Original Article
, Volume: 16( 2) DOI: 10.21767/0972-768X.1000252Ninhydrin Thiourea Derivative as Chemosensor for Visual Detection of Cu(II) in Aqueous Medium
- *Correspondence:
- Gururaja GN , Department of Chemistry, Goa University, Goa, India, Tel: 0832-6519048; E-mail: gururajagn@gmail.com
Received: February 11, 2018; Accepted: March 14, 2018; Published: March 19, 2018
Citation: Viegasa AM, Naika VB, Deshpandea K, et al. Ninhydrin Thiourea Derivative as Chemosensor for Visual Detection of Cu(II) in Aqueous Medium. Int J Chem Sci. 2018;16(2):252
Abstract
Visual detection of Cu(II) is significant as copper predominantly exists as Cu(II) in an aqueous medium. In contrast to thiourea where Cu(II) will be reduced to Cu(I) and bind to sulphur, thiourea derivative of ninhydrin showed synergistic effect with acetate anion and chelate well with Cu(II) and its chemoreceptor properties were explored. Binding nature of chemo-sensor (receptor) is successfully exploited to prepare eco-friendly paper strips and slides for the visual detection of Cu(II) in a wastewater sample.
Keywords
Receptor; Chemo sensor
Introduction
Transition metals are common in our environment and balanced concentration of them is obligatory for various biological processes [1]. Interestingly non-degradable metals are a major cause of ecological hazards. Prolonged exposure or excessive assimilation of these through food chain cause acute or chronic toxicity [2]. Utilization of industrial wastewater for irrigation and agriculture cause metal polluted lands and increased concentration of metal in the human body via the food chain [3]. Like few transition metals, copper with stable oxidation states occurs naturally in redox enzymes, which involves cyclic oxidation and reduction processes. Although Cu(II) is essential for various biological processes, significant Cu(II) pose health hazards such as prolonged exposure leads to irritation of nose and eyes, headache, stomachache, dizziness, diarrhoea and neurodegenerative diseases [4,5].
Experimental
Increased concerns about health issues make it essential to develop economically and environmentally benign technologies to identify toxic cations and anions. Although various sophisticated instruments and methods have been developed [6-12] with selectivity and sensitivity, the operational burden remains the main hurdle for extensive use. Recent time’s uses of organic chemoreceptors, colourimetric receptors have gained considerable momentum, which allows visual detection at milli and micro level without any operational constraint [12].
Ninhydrin and its derivatives attracted our attention due to their clinical applications and as sensors; optical receptors for various ions of biological and environmental significance [13]. Development of nonlinear optical devices and chemical sensors from these find immense applications [14-19]. Ninhydrin based colourimetric sensors have been known for visual detection of Cu(II) in an aqueous medium,19 these colourimetric sensors are Schiff base derivatives of ninhydrin which involves more than one synthetic procedures. Besides imine [13,19] based ninhydrin chemoreceptor which is known for coordination with cations/anions, use of other simple derivatives as colourimetric chemo sensors remain unexplored. We investigated these simple ninhydrin derivatives for analytical applications. Herein we report eco-friendly and non-toxic small organic molecule, thiourea derivative of ninhydrin as a sensor for selective visual detection of Cu(II) in aqueous medium and application as dipstick device (paper strips) for visual detection and analysis of Cu(II) in an aqueous medium.
Results and Discussion
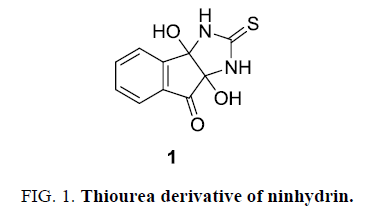
The objective for the present work originated from the fact that copper predominantly exists as Cu(II) in aqueous medium owing to the stability of Cu(II) over Cu(I). However, the presence of thiourea reduces Cu(II) to colourless Cu(I) and binds to sulphur of thiourea [20]. We envisioned that derivatives of thiourea with chromogenic substrate would impart properties [21] that would chelate well with copper ion and chemoreceptor properties could be explored. Thiourea-ninhydrin derivative 1 (3a,8a-dihydroxy-2-thioxo-2,3,3a,8a-tetrahydroindeno[1,2-d] imidazole-8(1H)-one) has been selected as model receptors as it holds ninhydrin as chromogenic moiety and thiourea as a counterpart (FIG. 1).
