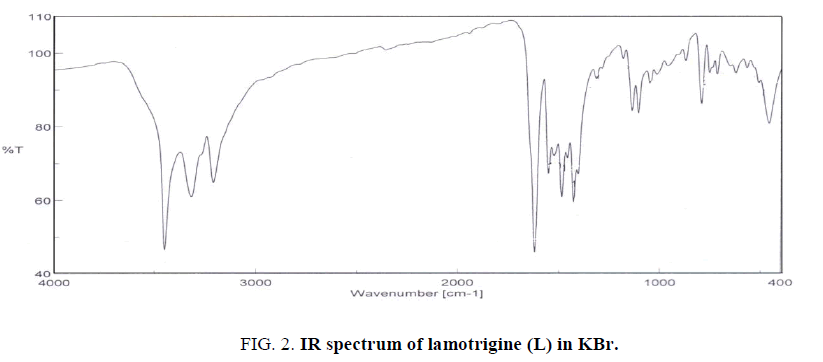
The IR spectrum of lamotrigine (L) in KBr shows several bands at 3450 (vs), 3319 (s), 3211(s), 1620 (vs) and 1554 (s) cm-1 (Figure. 2). These bands are assigned to νas(NH2, free), νs(NH2, free), νas(NH2, hydrogen-bonded) [12], ν(C=N) and ν(C=C) vibrations, respectively. The observation of weak broad bands in the 1948-1800 cm-1 region suggests the existence of inter and/or intra-hydrogen bonding of the type (N-H….N) as shown in Figure. 1.
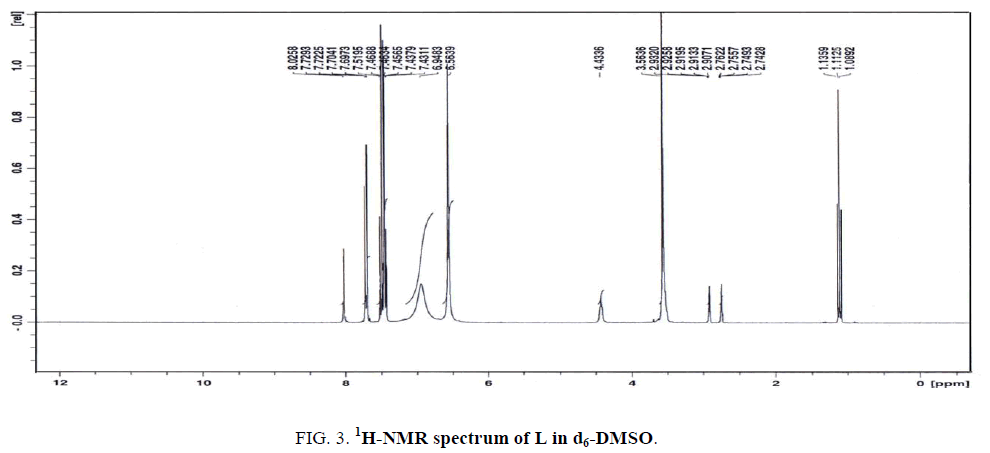
The 1H-NMR spectrum of L in d7-DMF (Figure. 3) displays five signals at 9.03, 7.52, 7.46, 6.95 and 6.56 ppm, relative to TMS. The first three signals are assigned to the protons of CH (C3-H19), (C2-H18) and (C1-H17), respectively. The latter two signals at 6.95 and 6.56 ppm are attributed to the protons of NH2 attached to (C8-N13) and (C10-N14), respectively. The signal at 6.59 ppm is assigned to the first NH2 signal (C8-N13) and observed downfield of TMS in comparison to the second NH2 signal (C10-N14) since the former group is hydrogen-bonded. All the results are taken an evidence of the existence of inter-or intra-hydrogen bonded within the molecule.
