Original Article
, Volume: 14( 2)Novel Cyanine Dyes Based on N-Bridgehead Heterobicyclic: Synthesis, Solvatochromism and Physicochemical Studies
- *Correspondence:
- Ahmed I. Koraiem, Faculty of Science, Department of Chemistry, Aswan University, Aswan, Egypt, E-Mail: imabdell@ncsu.edu
Received: May 30, 2018; Accepted: June 11, 2018; Published: June 18, 2018
Citation: Koraiem AI, El-Shafei A, Abdallah IM. Novel Cyanine Dyes Based on N-Bridgehead Heterobicyclic: Synthesis, Solvatochromism and Physicochemical Studies. Org Chem Ind J. 2018;14(2):125
Abstract
Cyanine dyes of zeromethine, monomethine, acyclic mero cyanine dyes incorporating pyrazolo (3,2-a) Pyridine, pyrollo (3,4-c) pyrazolo (3,2-a) pyridine or quinolino (1,2-a) imidazo (2.3-c) Pyrano (2,3-c) pyrazole and imidazo (1,2-a) quinoline respectively were synthesized and characterized. UV-Visible, elemental and spectral analysis. The absorption spectra properties of such selected dyes were investigated in 95% Ethanol to attempt and throw some light on the influence of such new heterocyclic nuclei and to compare or evaluate spectral behaviors. The solvatochromic behavior and colour changes of some selected newly synthesized cyanine dyes are observed in solvents having different polarities. This permits a selection of optimal solvent (fractional solvent) when such dyes are applied as photosensitizers. The spectral behavior of some selected cyanine dyes in mixed solvents of different polarities has been studied to estimate number of hydrogen bond formed between the solute & solvent molecules and to allow measurement of certain energies such as hydrogen bonding, orientation and free energies.
Keywords
N-Bridgehead heterobicyclic; Cyanine dyes; Solvatochromic behaviour; Hydrogen bonding; Orientation and free energies
Introduction
Special attention is given to the implementation, preparations and applications of polynuclear heterocyclic cyanine to show the various aspects in order to satisfy the great demand in industrial and various biological fields. Cyanine dyes are colorant compounds which represent one of the most versatile functional dyes [1]. They have considerable potential for application as laser dyes [2-5], as optoelectronic and photonic devices [6], as potential fluorescence sensors [7,8] or as electro-, chemo-, and photo-luminescent devices [9], in Staining of internal limiting membrane (ILM) [10], as fluorescent dyes in DNA detection [11,12], as anticancer agents [13-15], as materials for various applications such as vulcanizing accelerator agents and photographic sensitizers in solar cell [16].
N-Bridgehead heterocyclic cyanine dyes are an important class of dyes which characterized by the presence unit of aza-heterocyclic compound the nature of nitrogen atom inside the ring. N-bridge head heterocyclic compound used as precursors possess high site reactivity susceptible to be attack by either Electrophile/ Nucleophile in the substitution/addition reactions which give high stability nature for the dyes formed [17]. These dyes have many vital general applications this back to their higher stability which can be used as bioactive compounds such as the N-methyl-Daspartate antagonists [18]. Furthermore, N-Bridgehead heterocyclic cyanine dyes have a wide range of potential applications in fluorescent compounds, DNA-binding dyes [19] and organic materials [20]. According to their bioactivity and structural planarity, it is a reasonable idea to develop functional dyes based on these N-Bridgehead heterocycles.
A variety of cyanine dyes incorporating different N-bridgehead heterocyclic moieties have been reported such as oxazolo pyrimidine [21], quinolino oxazine [22], pyrazolo indolizine (benzoindolizine) [23] and indolizine (benzoindolizine) [24] heterocycles but few of them focused on the pyrazolo Pyridine, pyrollo pyrazolo pyridine, quinolino imidazo Pyrano pyrazole and imidazo quinoline N-bridgehead heterocycles.
This push our research group to develop and synthesis new highly stable zeromethine, monomethine, acyclic mero cyanine dyes incorporating pyrazolo(3,2-a)Pyridine, pyrollo(3,4-c)pyrazolo(3,2-a)pyridine or quinolino(1,2-a)imidazo(2.3-c)Pyrano(2,3-c)pyrazole and imidazo (1,2-a)quinoline respectively (34a-c), (39a, b)/(46a-c) and (43a-c) based on N-bridgehead heterobicyclic system. The absorption spectra of dyes in different solvents with different polarities were examined in the visible region. The spectral behavior of some selected newly synthesized cyanine dyes is observed in mixed solvents of different polarities to estimate number of hydrogen bond formed between the solute and solvent molecules in addition, allow measurement of certain energies such as hydrogen bonding, orientation and free energies.
Material and Methods
All melting points are uncorrected Elemental and spectral analysis was carried out at the microanalytical centre (Cairo University). The IR (vKBr) spectra were determined with Perkin Elmer Infrared 127ß spectrophotometer (Cairo-University). 1H–NMR spectra were recorded with a Bruker AMX-250 spectrometer (Cairo-University). Mass spectra were recorded on a HpMs 6988 spectrometer (Cairo University). The absorption spectra were recorded immediately after preparation of the solutions within the wavelength range (350-700) on 6405 UV/Visible recording spectrophotometers, Faculty of Science, Aswan University.
Synthesis and structural characterization
The required intermediates and final compounds were synthesized using the standard synthetic protocols. The procedures for the synthesis of intermediates and target dyes along with their structural characterization data are given below.
Synthesis of 4-acetyl-oxime-1-phenyl-3-methyl-2-pyrazolin-5-one (31): Prepared in a way similar to that described in [25], by treating 1 mol of 1-phenyl-3-methyl-4-acetyl-2-pyrazolin-5-one with a solution of hydroxylamine hydrochloride (2 mol) containing sodium acetate (3 mol). The mixture was heated on a water bath for about an hour, then pouring in water the corresponding oximes separated out which was recrystallized from alcohol.
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4-methylimino-Pyridin-1-ium Chloride salt (32): An Ethanolic solution mixture of (31, 0.01 mol.), pyridine and few mls of conc. HCl was refluxed on a water bath for an hour. The reaction mixture was filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (TABLE 1).