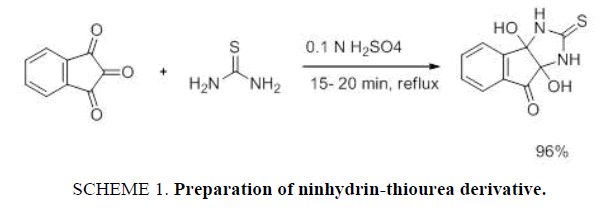
Ninhydrin thiourea derivative 1 has been synthesized from ninhydrin 2. Ninhydrin 2 reacted with thiourea 3 under an acidic condition to afford ninhydrin thiourea derivative 1 in 96% yields. The Ninhydrin thiourea derivative 1 has been recrystallised from hot methanol which melts with decomposition at 195°C-205°C. Powder XRD and spectral studies (ESI-S2) of these compounds are consistent with the expected product [22] (SCHEME 1).
Spectral property of receptor 1 was studied in various solvents. Spectral properties could not be recorded with water as precipitation of receptor 1 is observed. The similarity in absorbance is observed for methanol and ethanol and ethanol has been chosen as a solvent of choice for further studies. To evaluate the potential of 1 as colourimetric chemo sensor, initially, we investigated the interaction of 1 with various copper salts. Two equivalent metal salt solutions of CuCl2, CuSO4 .5H2O, Cu(NO3)2, Cu2(CH2COO)4 2H2O were separately added to 0.5 mM of receptor 1. To our surprise, none of the Cu(II) solutions showed remarkable colour change except for Cu2(CH2COO)4 2H2O, which showed distinct colour change from colourless to olive green. We expected that receptor 1 is sensing CH2COO- anion, as an acetate salt of Cu(II) showing distinct colour change. To examine under similar experimental conditions, 2-25 equivalent acetate solutions of Na(I), Ag(I), Pb(II) were separately added to 0.5 mM of receptor 1. However, no remarkable colour changes were observed, indicating receptor 1 is not selective to CH2COO- anion, but Cu(II) solutions show remarkable colour change for Cu2(CH2COO)4 2H2O. To end this ambiguity, separate set of experiments were performed to examine the role of CH2COO- anion in sensing of Cu(II) by receptor 1. In one set of experiment, 0.5 mM of receptor 1 is added separately to two equivalents of CuCl2, and 12-25 equivalents of CH2COONa respectively and in another set of experiment, CuCl2 and CH2COONa solutions were mixed in different proportions. However, no significant colour changes were observed in either experiment. But, when 0.5 mM of receptor 1 was added to two equivalents of CuCl2 followed by addition of 25 equivalents of sodium acetate, solution showed distinct colour change from colourless to olive green, demonstrating the synergistic effect of receptor 1 and CH2COO- in sensing Cu(II) ion (FIG. 2). Further, receptor 1 was considered with two equivalents of CuCl2 followed by addition of anions like Br-, SO42-, Cl- for sensing Cu(II), no visible colour change is observed.
Figure 2: Receptor 1 (0.5 mM) in ethanol, 2. Mixing proper proportions of 2. 1 (0.5 mM) and 1 mM CuCl2, 3. Receptor (0.5 mM) and 12.5 mM of NaOAc, 4. NaOAc (12.5 mM) and 1 mM CuCl2, 5. Receptor 1 (0.5 mM), 1 mM CuCl2 and 12.5 mM of NaOAc.
Synergistic effect of receptor 1 and CH2COO- in sensing Cu(II) ion was established by the spectral study of 0.5 mM of receptor 1 with 2 equivalents of Cu(II) salt solution and 25 equivalents of CH2COONa. UV-vis spectrum of 0.5 mM receptor 1 in ethanol (colourless) exhibited π-π* transition band at 289 nm. Addition of 2 equivalents of the CuCl2 solution showed analogous absorption pattern.