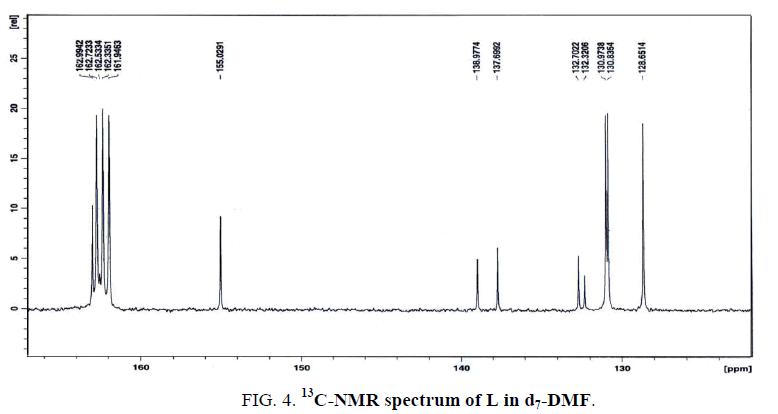
The 13C-NMR spectrum of L in d7-DMF (Figure. 4) displays seven signals at 155, 139.7, 137.6, 132.7, 132.3, 130.9 and 128.7, relative to TMS. The first three signals are assigned to the carbons of C8-N13 (hydrogen-bonded), C10-N14 (free) and C7, respectively. Also, the results are taken as strong evidence for the existence of hydrogen bonding (inter-or intra hydrogen-bonded) either from the data of IR and 1H-NMR. Finally, the latter four signals at 132.7, 132.3, 130.9 and 128.7 ppm are attributed to the carbons of C5, C6, C3, C2 and C1, respectively.
A comparison of the IR spectra of the free L with its complexes allows us to determine the mode of bonding. The IR spectra indicate that the L behaves in a monodentate manner in all metal complexes and coordinates via the NH2 group. The negative shift of the NH2 band to lower wavenumbers shows that this group is participate in coordination. Also, the other functional groups remain more or less at the same positions indicating that the other functional didn’t participate in bonding. Moreover, in comparing the IR spectra of L with its metal acetate complexes (Ni2+ and Hg2+) we observed that the difference between υas and υs of the acetate group is 199 and 212 cm-1 for Ni2+ and Hg2+ complexes, respectively indicating that this group behaves in a monodentate manner [13]. The band observed in the 410-490 cm-1 range is assigned to the υ (M-N) vibration [14].
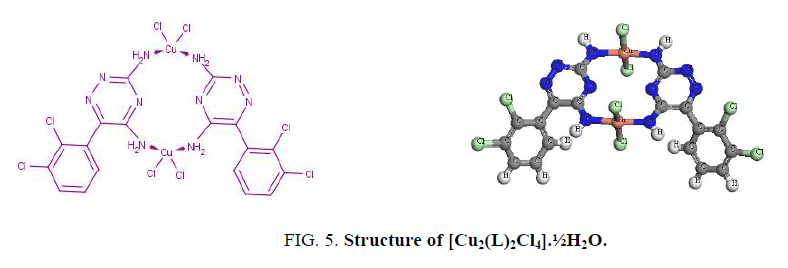
The UV spectra of lamoterigine in DMSO shows two bands at 32850 and 38460 cm-1 attributed to n → π* transitions for C=N and N=N groups, respectively [15] as shown in Figure. 1. The spectra of all complexes were carried out in DMSO. The electronic spectrum of the Cu2+ complex, [Cu2(L)2Cl4].½H2O, shows a broad band centered at 10870 cm-1 attributed to 2T2 → 2E2transition in a tetrahedral geometry around the Cu2+ ion [15] as shown in Figure. 5. The value of magnetic moment is 1.55 BM. is taken as additional evidence for the existence of small Cu-Cu interaction.
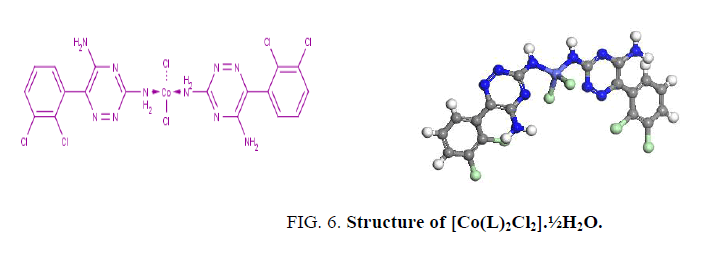
The electronic spectrum of [Co(L)2Cl2].1½EtOH shows multiple bands at 14,903 and 16290 cm-1 attributed to the 4A1 → 4T1 (P) transitions in a pseudo-tetrahedral geometry around the Co2+ ion [16,17]. The value of magnetic moment. (4.2 BM) is taken as additional evidence for the pseudo-tetrahedral geometry around Co2+ ion as shown in Figure 6.