| Comp No. | Nature of Products | % Calcd. (Found) | Absorption spectra in EtOH
lmax emax (nm) (cm2mol-1) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M.p. °C | Yield % | Color | Mol. Formula (Mol. Wt.) | C | H | N | |||
| 32 | 135 | 63 | Pale brown | C17H17ClN4O (328.8) | 62.10 62.101 | 5.21 5.219 | 17.04 17.046 | _ | _ |
| 33 | 140 | 72 | Red | C17H15ClN4O (326.8) | 62.48 62.478 | 4.83 4.826 | 17.14 17.15 | _ | _ |
| 34a | 110 | 54 | Red | C24H22IN5O (523.368) | 55.08 55.077 | 4.24 4.239 | 13.38 13.388 | 490 | 2543 |
| 34b | 110 | 60 | Red | C28H24IN5O (573.43) | 58.65 58.644 | 4.22 4.21 | 12.21 12.22 | 545 | 2870 |
| 34c | 125 | 64 | Red | C24H22IN5O (523.368) | 55.08 55.10 | 4.24 4.239 | 13.38 13.41 | 530 | 2821 |
| 35 | 162 | 65 | Pale Brown | C19H17ClN4O2 (368.9) | 61.87 61.88 | 4.65 4.649 | 15.19 15.21 | 470 | 2680 |
| 36a | 180 | 69 | Red violet | C27H26IN5O (563.6) | 57.56 57.55 | 4.65 4.649 | 12.43 12.44 | 465, 500 | 2170, 2340 |
| 36b | 220 | 70 | Violet | C31H28IN5O (613.491) | 60.69 60.66 | 4.60 4.56 | 11.42 11.429 | 470, 560 | 2540, 2812 |
| 36c | 195 | 66 | Red | C27H26IN5O (563.432) | 57.56 57.53 | 4.65 4.64 | 12.43 12.44 | 390, 520 | 2700, 2443 |
| 37 | 163 | 70 | pale brown | C19H18ClN5O2 (383.83) | 59.45 59.44 | 4.73 4.732 | 18.25 18.26 | _ | _ |
| 38 | 180 | 81 | Brown | C19H16ClN5O (365.81) | 62.38 62.38 | 4.14 4.15 | 19.14 19.2 | _ | _ |
| 39a | 152 | 85 | Red powder | C26H23IN6O (562.4) | 55.53 55.51 | 4.12 4.14 | 14.94 14.93 | 400, 505 | 1750, 2890 |
| 39b | 145 | 86 | Red crystals | C30H25IN6O (612.46) | 58.83 58.81 | 4.11 4.12 | 13.72 13.74 | 395, 445, 520 | 1750, 1550, 2920 |
| 40 | 100 | 82 | Reddish | C21H19IN4O2 (486.3) | 51.87 (51.87) | 3.94 (3.95) | 11.52 (11.53) | _ | _ |
| 41 | 129 | 60 | Brownish | C28H28I2N6O (718.37) | 46.81 46.80 | 3.93 3.95 | 11.70 11.71 | 400 | 1819 |
| 42 | 135 | 70 | Brown | C21H17IN4O (468.29) | 53.86 (53.85) | 3.66 (3.68) | 11.96 (11.96) | 390, 495 | 1816, 1872 |
| 43a | 140 | 73 | Red violet crystals | C28H24IN5O (573.42) | 58.65 (58.66) | 4.22 (4.22) | 12.21 (12.20) | 390, 490 | 1880, 2069 |
| 43b | 165 | 80 | Red | C32H26IN5O (623.48) | 61.64 (61.65) | 4.20 (4.23) | 11.23 (11.22) | 390, 500 | 1831, 2025 |
| 43c | 162 | 70 | Reddish | C28H24IN5O (573.42) | 58.65 (58.64) | 4.22 (4.23) | 12.21 (12.22) | 390, 493 | 1840, 2050 |
| 44 | 115 | 76 | Brownish | C23H19IN4O2 (510.32) | 54.13 (54.14) | 3.75 (3.75) | 10.98 (10.99) | 390, 450 | 1856, 1791 |
| 45 | 190 | 80 | Brown | C23H17ClN4O (400.86) | 68.91 (68.89) | 4.27 (4.29) | 13.98 (13.99) | 390, 480 | 1900, 1909 |
| 46a | 180 | 68 | Brown crystals | C30H24IN5O (597.448) | 60.31 (60.30) | 4.05 (4.06) | 11.72 (11.73) | 390, 483 | 1863, 1079 |
| 46b | 200 | 73 | red crystals | C34H26IN5O (647.5) | 63.07 (63.07) | 4.05 (4.06) | 10.82 (10.84) | 390, 485 | 1850, 1921 |
| 46c | 210 | 65 | Brown crystals | C30H24IN5O (597.448) | 60.31 (60.32) | 4.05 (4.04) | 11.72 (11.70) | 390, 490 | 1865, 2212 |
TABLE 1. Characterization data of the synthesized precursors and the target dyes.
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4-[3H-pyrazolo(3,2-a)Pyridin-1-ium chloride salt (33): Fusion of (32, 0.01 mol.) with piperidine for few minutes, then dissolve in ethanol and refluxes for 3-4 hours. The reaction mixture was concentrated to one third of its volume, cooled and precipitated by dilution with ice-water mixture. The precipitates were then collected and crystallized from ethanol to afford (33) (TABLE 1).
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4-pyrazolo(3,2-a)Pyridin-zero-3[4(1)] methine cyanine dyes (34a-c): An Ethanolic solution mixture of (33B, 0.01 mol.) and pyridin [quinolin]-4(1)-ium-ethiodide salts (0.01 mole) in piperidine was refluxed on a water bath for one hour. The reaction mixture was filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (TABLE 1).
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyl-pyrazolo(3,2-a) Pyridin-1-ium Chloride salt (35): An Ethanolic solution mixture of (33B, 0.005 mol) and acetic anhydride (10 ml) was refluxed for 3 hours. The reaction mixture was filtrated from unreacted materials. The filtrate was concentrated and cooled concentrated to one third of its volume, cooled and precipitated by adding of ice-water mixture. (35) (TABLE 1).
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4[pyrazolo(3,2-a)pyridin-β-methyl-di-3[2(4)]methine cyanine dyes (36a-c): An Ethanolic solution of (35, 0.01mol) and 2-methyl-pyridin [quinolin]-2(4)-ium-1-ethiodide salts (0.01mol) in few drops of piperidine was refluxed for 3-5 hrs. The reaction mixture was filtrated from unreacted materials. The filtrate concentrated to one third of its volume, cooled and acidified with acetic acid. The precipitated products after dilution with water were separated, filtrated, crystallized from ethanol (TABLE 1).
Synthesis of 3-Methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyloximino-pyrazolo(3,2-a)pyridin-1-ium Chloride salt (37): An Ethanolic solution of (35, 0.01mol), hydroxylamine hydrochloride (2 moles) and sodium acetate (3 moles) in few drops of piperidine was refluxed on water bath for an hour. The reaction mixture was filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (TABLE 1).
Synthesis of 3-Methyl-1-phenyl-Spiro-[2-methyl-pyrazolin-5-one-4,2-pyrollo(3,4-c) pyrazolo (3,2-a)pyridin-1-ium Chloride salt (38): Fusion of (37, 0.01mol) with piperidine for few minutes then dissolved in ethanol and reflux for 3 hours. The reaction mixture was filtrated from unreacted materials, concentrated to half of its volume, cooled and precipitated with ice water then recrystallized from ethanol. (38) (TABLE 1).
Synthesis of 3H-Spiro-[3-methyl-1-phenyl-pyrazolin-5-one]-4,2-pyrollo(3,4-c)pyrazolo(3,2-a)pyridine zero-4-[4(1)] methine cyanine dyes (39a-b): A mixture of (38) (0.01 mole) and pyridin[quinolin]-4(1)-ium-1(2)-ethiodide salts (0.01 mole) was dissolved in ethanol (30 ml) in the presence of few drops of piperidine and heated on a water bath for about an hour. The reaction mixtures were filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (39a, b) (TABLE 1).
Synthesis of 1-phenyl-3-methyl-pyrazolino-4-keto-α-methylene-N-quinolin-1-ium-iodide salt (40): An ethanol solution of (31) (0.01mol) with (0.01mol) of [quinoline] and (0.01mol) of iodine were refluxed for 3-5hrson a hot plate. The reaction mixtures were filtrated from unreacted materials. The filtrate concentrated to one third of its volume, cooled. The precipitated products after dilution with water were separated, filtrated, crystallized from ethanol (TABLE 1).
Synthesis of 1-phenyl-3-methyl-pyrazolino-4-ketooxime-α-methylene-N-quinolin-1-ium-iodide salt (41): A mixture of (40) (1 mole), hydroxylamine hydrochloride (2 moles) and sodium acetate (3 moles) was dissolved in ethanol (30 ml) and heated in a water bath for about an hour. The reaction mixtures were filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (TABLE 1).