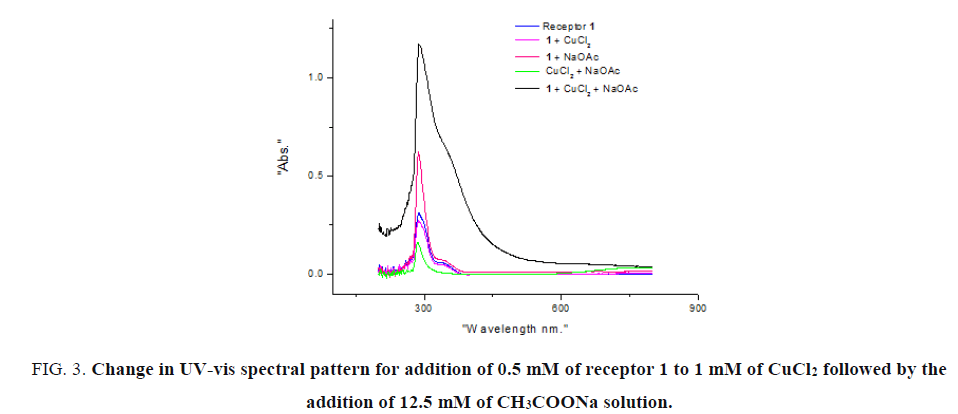
Similarly, when 25 equivalents of CH2COONa solution was added to receptor 1 no substantial increase in the intensity was observed. Notably when both CuCl2 and CH2COONa solutions were added to receptor 1 colour change from colourless to olive green and marked an increase in the intensity of absorption was observed (FIG. 3). Intramolecular charge transfer (ICT) band at 530-540 nm are generally observed along with π-π* transition band at 289 nm. However, sensitivity of the instrument used was inadequate to record such weak transition for these dilute solutions.
Figure 3: Change in UV-vis spectral pattern for addition of 0.5 mM of receptor 1 to 1 mM of CuCl2 followed by the addition of 12.5 mM of CH3COONa solution.
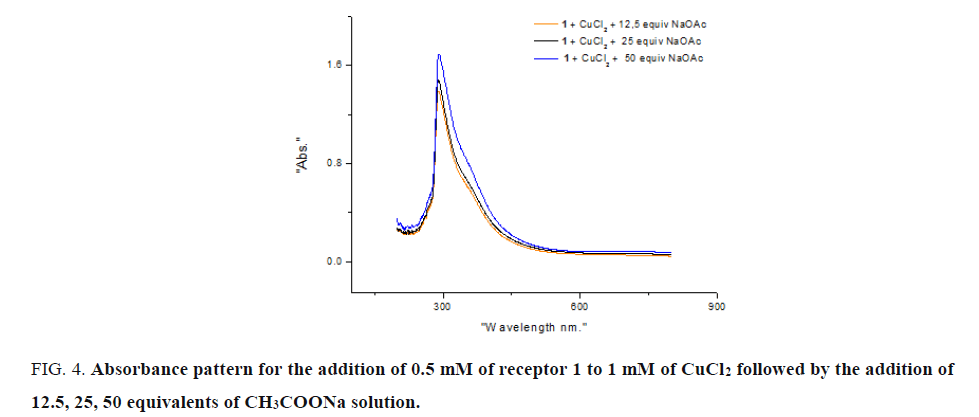
The scope of visual detection with receptor 1 was optimized for different concentrations of CH2COONa. Receptor 1 (0.05 mM) and corresponding two equivalents of CuCl2 solution were added to 12.5, 25, 50 equivalents of CH2COONa solution. Absorbance pattern was similar in all cases and a small increase in intensity for 25 and 50 equivalents CH2COONa may be attributed to the presence of CH2COO- ion. At lower concentration of CH2COO- solution, change of intensity of receptor 1 was not observed and visible colour change was observed corresponding to 12.5 equivalents (FIG. 4).
Figure 4: Absorbance pattern for the addition of 0.5 mM of receptor 1 to 1 mM of CuCl2 followed by the addition of 12.5, 25, 50 equivalents of CH3COONa solution.
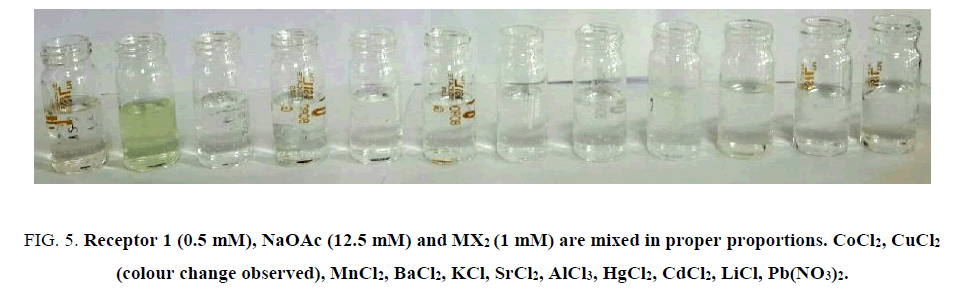
To study the selectivity of receptor 1 with specific metal ion, receptor 1 (0.5 mM) is added to two equivalents of different metal ions solutions. Significant colour change is not observed for any metal ions, however, followed by addition of 25 equivalent of CH2COONa, distinct colour change is observed only for Cu(II) (FIG. 5). Similar results are obtained when different concentrations of reagents were considered.