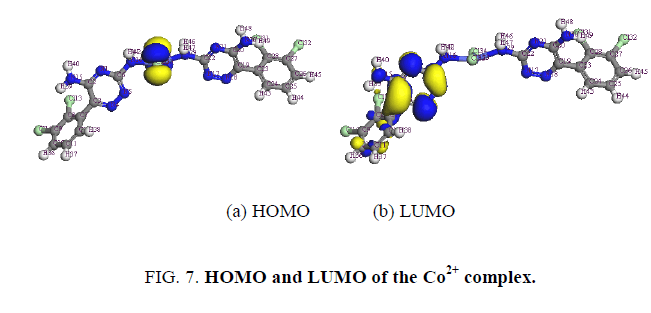
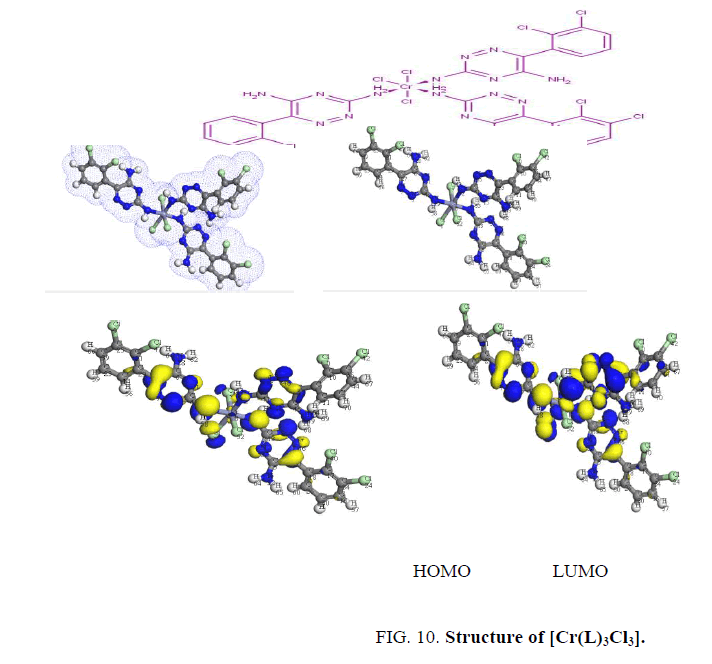
The determination of energies of the HOMO (π-donor) and LUMO (π-acceptor) are important parameters in quantum chemical calculations. The HOMO (Highest Occupied Molecular Orbitals) is the orbital that primarily acts as an electron donor and the LUMO (Lowest Unoccupied Molecular Orbital) is the orbital that largely act as the electron acceptor. These molecular orbitals are also called the frontier molecular orbitals (FMOs) as shown in Figure. 7.
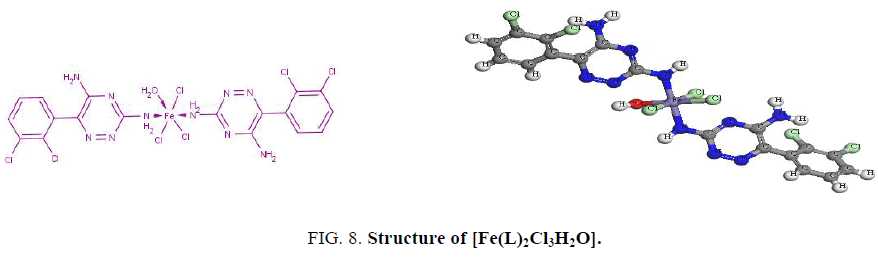
The electronic spectrum of the Fe3+ complex, [Fe(L)2Cl3H2O], shows a band at 17860 cm-1 assigned to 6A1g → 4T2g, 4T1g transitions in an octahedral geometry around the Fe3+ ion [14,16]. The value of magnetic moment. (5.6 BM) supports a high-spin octahedral geometry as shown in Figure. 8. The band observed at 37740 cm-1 is assigned to charge-transfer of the type L → M. The low value of the molar conductance (3.6 ohm-1.cm2 mol-1) indicates that the three chloride ions reside inside the coordination sphere [11] as shown in Figure. 8.
The magnetic moment value of the Ni2+ complex (3.3 BM) as well as the observation of multiplet bands centered at 15060 cm-1 in the electronic spectrum of the Ni2+ complex, [Ni2(L)(Ac)4(H2O)2].4H2O, assigned to 3A2 → 3T1 (P) transition suggests a tetrahedral geometry around the two Ni2+ ions [16] as shown in Figure. 9. Also, the molar conductance value (13 ohm-1. cm2. mol-1) indicates that the acetate ions are non-conducting [11].