Synthesis of 3-H-2-[1-phenyl-3-methyl-pyrazoline-5-one]imidazo(1,2-a)quinoline-1-ium-iodide salt (42): Fusion of (41) with piperidine for about an hour then dissolved the reaction mixture in ethanol and reflux for 3 hours. The reaction mixture concentrated to half of its volume, cooled and precipitated with ice water then recrystallised from ethanol (TABLE 1).
Synthesis of 2-[1-phenyl-3-methyl-pyrazoline-5-one]imidazo(1,2-a)quinolin-mono-3-[4(1)]methine cyanine dyes (acyclic merocyanine dyes) (43a-c): Ethanolic solution of (42) (0.01mol) and (0.01mol) of pyridin[quinolin]-4(1)-ium-1(2)-ethiodide salts in the presence of few drops of piperidine were refluxed for 5-7 hrs on a hot plate. The reaction mixtures were filtrated from unreacted materials. The filtrate concentrated to one third of its volume, cooled and acidified with acetic acid. The precipitated products after dilution with water were separated, filtrated, crystallized from ethanol (TABLE 1).
Synthesis of 2-[1-phenyl-3-methyl-pyrazoline-5-one]-3-acetyl-imidazo (1,2-a) quinoline-1-ium-iodide salt (44): A mixture of compound (42) (0.005 mol) and acetic anhydride (10 ml). The reaction mixture was refluxed for 3 hours, concentrated to one third of its volume, cooled and precipitated by adding of ice-water mixture (TABLE 1).
Synthesis of 1-phenyl-3,11-dimethyl-quinolino(1,2-a)imidazo(2.3-c)Pyrano(2,3-c)pyrazole-12-ium-chloride salt (45): Ethanolic solution of (0.01mole) of (44) and few drops of concentrated HCl was heated on a water bath for about an hour. The reaction mixture were filtrated from unreacted materials. The filtrate was concentrated and cooled. The precipitated products after dilution with water were separated, filtrated, washed with water several times and crystallized from ethanol (TABLE 1).
Synthesis of 1-phenyl-3-methyl-quinolino(1,2-a)imidazo(2.3-c)pyrano (2,3-c)pyrazole mono-11[1(4)]-methine cyanine dyes (46a-c): Ethanolic solution of (45) (0.01mol) and (0.01mol) of pyridin[quinolin]-4(1)-ium-1(2)-ethiodide salts in the presence of few drops of piperidine were refluxed for 5-7 hrs. The reaction mixture was filtrated from unreacted materials. The filtrate concentrated to one third of its volume, cooled and acidified with acetic acid. The precipitated products after dilution with water were separated, filtrated, crystallized from ethanol (TABLE 1).
Structural characterizations: The structure of the precursors and the target dyes were confirmed by spectral data IR, 1H-NMR and Mass analyses as shown below.
Compound (32): FT-IR (vKBr cm-1) showed general absorption bands at 1494.56 cm-1 (C=N), 1601.59 cm-1 (C=C) conjugated, 1716.65 cm-1 (C=O), well defined bands at 2921.63 cm-1 (heterocyclic quaternary salt), 3064.33 cm-1 (stretching CH). Mass spectra reveals molecular ion peaks at m/z =330 and base peak at m/z =77.
Compound (33): FT-IR vKBr cm-1) showed general absorption bands at 1495.53cm-1 (C=N), 1602.56 cm-1 (C=C) conjugated, 1710.55 cm-1 (C=O), 2924.52 cm-1 (heterocyclic quaternary salt), 3063.37 cm-1 (stretching CH). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z =330 and base peak at m/z=77.
Compound (34b): 1H-NMR (DMSO, 300 MHz) spectra showed the general signals at δ 1.23 (s, 3H, CH3-pyrazole), 2.2-2.27 (t, 3H, CH3), 2.3-2.5(q, 2H, CH2), 3.35 (s, 1H, CH-pyrazole), 7.17-7.82 (m, 13H, 3Ar). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z =575 and base peak at m/z =539.
Compound (35): FT-IR (vKBr cm-1) showed general absorption bands at 1490.7 cm-1 (C=N), 1616.06 cm-1 (C=C) conjugated, 1714.04 cm-1 (COCH3), 2924.52 cm-1 (heterocyclic quaternary salt). 1H-NMR (DMSO, 300 MHz) spectra showed the general signals at 0.818 (s, 3H, CH3-pyrazole), 1.19 (s, 3H, CH3) acetyl group, 1.553 (s, 1H, CH), 1.87 (s, 1H, CH), 7.04-7.96 (m, 9H, two Ar). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=370 and base peak at m/z=149.
Compound (36a): FT-IR (vKBr cm-1) showed general absorption bands at 3043.62 cm-1 (stretching CH), 2929.28 cm-1 (heterocyclic quaternary salt), 1702.82 cm-1 (CO), 1497.45 cm-1 (C=N) and 1597 cm-1 (C=C) conjugated. Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=565 and base peak at m/z=250.
Compound (37): FT-IR (vKBr cm-1) showed general absorption bands at 3407.6 cm-1 (OH), 3063.37 cm-1 (stretching CH), 2921.63 cm-1 (heterocyclic quaternary salt), 1497.45 cm-1 (C=N) and 1600 cm-1 (C=C) conjugated. Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=386 and base peak at m/z=64.
Compound (38): FT-IR (vKBr cm-1) showed general absorption bands at 3053.7 cm-1 (stretching CH) and 2925.2 cm-1 (heterocyclic quaternary salt). 1668.8 cm-1 (CO), 1495.5 cm-1 (C=N), 1604 cm-1 (C=C) conjugated, Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=367 and base peak at m/z =80.
Compound (42): FT-IR (vKBr cm-1) showed general absorption bands at 1713.44 cm-1 (C=O), 2927.41 cm-1 (heterocyclic quaternary salt), 15971.73 cm-1 (C=C) conjugated, 1496.49 cm-1 (C=N), 755.959 cm-1 (Aromatic compound). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=469 and base peak at m/z =116.
Compound (43b): FT-IR (vKBr cm-1) showed general absorption bands at 3424.96 cm-1 (OH), 2923.56 cm-1 (heterocyclic quaternary salt), 1597.73 cm-1 (C=C) conjugated, 1453.1 cm-1 (C=N), 753.06 cm-1 (Aromatic compound). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=624 and base peak at m/z =77.
Compound (44): FT-IR (vKBr cm-1) showed general absorption bands at 3422.069 cm-1 (NH), 2923.56 cm-1 (heterocyclic quaternary salt), 1600.63 cm-1 (C=C) conjugated, 1493.6 cm-1 (C=N), 754.995 cm-1 (Aromatic compound). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=510 and base peak at m/z =84.
Compound (45): FT-IR (vKBr cm-1) showed general absorption bands at 2924.3 cm-1 (heterocyclic quaternary salt), 1595.3 cm-1 (C=C) conjugated, 1494.5 cm-1 (C=N), 748.5 cm-1 (Aromatic compound). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=402 and base peaks at m/z =346.
Compound (46b): FT-IR (vKBr cm-1) showed general absorption bands at 2927 cm-1 (heterocyclic quaternary salt), 1608.63 cm-1 (C=C) conjugated, 1495.2 cm-1 (C=N), 745.8 cm-1 (Aromatic compound). Mass spectra reveals molecular ion peaks at a molecular ion peaks at m/z=a molecular ion peaks at m/z =648 and base peak at m/z =120.