Figure 5: Receptor 1 (0.5 mM), NaOAc (12.5 mM) and MX2 (1 mM) are mixed in proper proportions. CoCl2, CuCl2 (colour change observed), MnCl2, BaCl2, KCl, SrCl2, AlCl3, HgCl2, CdCl2, LiCl, Pb(NO3)2.
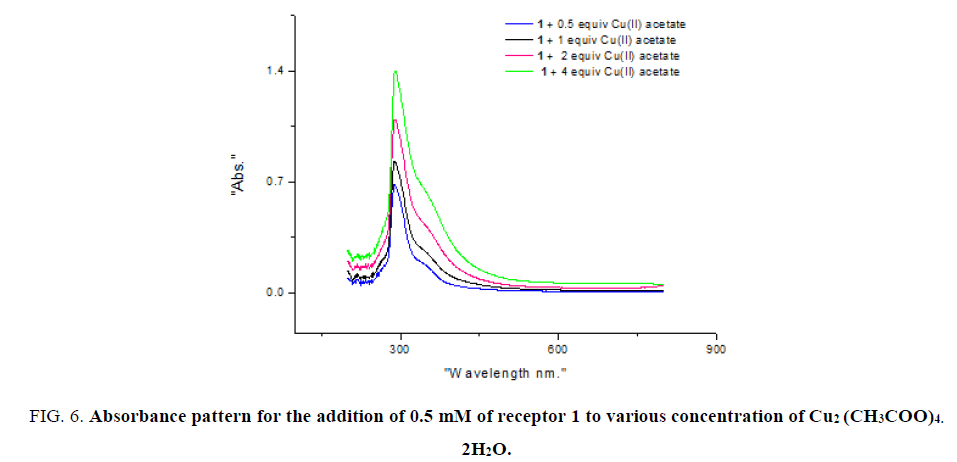
As absorption properties of receptor 1 with CuCl2 ion is significant in the presence of CH2COO- ion. Varying concentrations of Cu2(CH2COO)4.2H2Owere added to 0.5 mM of receptor 1. When two equivalents of Cu2(CH2COO)4.2H2Owere added to 0.5 mM of receptor 1 distinct colour change to olive green is observed (FIG. 6). Notably below this concentration, no detectable colour change was observed.
Figure 6: Receptor 1 (0.5 mM), NaOAc (12.5 mM) and MX2 (1 mM) are mixed in proper proportions. CoCl2, CuCl2 (colour change observed), MnCl2, BaCl2, KCl, SrCl2, AlCl3, HgCl2, CdCl2, LiCl, Pb(NO3)2.
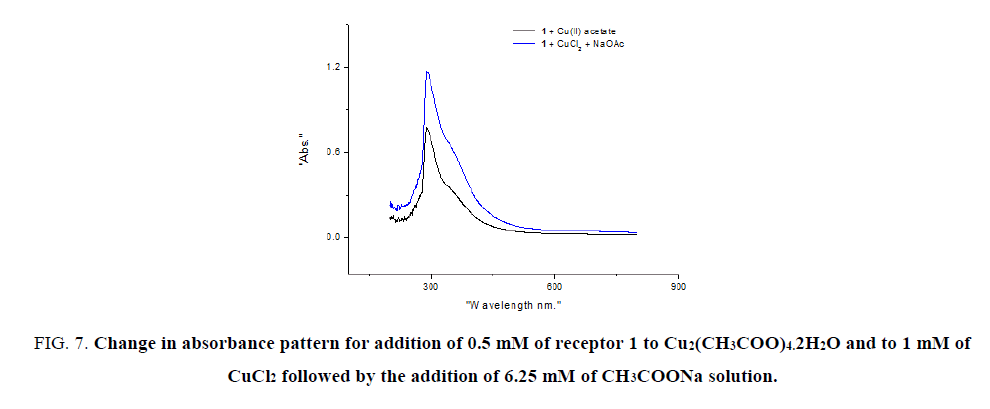
To study the effect of CH2COO- ion, receptor 1 with Cu2(CH2COO)4. 2H2Oand CuCl2, CH2COONa in two separate set of the experiment were considered. Spectral studies reveal a considerable difference in intensity of absorption for both experiments (FIG. 7).