Finally, the Cr3+ complex, [Cr(L)3Cl3], shows two bands at 16500 and 22222 cm-1 assigned to 4A2g → 2T2g, 2Eg and 4A2g → 4T2g transitions in an octahedral geometry around the Cr3+ ion [16]. The value of magnetic moment (3.6 BM) is taken as additional evidence for octahedral geometry around the Cr3+ ion [18,19] as shown in Figure. 10. The low molar conductance value (7 ohm-1.cm2 mol-1) suggests that the chloride ions are non-conducting [11,20].
DFT method concepts can indicate the chemical reactivity and site selectivity of the molecular systems. The energies of frontier molecular orbitals (EHOMO+ELUMO) and energy band gap (EHOMO-ELUMO) explains the eventual charge transfer interaction within the molecule [21,22].
Thermal studies
The steps of decomposition, temperature extent and decomposition products and the percentages of weight loss for the metal complexes are reported in Table 2. The results are in good agreement with the proposed structures. Also, the data suggest that the complexes are stable up to 800°C since the complexes didn’t decomposed completely to get the metal oxides and/or the nitride.
| No. | Compound | Decomposition steps |
Temperature range (°C) | Removed species | Wt. loss | |
|---|---|---|---|---|---|---|
| % Calcd. | % Found | |||||
| 1 | [Cu2(L)2Cl4].½H2O (C18H15Cu2Cl8 N10O½) |
1st | 30.00-433.75 | ½EtOH + C2H2N6 | 15.07 | 15.06 |
| 2nd | 435.00-602.50 | HN3 | 5.45 | 5.40 | ||
| 3rd | 604.91-796.25 | C9H4N | 15.96 | 16.05 | ||
| Residue | Cu2C7H7Cl8 | 63.52 | 63.49 | |||
| 2 | [Co(L)2Cl2].1½EtOH (C21H23Cl6CoN10O1½) |
1st | 36.25-471.44 | ½EtOH + N2CH5 | 9.58 | 9.51 |
| 2nd | 472.50-796.25 | C2O | 5.63 | 5.18 | ||
| Residue | CoC17H15Cl6N8 | 84.80 | 85.32 | |||
| 3 |
[Ni2(L)(Ac)4(H2O)2].6H2O
(C17H31Ni2Cl2N5O14) |
1st | 32.12-380 .00 | H2O+C2N2 | 9.76 | 9.44 |
| 2nd | 380.00-740.00 | CH4 N2 | 6.14 | 6.27 | ||
| Residue | Ni2C14H25Cl2NO13 | 84.10 | 84.29 | |||
| 4 | [Fe(L)2Cl3H2O] (C18H16FeCl7N10O) |
1st | 31.25-302.95 | H2O + C2N3 | 12.14 | 11.81 |
| 2nd | 301.25-503.75 | N4H | 8.24 | 8.23 | ||
| 3rd | 502.50-722.50 | C8N3 | 19.95 | 19.80 | ||
| Residue | FeC8H13Cl7 | 59.68 | 60.16 | |||
| 5 | [Cr(L)3Cl3] (C27H21Cl9CrN15) |
1st | 44.84-453.75 | CH2N10 | 16.63 | 16.60 |
| 2nd | 453.75-795.00 | N3H | 4.64 | 4.69 | ||
| Residue | CrC26H18Cl9N2 | 78.73 | 78.71 | |||
Table 2: Decomposition steps and weight loss of the metal complexes.