Solvatochromic studies: The organic solvents were used of spectroscopic grade which purified according to the recommended methods. The electronic absorption spectra of the studied dyes in different organic solvents were recorded within the wavelength (350-700 nm) on 6405 UV/Visible recording spectrophotometers using 1cm quartz cell. The stock solution of the dye was of the order 10-3 mol-dm-3. Solutions of low molarities used in spectral measurements were obtained by accurate dilution
Preparation of working solutions: For studying the effect of pure solvents in the UV and visible range: An accurate volume of the stock solution (10–3 mol-1cm-3 in ethanol) of the dyes were diluted to appropriate volume in order to obtain the required concentration. The spectra were recorded immediately after mixing in order to eliminate as much as possible the effect of time. Furthermore, to study the spectral behaviour in mixed solvents in the visible region: An accurate volume of stock solution of the dyes was placed in 10 ml measuring flask containing the required volume of ethanol, then completed to the mark with the other solvent.
Results and Discussions
Synthesis
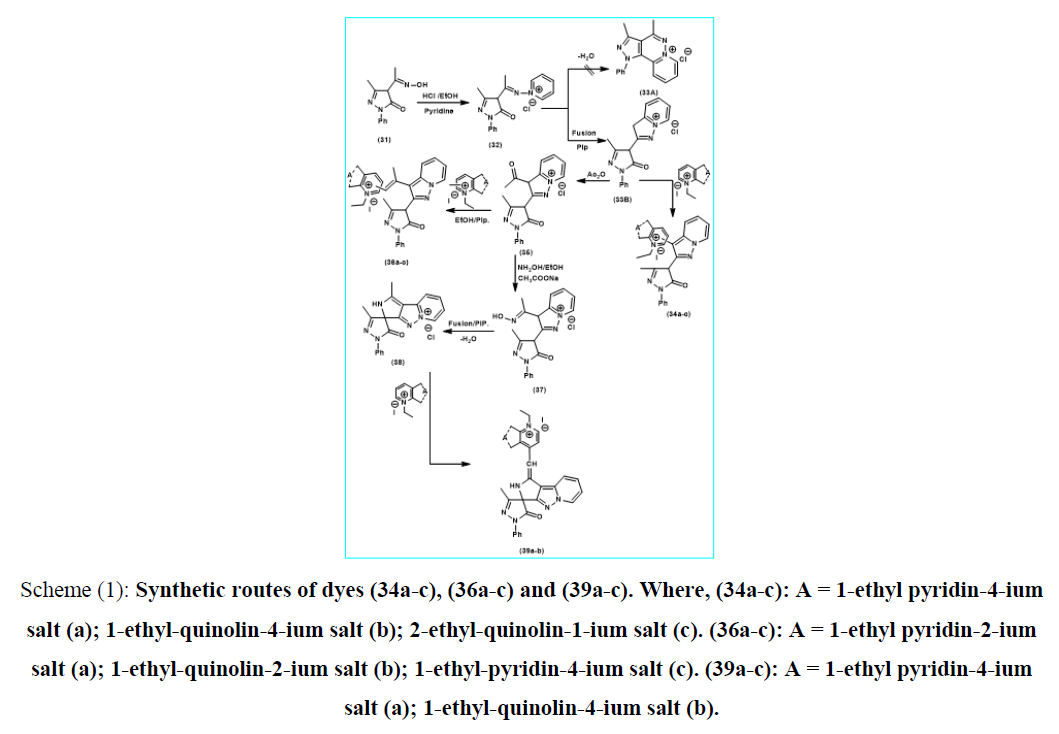
The reaction of 4-acetyl-oximino-3-methyl-1-phenyl-2-pyrazolin-5-one (31) [17] with pyridine in equimolar ratio using hydrochloric acid catalysis afforded 3-methyl-1-phenyl-pyrazolin-5-one-4-methylimino-pyridin-1-ium-chloride salt (32) which undergoes oxidative elimination in piperidine catalysis to afford 3-Methyl-1-phenyl-pyrazolin-5-one-4-[3H-pyrazolo(3,2-a) pyridin-1-ium-chloride salt (33B). The Intramolecular heterocyclization for the formation of (33B) seem to be an easier for the orientation towards acyclic oxidative elimination between acyclic methyl groups rather than cyclo-dehydration process of Enol form of endocyclic pyrazolino-5-one carbonyl group and active position-2-heterocyclic quaternary salt to form the expected (33A), The formation was suggested to proceed through formation of one form (33B) which was easier to be available than those of isomeric structure analogous (33A). On triturating of (33B) with saturated solution of KI followed by dissolving the resulted compound in conc. H2SO4 liberating iodine vapor on warming. This is a criterion for the presence of the chloride anion replaced by iodide analogous. The reaction of 3-methyl-1-phenyl-pyrazolin-5-one-4-[3H-pyrazolo(3,2-a)pyridin-1-ium-chloride salt (33B) with N-ethyl pyridine (quinolin)-4(1)-ium ethyl iodide salts in, equimolar amount, under piperidine catalysis afforded 3-methyl-1-phenyl-pyrazolin-5-one-4-pyrazolo(1,5-a)pyridin-zero-3[4(1)] methine cyanine dyes (34a-c). Acetylating reaction of equimolar amount of 3-methyl-1-phenyl-pyrazolin-5-one-4-[3H-pyrazolo (3,2-a)pyridin-1-ium-chloride salt (33B) using acetic anhydride afforded 3-methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyl-pyrazolo (3,2-a)pyridin-1-ium-chloride salt (35) which on triturating with saturated solution of KI followed by dissolving of the resulted in conc.H2SO4 liberated iodine vapor on warming. This is a criterion for the presence of the chloride anion replaced by iodide analogous. Reaction of 3-methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyl-pyrazolo(3,2-a)Pyridin-1-ium- chloride salt (35) with 2(4)-methyl-pyridin (quinolin)-2(4)-ium-ethyl iodide in, equimolar amount, under piperidine catalysis afforded 3-methyl-1-phenyl-pyrazolin-5-one-4-[pyrazolo(3,2-a) pyridin-β-methyl-di-3[2(4)]methine cyanine dyes (36a-c). Condensation reaction of 3-methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyl-pyrazolo(3,2-a) pyridin-1-ium-chloride salt (35) with hydroxyl amine hydro-chloride in, equimolar amount, under anhydrous sodium acetate catalysis afforded 3-methyl-1-phenyl-pyrazolin-5-one-4-[3-acetyloximino-pyrazolo(3,2-a)pyridin-1-ium-chloride salt (37). Thermal piperidine catalysis of (37) in, equimolar amount, undergoes dehydration process to afford 3-methyl-1-phenyl-spiro-[2-methyl-pyrazolin-5-one-4,2-pyrollo(3,4-c)pyrazolo(3,2-a)pyridin-1-ium-chloride salt (38) as shown in (scheme 1) depicted below. On the other hand, Monomethine cyanine dyes (39a, b) formed by reaction of 4-Methyl-3H-Spiro-[3-methyl-1-phenyl-pyrazolin-5-one]-4,2-pyrollo(3,4-c) Pyrazolo (3,2-a)pyridin-1-ium Chloride salt (38),with pyridine(quinolin)-2(4)ium-1-ethiodide salt to afford 3H-Spiro-[3-methyl-1-phenyl-pyrazolin-5-one]-4,2-pyrollo(3,4-c)pyrazolo (3,2-a) pyridine mono-4[1(4)]-methine cyanine (39a, b). It’s represent dehydrogenation reaction between active methyl group in compound (38) and 4-position in pyridine or quinoline followed by rearrangement involving loss of (HCl). It was obvious that, the stability of (33B) is due to mesmeric effect supporting the existence of stable chloride anion as was observed in. The stability of (38) is due to both communicative hyper-conjugative of pyrrole methyl group and the two mesmeric effects supporting the existence of stable chloride anion.