Figure 7: Change in absorbance pattern for addition of 0.5 mM of receptor 1 to Cu2(CH3COO)4.2H2O and to 1 mM of CuCl2 followed by the addition of 6.25 mM of CH3COONa solution.
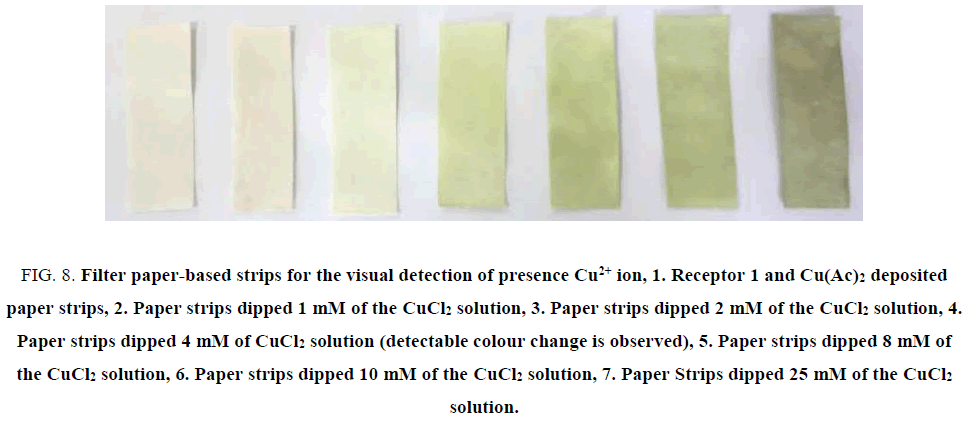
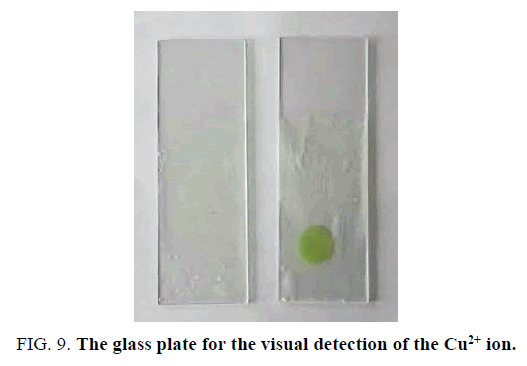
Different Cu(II) solutions (as their chlorides) were applied on previously dried receptor containing paper strips. As we can see a change in the colour corresponding to two mM solution, however for four mM solution distinct colour change is observed (corresponding to 2.38 × 10-4 g of Cu(II) in solution) (FIG. 8). These filter paper-based strips offer many advantages such as cheap, easy to operate, selective with distinct colour change with different concentration of Cu(II). Similarly, previously dried receptor slide was also tested for the presence of Cu(II) (FIG. 9). Model industrial waste Cu(II) solution was prepared by dissolving copper salt with other metal ions like Pb(II), Ni(II), Fe(III) solution was used for selective detection of the presence of cations. When glass plate and paper strips were used, selective distinct colour change for Cu(II) is observed (FIG. 10).
Figure 8: Change in absorbance pattern for addition of 0.5 mM of receptor 1 to Cu2(CH3COO)4.2H2O and to 1 mM of CuCl2 followed by the addition of 6.25 mM of CH3COONa solution.
Conclusion
Visual detection of Cu(II) is significant as copper predominantly exists as Cu(II) in an aqueous medium. In the presence of thiourea, Cu(II) will be reduced and bind to sulphur. However, thiourea derivative of ninhydrin showed synergistic effect with acetate anion and chelate well with Cu(II) and its chemoreceptor properties were explored. Binding nature of receptor is successfully exploited to prepare eco-friendly paper strips and slides as sensors for the visual detection of Cu(II) in wastewater sample. The development of the nonlinear optical device and immobilized sensors from these non-toxic derivatives offer a wide range of applications
Acknowledgment
GNG acknowledges Prof. V. S. Nadkarni, Goa University for providing NMR facility and for helpful discussions.
References
- Cowan JA. Inorganic Biochemistry: An Introduction. Wiley-VCH, New York. 1997;pp:133-4.