In vitro anticancer activity
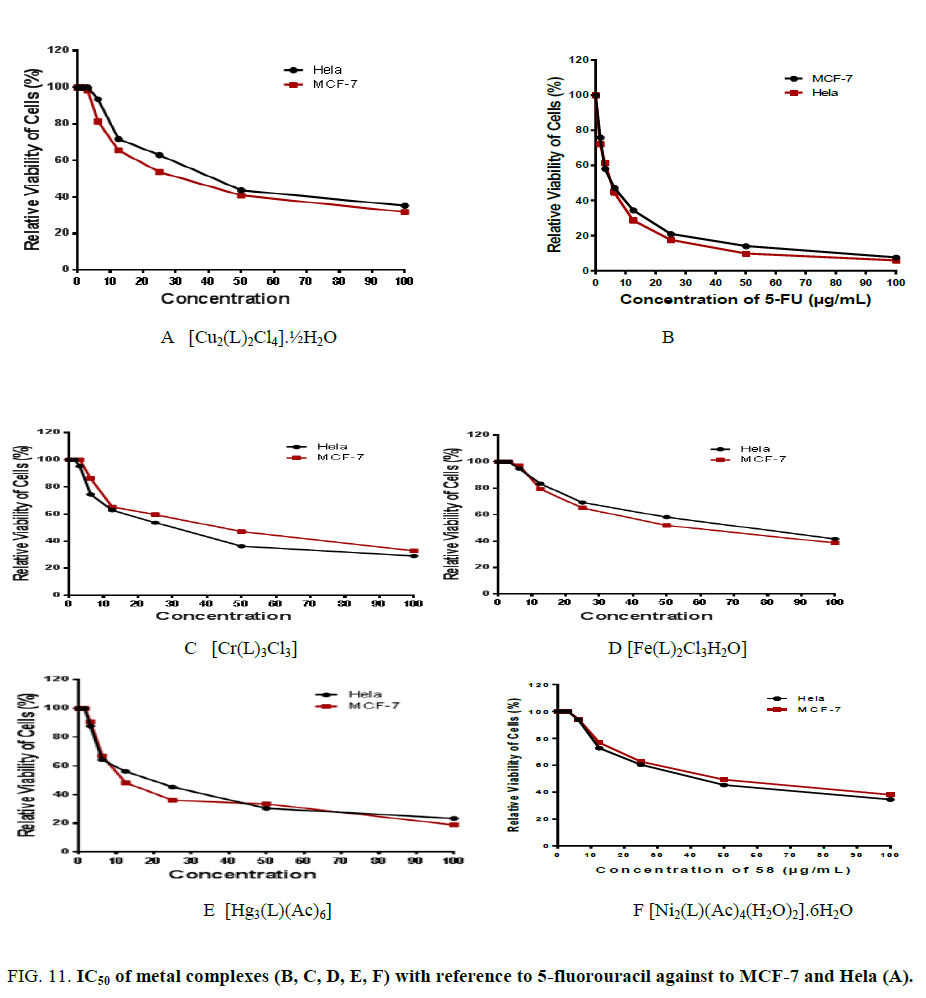
In vitro cytotoxicity tests were conducted utilizing five complexes derived from lamotrigine (L) against human tumor cell lines of the types MCF-7 and Hela normal cell line using a colorimetric assay (MTT assay) that is a measurement for mitochondrial dehydrogenase activity as an indication of cell viability [21,22]. The activities correspond to the viability of cancer cell growth. In parallel, the impact of widely utilized anticancer drug, 5-fluorouracil, has been also assayed as a standard. The IC50 values were estimated from the plot between the percent cell viability and concentration. The IC50 of both cell lines for the five complexes covered a large range of activity ranging from 8.2 up to 46.3 μg/ml as shown inFigure. 11(A, B, C, D, E, F). The results suggest that the five complexes monitored antitumor activity against the cell lines without damaging the normal cells. There is obviously direct correlation between strong interaction of metal ion complex with DNA and ant proliferative activity. The results reveal that [Fe(L)2Cl3H2O] and [Cr(L)3Cl3] complexes have high potential activity than [Cu2(L)2Cl4].½H2O, [Ni2(L)(Ac)4(H2O)2].4H2O and [Hg3(L)(Ac)6] complexes.
Figure 11: IC50 of metal complexes (B, C, D, E, F) with reference to 5-fluorouracil against to MCF-7 and Hela (A).
The results are explained on the basis of three factors. Firstly, the ionic radii of the Cr3+ and Fe3+ ions are smaller than the other divalent ions (Cu2+, Ni2+and Hg2+) which permit the penetration of the trivalent ions (Cr3+ and Fe3+) to the membrane of the cells more than the divalent ions and coordinated to DNA. Secondly, both the Cr3+ and Fe3+complexes contain only one metal ion while the rest involve two or three ions as shown from the chemical formulae. The existence of more than one metal ion may cause a damage of the cell membrane. Finally, the stereochemistry of the metal complexes plays role on targeting cells in which the octahedral structure is more effective than the other geometries. All these observations suggest that the DNA may be the targeting molecules of the metal complexes anti-carcinogenic action and the metal complexes bind significantly enhances the drugs anticancer activity. This means that the complex is essential in designing and synthesizing novel anticancer drugs. So, it is unmistakably watched that coordination with Cr3+ and Fe3+ ions has a synergistic impact on the cytotoxicity. Also, it means that the chemical structure of compounds is very important to explain the complex biological activity and it can be essential in designing and synthesizing novel anticancer drugs.