Scheme (1): Synthetic routes of dyes (34a-c), (36a-c) and (39a-c). Where, (34a-c): A = 1-ethyl pyridin-4-ium salt (a); 1-ethyl-quinolin-4-ium salt (b); 2-ethyl-quinolin-1-ium salt (c). (36a-c): A = 1-ethyl pyridin-2-ium salt (a); 1-ethyl-quinolin-2-ium salt (b); 1-ethyl-pyridin-4-ium salt (c). (39a-c): A = 1-ethyl pyridin-4-ium salt (a); 1-ethyl-quinolin-4-ium salt (b).
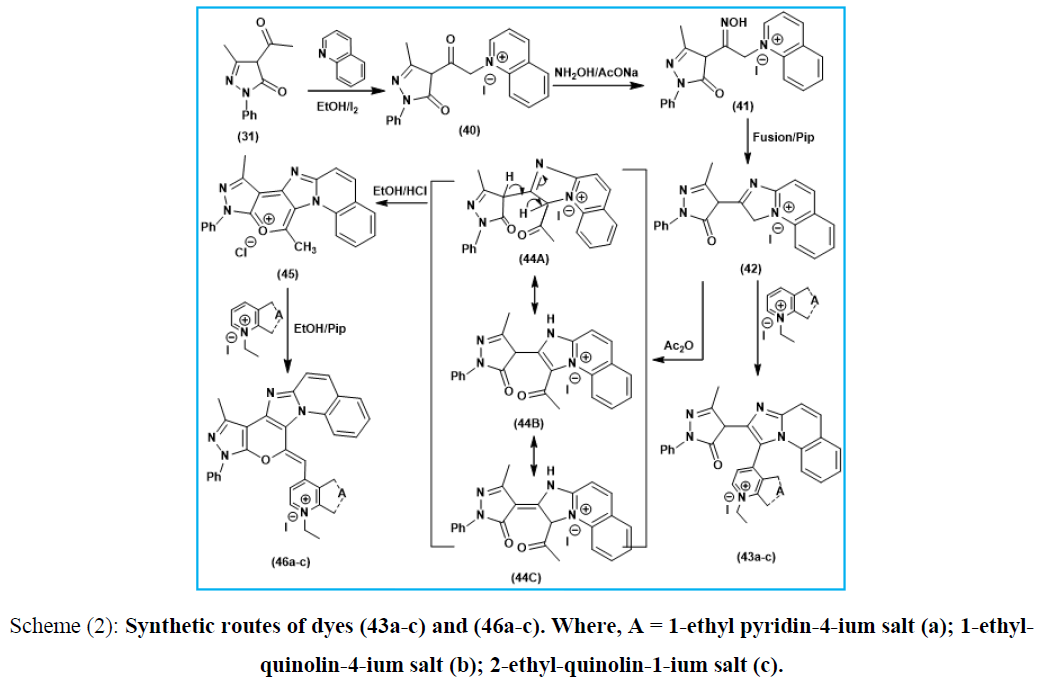
Furthermore, N-bridgehead heterobicyclic cyanine dyes were synthesized starting by the reaction ethanolic solution of 4-acetyl-1-phenyl-3-methyl-2-pyrazolin-5-one (31) with quinoline and iodine to afford 1-phenyl-3-methyl-pyrazolino-4-keto-α-methylene-N-quinolin-1-ium-iodide salt (40), reaction of (40) with hydroxyl amine in presence of anhydrous sodium acetate afford 1-phenyl-3-methyl-pyrazolino-4-ketooxime-α-methylene-N-quinolin-1-ium-iodide salt (41) which undergo ring closure by loss of water molecule under fusion condition and in presence of pipredine catalyst to afford 3-H-2-[1-phenyl-3-methyl-pyrazoline-5-one] imidazo(1,2-a) quinoline-1-ium-iodide salt (42). Reaction ethanolic solution of compound (42) with pyridin[quinolin]-4(1)-ium-1(2)-ethiodide salts in piperidine catalyst involving formation of 2-[1-phenyl-3-methyl-pyrazoline-5-one]imidazo(1,2-a)quinoline-mono-3-[4(1)]methine cyanine dyes (acyclic merocyanine dyes) (43a-c). On the other hand compound (42) undergo thermal acetylation by the effect of acetic anhydride to afford 2-[1-phenyl-3-methyl-pyrazoline-5-one]-3-acetyl-imidazo(1,2-a) quinoline-1-ium-iodide salt (44) followed by dehydration and ring closure under the effect of concentrated HCl to afford 1-phenyl-3,11-dimethyl-quinolino(1,2-a)imidazo(2.3-c)pyrano(2,3-c) pyrazol-12-ium-chloride salt (45), then followed by the reaction with under piperidine condition to afford 1-phenyl-3-methyl-quinolino(1,2-a)imidazo(2.3-c) pyrano (2,3-c)pyrazolo mono-11[1(4)]-methine cyanine dyes (46a-c).Acetylating of the later (42) was conducted using acetic anhydride to give the corresponding 2-[1-phenyl-3-methyl-pyrazoline-5-one]-3-acetyl-imidazo (1,2-a)quinoline-1-ium-iodide salt (44). Enolization of the later (44) afforded the Enol form intermediate which undergo ring closure using Conc.HCl/Ethanol conditions to give 1-phenyl-3,11-dimethyl-quinolino(1,2-a)imidazo(2.3-c)pyrano(2,3-c)pyrazole-12-ium-chloride salt (45), as depicted in (Scheme 2).
Scheme (2): Synthetic routes of dyes (43a-c) and (46a-c). Where, A = 1-ethyl pyridin-4-ium salt (a); 1-ethyl-quinolin-4-ium salt (b); 2-ethyl-quinolin-1-ium salt (c).