- Mudgal V, Madaan N, Mudgal A, et al. Effect of toxic metals on human health. The Open Nutraceuticals Journal. 2010;3(1):94-9.
- Qadir M, Scott CA. Non-Pathogenic Trade-Offs of Wastewater Irrigation. Drechsel P, Scott CA, Raschid-Sally L, Redwood M, Bahri A, Earthscan, editors. London. 2010;101.
- Jarup L. Impact of environmental pollution on health; balancing risk. Br Med Bull. 2003;68:167-82.
- Sud D, Mahajan G, Kaur MP. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: A review. Bioresource Technology. 2008;99(14):6017-27.
- Kato T, Nakamura S, Morita M. Determination of nickel, copper, zinc, silver, cadmium and lead in seawater by isotope dilution inductively coupled plasma mass spectrometry. Analy Sci. 1990;6(4):623-6.
- Yin BC, Zuo P, Huo H, et al. DNAzyme self-assembled gold nanoparticles for determination of metal ions using fluorescence anisotropy assay. Analy Biochem. 2010;401(1):47-52.
- Hong S, Kang T, Moon J, et al. Surface plasmon resonance analysis of aqueous copper ions with amino-terminated self-assembled monolayers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;292(2-3):264-70.
- Gattás-Asfura KM, Leblanc RM. Peptide-coated CdS quantum dots for the optical detection of copper (II) and silver (I). Chemical Communications. 2003(21):2684-5.
- Zhang YJ, He XP, Hu M, et al. Highly optically selective and electrochemically active chemosensor for copper (II) based on triazole-linked glucosyl anthraquinone. Dyes and Pigments. 2011;88(3):391-5.
- Hu B, Su Q, Lu P, et al. BODIPY modified 9-cycloheptatrienylidene fluorene derivatives: Fluorescent ?turn-on? for detecting Cu2+ with acidity independence. Sensors and Actuators B: Chemical. 2012;168:310-7.
- Xu Z, Yoon J, Spring DR. A selective and ratiometric Cu2+ fluorescent probe based on naphthalimide excimer?monomer switching. Chemical Communications. 2010;46(15):2563-5.
- Kumar A, Kumar V, Upadhyay KK. A ninhydrin based colorimetric molecular switch for Hg2+ and CH3COO-/F-. Tetrahedron Letters. 2011;52(50):6809-13.
- Jung HS, Park M, Han JH, et al. Selective removal and quantification of Cu (II) using fluorescent iminocoumarin-functionalized magnetic nanosilica. Chemical Communications. 2012;48(42):5082-4.
- Gunnlaugsson T, Leonard JP, Murray NS. Highly selective colorimetric naked-eye Cu (II) detection using an azobenzene chemosensor. Organic Letters. 2004;6(10):1557-60.
- Gao JQ, Duan MY, Zhang XY, et al. Effects of frequency and intensity of drying-rewetting cycles on Hydrocotyle vulgaris growth and greenhouse gas emissions from wetland microcosms. CATENA. 2018;164:44-9.
- Kumar A, Kumar V, Diwan U, et al. Highly sensitive and selective naked-eye detection of Cu2+ in aqueous medium by a ninhydrin-quinoxaline derivative. Sensors and Actuators B: Chemical. 2013;176:420-7.
- Mruthyunjaya HC, Murthy AV. Oxidation of coordinated thiourea in copper (I)-thiourea complexes by copper (II)-perchlorate in acetonitrile. Analy Chem. 1969;41(1):186-8.
- Katritzky AR, Gordeev MF. New 1H-benzotriazole-mediated synthesis of N, N'-disubstituted thioureas and carbodiimides. J Chem Soc. 1991(9):2199-203.
- Ghalib RM, Hashim R, Alshahateet SF, et al. Synthesis, supramolecularity and in vitro antimicrobial activity of 3a, 8a-dihydroxy-2-thioxo-1, 3, 3a, 8a-tetrahydroindeno [1,2-d] imidazol-8 (2H)-one. J Mol Struc. 2011;1005(1):152-5.
- Lakshmi S, Geetha S. Synthesis, characterization and biological studies of tridentate amino acid (L-tryptophan) Schiff base transition metal complexes. Chem Pharm Res. 2016;8:668-74.
- Xiong JJ, Huang PC, Zhang CY, et al. Colorimetric detection of Cu2+ in aqueous solution and on the test kit by 4-aminoantipyrine derivatives. Sensors and Actuators B: Chemical. 2016;226:30-6.