Conclusion
New mono, di and tri-metallic metal complexes derived from lamotrigine (L) were synthesized and characterized by elemental analyses, conductance, spectral (IR, UV-Vis., 1H-NMR and 13C-NMR), magnetic and thermal measurements. A distorted-octahedral geometry is proposed for the Cu2+ complex, tetrahedral for Ni2+ and Co2+ and octahedral structures around the Cr3+ and the Fe3+. TGA data suggest the mechanism of decomposition for the metal complexes and indicates also the stability of these complexes at high temperature. The cytotoxic activity assay of the Cr3+ and Fe3+ complexes have the highest cytotoxic activity against human tumor cell lines of the types MCF-7 and Hela normal cell line, while other complexes showed low cytotoxic activity. The role of oxidation state of the metal ions, nature and geometry of complex is proposed.
References
- Cheung H, Kamp D, Harris E.An in Vitro Investigation of the action of Lamotrigine on Neuronal Voltage-Activated Sodium Channels. Epilepsy Res.1992;13:107.
- Bourin M, Masse F,Hascoët M. Evidence for the activity of lamotrigine at 5-HT1Areceptors in the mouse forced swimming test.J PsychiatryNeurosci. 2005;30:275.
- Cunningham MO, Jones RSG.Tamotriginedecreases spontaneous glutamate release and increases GABA release in the rat EEntorhinalcortexin Vitro.Neuropharmacology.2000;39:2139.
- Besag FMC, Wallace SJ, Dulac FO, et al. Lamotrigine for the treatment of epilepsy in childhood.J Pediatr. 1995;127:991.
- Goldsmith DR, Wagstaff AJ, Ibbotson T, et al.Lamotrigine: A review of its use in bipolar disorder.Drugs.2003; 63:2029.
- Calabrese JR, Bowden CL, Sachs G, et al. A placebo-controlled18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar i disorder. JClin Psychiatry.2003;64:1013.
- Bowden CL, Calabrese JR, Ketter TA, et al.Impact of lamotrigine and lithium on weight in obese and nonobese patients with bipolar i disorder.AmerJ Psychiatry.2006;163:1199.
- Felicity Ng, Hallam K, Lucas N, et al.The role of lamotrigine in the management of bipolar disorder.Neuropsychiatr Dis Treat. 2007;3:463.
- Lewis L, Wilkins RG. Modern coordination chemistry. IOAJ. New York, 1960.
- Vogel AI. A text book of quantitative inorganic chemistry, Longmans, London, 1961.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. CoordChem Rev. 1971;7:81.
- Nakamoto K.Infrared and raman spectra of inorganic and coordination compounds. John Wiley, 2009.
- Groenewold GS, de Jong WA, Oomens J Michael, et al. Variable denticity in carboxylate binding to the uranyl coordination complexes. J Am Soc Mass Spectr. 2010;2:719.
- Ferraro JR, Walker WR.Infrared Spectra of Hydroxy-Bridged Copper (II) Compounds.Inorg Chem.1965;4:1382.
- Silverstein RM, Bassler GC, Spectroscopic Identification of Organic Compounds, Wiley, New York, 1967.
- Lever ABP,Inorganic Electronic Spectroscopy. Elsevier, Amsterdam, 1968.
- Brader ML, Kaarsholm NC, Harnung SE, et al. Ligand Perturbation Effects on a Pseudo-Tetrahedral Co(II)(His)3-Ligand Site. J Biolog Chem. 1997;272:1088.
- H,VeraniCN,Chaudhuri P,et al.molecular and electronic structure of octahedralo-aminophenolato ando-iminobenzosemiquinonato complexes of V(V), Cr(III), Fe(III) and Co(III). experimental determination of oxidation levels of ligands and metal ions.Inorg Chem. 2001;40:4157.
- Daines TL.the chemistry involved in the preparation of a paint pigment. an experiment for the freshman laboratory. J Chem Educ. 1976;53:117.
- Pearson RG.Absolute electronegativity and hardness: Applications to organic chemistry.J Org Chem.1989;54:1423.
- T. Mosmann. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays.J Immunol Methods. 1983;65:55.
- Denizot F. Lang R.Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J ImmunolMethods. 1986;89:271.