Colour and Spectral Behaviour
Ethanolic solution of (34a-c), (36a-c), (39a, b), (43a-c) and (46a-c) are highly colored ranging from brownish red to intense red, easily (partially) soluble in polar (non) organic solvents exhibiting colored solutions (reddish/red) concomitant with slight or intense greenish-red fluorescence depending upon the solvent used. They are soluble in conc. H2SO4 liberating iodine vapor on warming. Their Ethanolic solutions gave permanent colours in basic media which reversibly discharged on acidification. They possess interachargable colours solution (brownish-red/intense redyellow) in basic and acidic medium. The absorption spectra of (34a-c), (36a-c), (39a, b), (43a-c) and (46a-c) in 95% ethanol showed absorption bands undergo batho (hypso) chromically shifted depending upon the nature of heterocyclic quaternary residue A and their linkage position. Thus, the visible absorb-maximum of dye 34a [A=pyridin-4-ium ethiodide] showed λmax=490nm. Substitution of [A=pyridin-4-ium ethiodide] in dye 34a by [A=quinolin-4-ium ethiodide] in dye 34b resulted in bathochromic shift of Δλmax=55 nm concomitant with an increasing in number of absorption bands in dye 34b. This is due to the more extensive π-delocalization and extra conjugation in quinoline ring. Additionally, changing the linkage position of pyridin-4-ium salt in dye 34a to quinolin-4-ium salt in dye 34c causes bathochromic shift by Δλmax=40 nm. This is due to an extended π-delocalization within quinolin-4-ium salt in dye 34c. Moreover, the absorption spectra of (36a-c) showed absorb-maximum of dye 36a [A=pyridin-2-ium-ethiodide] showed λmax=500 nm. Substitution of [A=pyridin-2-ium ethiodide] in dye 36a by [A=quinolin-2-ium ethiodide] in dye 36b resulted in bathochromic shift of Δλmax= 60 nm concomitant with an increasing in number of absorption bands in dye 36b. This is due to the more extensive extra conjugated π-delocalization n the quinoline ring. Additionally, changing the linkage position of pyridin-2-ium in dye 36a to pyridin-4-ium analogue salt in dye 36c causes bathochromic shift of Δλmax=20 nm. This is due to the extended of π-delocalization within pyridin-4-ium ethiodide in dye 36c. On comparison between the absorption spectra of (34a-c & 36a-c), it was obvious that dyes (34a-c) showed absorption band more hypsochromic shift relative to that absorption band of dyes (36a-c). This may due to the extended π-delocalization in dyes (36a-c). Furthermore, the absorption spectra of (39a,b) showed absorb-maximum of 39a [A=pyridine-4-ium ethiodide] showed (λmax=400, 505 nm. εmax=1750, 2890 m-1cm-1) for 39a and dye 39b [A=quinoline-4-ium ethiodide] exhibited (λmax=395, 445, 520 nm; εmax=1750, 1550 & 2920 m-1cm-1) resulted in bathochromic shift of Δλmax=15 nm concomitant with the increasing number of absorption bands in the case of compound 39b. This is due to the more extensive π-delocalization and extra conjugation in the quinoline ring. In addition, the absorption spectra of (43a-c) showed absorb-maximum of dye 43a [A= pyridin-4-ium ethiodide] showed λmax=490 nm; εmax 2069 m-1cm-1). Substituting of [A=pyridin-4-ium ethiodide] in dye 43a by [A=quinolin-4-ium ethiodide] in dye 43b exhibit (λmax =500nm; εmax=2025 m-1cm-1) resulted in bathochromic shift of Δλmax=10 nm. This is due to the more extensive π-delocalization and extra conjugation in the quinoline ring. Additionally, changing the linkage position of [A=quinolin-4-ium ethiodide] in 43b to the 1-ium analogue salt in 43c exhibited (Δλmax=493 nm; εmax 2050 m-1cm-1) resulted in hypsochromic shift Δλmax=3nm. This can be related to decreasing the length of conjugated π-delocalization in quinolin-1-ium dye 43c than that quinolin-4-ium analogous in dye 43b. As well as, the absorption spectra of (46a-c) showed absorb-maximum of dye 46a [A=pyridin-4-ium ethiodide] showed (λmax483 nm; εmax 1079 m-1cm-1). Substitution of [A=pyridin-4-ium ethiodide] in dye 46a by [A=quinolin-4-ium ethiodide] in dye 46b showed (λmax=485 nm; εmax 1921 m-1cm-1) resulted in bathochromic shift of Δλmax=2 nm. This is due to the more extensive π-delocalization and extra conjugation in the quinoline ring. Additionally, changing the linkage position of [pyridine-4-ium] salt in 46a to [quinoline-4-ium salt] in 46c showed (λmax=490 nm; εmax 2212 m-1cm-1) resulted in bathochromic shift Δλmax=7 nm. This is due to the extended of π-delocalization within quinoline-4-ium salt in 46c, (TABLE 1).
Solvatochromic Behaviour
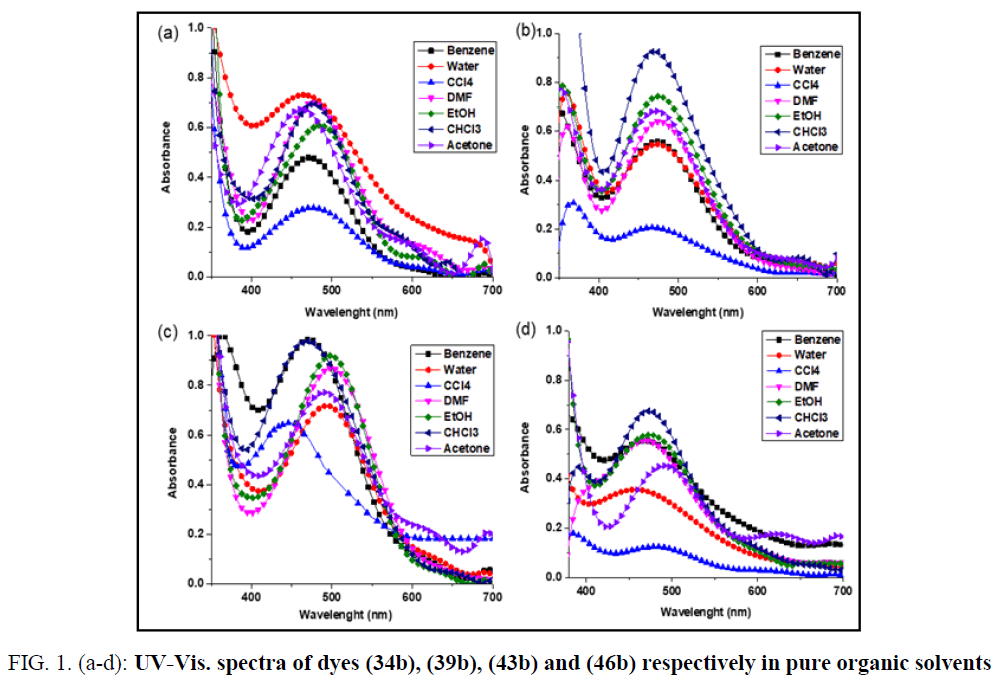
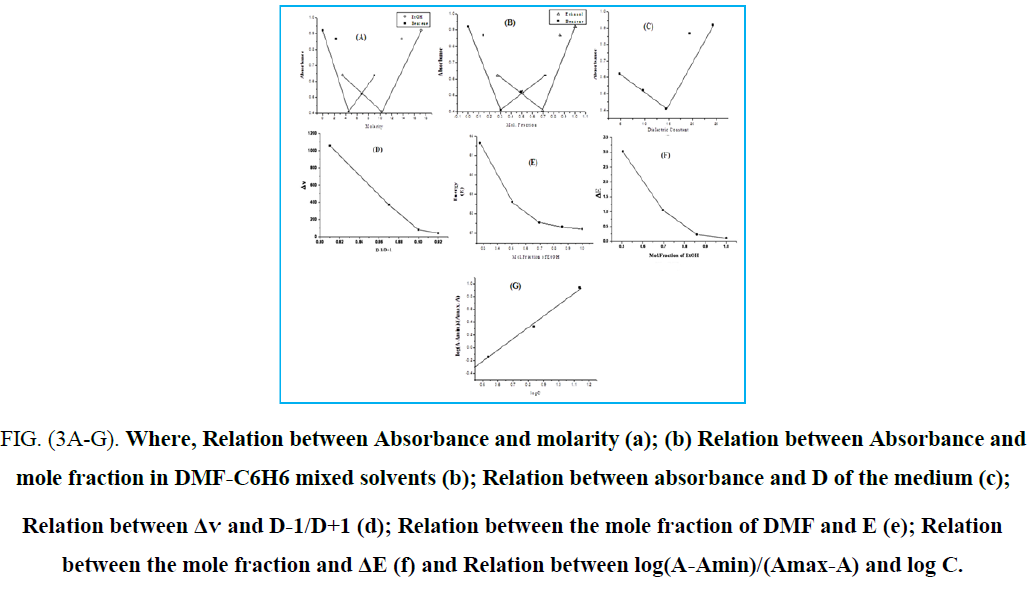
The colour changes of cyanine dyes with solvents called (solvatochromism) [27]. It is clear that Solvatochromic dyes generally exhibit steady bathochromic (positive solvatochromism) or hypsochromic (negative solvatochromism) shifts in solvents of various polarities which can attributed to the solvent polarity change the electron densities of cyanine dyes. The solvatochromism is caused by differential solvation of the ground and Franck-Condon excited state [28], due to the absorption of electromagnetic radiation in the UV-vis region If the ground state is more stabilized than the excited state due to solvation by solvents of increasing polarity, negative solvatochromism is exhibited and vice versa. The visible absorption spectra of 34b, 39b, 43b and 46b in the wavelength range 400-700 nm, have been studied in different organic solvents (H2O, DMF, EtOH, acetone, CCl4, CHCl3, & C6H6) respectively. The colour changes of these dyes with solvents having different polarities. This is constructed with the intention to illustrate the solvatochromic behaviour of these dyes λmax & λmax) values of the intramolecular charge transfer bands are given in TABLE 2. These dyes are showed positive solvatochromism with increased solvent polarity, which depend on the structure and the type of dye. This indicates that the polar excited states of these cyanine dyes are stabilized by polarization interaction forces as the polarizability of the solvent is increased. This behavior occurs as a result of electrostatic interactions of the distributed cationic charges with the dipoles of the solvated molecules which lead to formation of specific solvated forms of dyes. The absorption spectra of the dyes in ethanol are characterized by the presence of one or two essential bands which reflects the presence of intermolecular charge transfer. This intermolecular charge transfer had arisen from transferring the electron lone pair of the nitrogen atoms of the heterocyclic ring system towards the positively charged residue along the conjugated chain between both. The representing graphs disclosed that these electronic charge transfer bands exhibit a hypsochromic shifts in ethanol relative to DMF, CHCl3 & CCl4. This shift can be attributed to the following factors: The bathochromic shift occurred in DMF relative to ethanol is mainly a result of the increase in solvent polarity due to increasing the dielectric constant of the former. The hypsochromic shifts appeared in ethanol relative to CHCl3 & CCl4 is generated from the solute-solvent interaction through intermolecular hydrogen bonding between ethanol and the lone pair of electrons within the heterocyclic ring system. Otherwise, this decreases the mobility of the electron cloud over the conjugated pathway towards the positively charged center. It was worth mentioning that the intermolecular hydrogen bonding between CHCl3 molecules and the lone pair of electrons of nitrogen atoms of the heterocyclic ring system is difficult due to the sterric hindrance of the three bulk chlorines. Moreover, the solute solvent interactions in cases of CHCl3 & CCl4 generated a residual negative charge on the nitrogen atoms of the heterocyclic ring system which intern facilitated the electronic charge transfer to the positively charged center and this explain the bathochromic shifts in these solvents relative to ethanol. The unexpected hypsochromic shifts in the absorption spectral maxima in water relative to ethanol and its lower extinction coefficients were mainly ascribed to the ease of interactions of water molecules, through intermolecular hydrogen bonding, with the lone pair of electrons of the nitrogen atoms of the heterocyclic ring system, through intermolecular hydrogen bonding, which intern preclude the charge transfer from the heterocyclic ring system to the positively charged residue along the conjugated bridge, (FIG. 1. (a-d)).
FIG. 1. (a-d): UV-Vis. spectra of dyes (34b), (39b), (43b) and (46b) respectively in pure organic solvents
| Dye | C6H6 | Water | CCl4 | DMF | EtOH | CHCl3 | Acetone | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lmax | emax | lmax | emax | lmax | emax | lmax | emax | lmax | emax | lmax | emax | lmax | emax | |
| 34a | 471 | 478.7 | 465 | 731.2 | 474 | 277.9 | 477 | 699.9 | 484 | 608 | 480 | 694.7 | 461 | 672.9 |
| 39b | 474 | 559.7 | 474 | 546.7 | 467 | 206.2 | 478 | 639.8 | 474 | 742.9 | 470 | 927.5 | 473 | 682.7 |
| 43b | 470 | 984.8 | 494 | 717.8 | 449 | 647.9 | 501 | 869.5 | 500 | 918.9 | 470 | 977.5 | 492 | 771.5 |
| 46b | 466 | 554.4 | 460 | 355.9 | 482 | 124.3 | 469 | 555.7 | 475 | 578.3 | 472 | 674.7 | 485 | 449.2 |
TABLE 2. Values of absorption (nm) and extinction coefficients (M-1cm- 1) of (34a, 39b, 43b, and 46b) in pure organic solvents
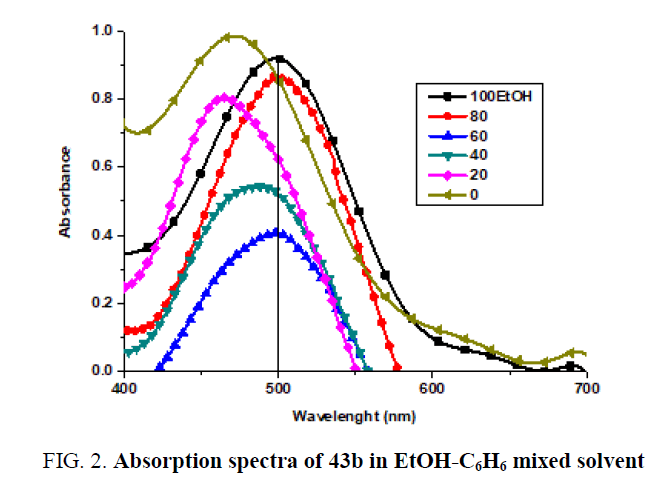
Absorption spectra in mixed solvent: The absorption spectra of 1 × 10-4 M of (43b) in ethanol in the presence of different concentrations of C6H6 are shown in (FIG. 2).
It is shown that in presence of 17.17 M of ethanol, the spectrum exhibits a band located at 500 nm. In the presence of 1.72M of ethanol, the band is shifted to 464 nm concomitant with a gradual blue shift. Also, an increase in band intensity at fixed wavelength (500 nm) is observed on increasing of benzene concentration (FIG. 3A). The increase in absorbance as well as the gradual blue shift in the maximum absorption wavelength on increasing the C6H6, content can be ascribed to the gradual formation of the complex species through intermolecular hydrogen-bond. The graphical representation of absorbance at 500 nm against the mole fraction of ethanol FIG. 3B, reveals that the absorbance increases gradually with increasing mole fraction. To investigate the effect of the dielectric constant of the medium on the band shift (Δv), On plotting versus (D is dielectric constant of the solvent added), a straight line is obtained which at 6.88 M ethanol give another straight-line (FIG. 3D). Furthermore, a broken line is obtained on plotting the absorbance against the dielectric constant of the medium (FIG. 3C). Such behaviour indicates that factors other than the change in the dielectric constant of the medium are responsible for the shift of λmax at lower and higher percentage of ethanol. These factors mainly include the solute-solvent interaction through intermolecular hydrogen bond which leads to the formation of some molecular complex. On plotting the excitation energy (E) versus the mole fraction of ethanol (FIG. 3D), a broken line with three segments is obtained. The first segment represents the orientation energy of the solvent molecules around the solute. The second segment corresponds to the molecule complex formation, where the third one represents the steady state of energy attained after complete formation of the molecular complex. From the above relations, it is clear that the position of the bands and consequently the excitation energy depends not only on the mole fraction of ethanol, but also on the following: (a) Solvation energy. (b) Orientation of solvent molecules around the solute molecule in the ground states. (c) Dipole moment of the solute in both ground and excited states. (d) Dipole-dipole interaction between solute and solvents (e) The strength of H-bond between solute and solvent in both ground and excited states. In pure EtOH solution, the dye molecule form solvent cage, which is affected on adding C6H6. At lower C6H6 content, ethanol molecules will have distributed themselves uniformly on all the solvation sheaths around the molecules. The added molecules may first enter the outer solvation sheaths and then will introduce themselves in the first sheaths as their proportions are increased. This is probably due to the fact that addition of ethanol permits the formation of a solvent cage around the solute molecules, through intermolecular hydrogen-bonding which previously discussed (FIG. 3E). it is possible to evaluate the excitation energy of the solute in pure C6H6 is equal to 61 K Cal mol-1 whereas the value in pure EtOH amounts to 57.2 K Cal mol-1. The difference between the excitation energy in pure EtOH and that corresponding to the first inflection point amounts to 1.406 K Cal mol-1. This value may correspond to the orientation energy of the solvent molecules around the solute molecules. The value of the stability constant (Kf) of the complex with C6H6 was determined from the spectral behaviour in mixed solvents using the relations previously described (FIG. 3G). The value of Kf, ΔG (free energy change of formation) and n (number of C6H6 molecules complexes with the solute) indicate that 1:1 complex is formed. The value of Kf is dependent on both solute and solvent used (TABLE 3).
FIG. (3A-G). Where, Relation between Absorbance and molarity (a); (b) Relation between Absorbance and mole fraction in DMF-C6H6 mixed solvents (b); Relation between absorbance and D of the medium (c); Relation between Δѵ and D-1/D+1 (d); Relation between the mole fraction of DMF and E (e); Relation between the mole fraction and ΔE (f) and Relation between log(A-Amin)/(Amax-A) and log C.
| Comp. No. | Solvent System | Excitation energy K Cal mol-1 | Orient energy K. Cal mol-1 | H-bond energy K.Cal.mol-1 | Total energy K.Cal.mol-1 | N | Log Kf (-) | Kf (-) | ΔG K.Cal.mol-1 (+) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pure Solvents | ||||||||||
| (43b) | (EtOH-Benzene) | 57.2 (EtOH) | 61 (Benzene) | 1.406 | 0.345 | 1.751 | 1 | 1.569 | 37.06 | 0.3697 |
TABLE 3. Commutative data obtained for dye (43b) in mixed solvents.
Conclusions
Highly stable series of novel zeromehine, monomethine and mero cyanine dyes stabilized by C-N bond were synthesized based on N-Bridge head heterocycles. The absorption spectra of the synthesized dyes were investigated in different organic solvents and a mixed solvent system. The results indicated that the dyes undergo bathochromic or hypsochromic shift depending basically on the structure of dye, the type of solvent and length of π-conjugation within the structure.
References
- Koraiem AI, El-Shafie AM, Abdellah IM, et. al. Microwave assisted synthesis and solvato (media)-chromic behaviour of some new series photosensitizing dyes. J Applicable Chem. 2018;7:309-24.
- Czerney P, Grane G, Birckner E, et al. Molecular engineering of cyanine-type fluorescent and laser dyes J Photochem Photobiol A Chem. 1995;89:31-6.
- Widengren J, Schwille P. Characterization of photo induced isomerization and back-isomerization of the cyanine dye Cy5 by fluorescence correlation spectroscopy. J Phys Chem A, 2000;104:6416-28.
- Khairutdinov RF, Serpone N. Photophysics of cyanine dyes: Subnanosecond relaxation dynamics in monomers, dimers, and H-and J-aggregates in solution J Phys Chem B. 1997;101: 2602 -10.
- Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods; 2006;3:793.
- Pokhriyal A, Lu M, Huang CS, et al. Multicolor fluorescence enhancement from a photonics crystal surface. Appl Phys Lett.2010;97:121108.
- Yin J, Kwon Y, Kim D, et al. Cyanine-based fluorescent probe for highly selective detection of glutathione in cell cultures and live mouse tissues. J Am Chem Soc. 2014;136:5351-8.
- Guo Z, Kim G, Shin I, et al. A cyanine-based fluorescent sensor for detecting endogenous zinc ions in live cells and organisms. Biomaterials. 2012;33:7818 -27.
- Suleimanov I, Molnár G, Salmon L,et al. Near‐infrared luminescence switching in a spin‐crossover polymer nanocomposite. Eur J Inorg Chem. 2017;28:3446.
- Williams GA. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. J Ophthalmol. 2006;90:1216.
- Zhang XH, Wang LY, Nan ZX. Microwave-assisted solvent-free synthesis and spectral properties of some dimethine cyanine dyes as fluorescent dyes for DNA detection. Dye Pigment. 2008;79:205 -9.
- He S, Song B, Li D, et al. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv Funct Mater. 2010;20:453 -9.
- Ning J, Huang B, Wei Z, et al. Mitochondria targeting and near-infrared fluorescence imaging of a novel heptamethine cyanine anticancer agent. Mol Med Rep. 2017;15:3761-6.
- Bhattarai P, Dai Z. cyanine based Nanoprobes for Cancer Theranostics. Adv Healthc Mater. 2017;6:1700262.
- Deng Y, Huang L, Yang H, et al. Cyanine‐anchored silica nanochannels for light‐driven synergistic thermo‐chemotherapy. Small.2017;13:1602747.
- Renikuntla BR, Rose HC, Eldo J, et al.. Improved photostability and fluorescence properties through polyfluorination of a cyanine dye. Org Lett. 2004;6:909-12.
- Heaney F, McCarthy T, Mahon M, et al. Bridgehead nitrogen heterocycles which contain the quinazoline moiety–synthesis and cycloaddition of 1,2-dihydroquinazoline 3-oxides. Org. Biomol. Chem.2005;3:4351-61.
- Abengózar A, Abarca B, Cuadro AM, et al. Azonia aromatic cations by ring‐closing metathesis: synthesis of azaquinolizinium cations. Eur J Org Chem. 2015;19: 4214-23.
- Aliyeu T M, Berdnikova D V, Fedorova OA. Regiospecific photocyclization of mono-and bis-styryl-substituted n-heterocycles: a synthesis of dna-binding benzo [c] quinolizinium derivatives. J Org Chem.2016;81:19 -85.
- Zhang G, Yang L, Wang Y, et al. An efficient Rh/O2 catalytic system for oxidative C–H activation/annulation: evidence for Rh (I) to Rh (III) oxidation by molecular oxygen. J Am Chem Soc. 2013;135: 24-3.
- Abd El-Aal RM, Koraiem AIM, N.M.S El-Deen. Color Technol. 2005;121:4.
- Abd El-Aal RM. The synthesis of some bridgehead heterocyclic monomethine cyanine dyes. Dye Pigment.1998;39: 267-80.
- Koraiem AIM, Abd El-Aal RM, Salah El-Deen NM. The use of N-bridgehead heterocyclic indolizinium ylide in the synthesis of aza-cyanine dyes. Dye Pigment. 2006;68:235-42.
- Koraiem AI, El-Shafie AM , Abdellah IM. Synthesis and physicochemical properties of novel zero methine cyanine dyes based on N-bridgehead indolizine (benzoindolizine) heterocycles. IJARSET. 2018;5.
- Thakera BT, Suratia KR, Patela SV, et al. Synthesis, spectral, thermal, and antibacterial investigation of mixed ligand complexes of oxovanadium (IV).J Saudi Chem Soc. 2006;10:447-33.
- Dietz F, Mueller G, Bach G, I. Von Grossmann, J Signal Am.1975;3.
- Bayliss NS, McRae EG. Solvent effects in the spectra of acetone, crotonaldehyde, nitromethane and nitrobenzene. J Phys Chem. 1954;58:1002-11.