Mini Review
, Volume: 17( 1)Nanodrug Delivery Systems-A Review
- *Correspondence:
- MN Anusha
Department of Oral Pathology and Microbiology, Bharath University, Chennai, Tamil Nadu, India
Tel: 9790325286
E-mail: anushanedu97@gmail.com
Received: December 30, 2022, Manuscript No. TSBC-23-85116; Editor assigned: January 02, 2023, PreQC No. TSBC-23-85116 (PQ); Reviewed: January 16, 2023, QC No. TSBC-23-85116; Revised: March 02, 2023, Manuscript No. TSBC-23-85116 (R); Published: March 10, 2023, DOI: 10.37532/0974-7427.2023.17(1).177
Citation: Anusha MN, Babu NA, Masthan KMK, et al. Nanodrug Delivery Systems-A Review. Biochem Ind J. 2023;17(1):177.
Abstract
The chief methods of drug delivery are oral route and injections and it is been limited with the advent of new drugs. Such drugs aren’t effective with the same route of administration when concerned to a particular therapy. New biologic medications, such as proteins and nucleic acids, necessitate innovative delivery strategies that reduce adverse effects and improve patient compliance. Nanometer sized drug particles have unique properties that can contribute to improved performance in a number of dosage formats. This size range of particles is resistant to settling and can have increased saturation solubility, quick dissolution and enhanced adherence to biological surfaces, resulting in a faster commencement of therapeutic action and improved bioavailability. Nanotechnology is being used by scientists to tackle both traditional and innovative drug delivery applications.
Keywords
Drug delivery; Nanodrug; Nanotechnology; Nanoparticles; Bioavailability
Introduction
Nanoscience deals with the study of the distinctive characteristics of minute materials ranging between 1 nm-100 nm. The application of these materials to construct or modify a novel object is called nanotechnology [1]. Nanotechnology is now raising the bars by enabling new methods in drug delivery, tissue regeneration, manufacturing medical devices and many other inspiring evolutions. Drug delivery is manipulated technologies for the accurate release of drugs at the appropriate site. Nanotechnology has supplemented its role in all these spheres. This article is a review about the basics and evolution of nanotechnology, types of nanoparticles their method of manufacturing, nano diagnostics, drug delivery technologies, barriers to oral drug delivery and the probable risks of nanotechnology [2].
Literature Review
Evolution of nanotechnology
Nanoparticles and structures have been used by humans throughout history, including in the fourth century AD by the Roman. This demonstrates one of the most interesting examples of nanotechnology in history. The Lycurgus cup, from the British museum collection, is an excellent example of ancient glassmaking technology. The dichroic glass is the oldest known example of a type of glass that can selectively reflect different colors of light. Dichroic glass describes two different types of glass that change color in certain lighting conditions [3]. The scientists analyzed the cup using a Transmission Electron Microscope (TEM) in 1990 to explain the phenomenon of dichroism. The observed dichroism (two colors) is due to the presence of nanoparticles with a diameter of 50 nm-100 nm X-ray analysis of the nanoparticles showed that they are made of a silver and gold alloy with a ratio of 7:3. In addition, they contain about 10% copper.
The red colour of the Au nanoparticles is a result of light absorption. The red purple colour is due to the absorption of larger particles by the dye, while the green colour is due to the scattering of light by smaller particles of silver nanoparticles. The Lycurgus cup is a well-known synthetic nanomaterial that is ancient. The effect of nanoparticles fusion in glass is seen in medieval church windows, where a red and yellow light is produced due to the combination of Au and Ag nanoparticles [4].
Richard P Feynman developed the concept of nanotechnology in 1959. In a 1974 publication, Norio Taniguchi of Tokyo Science university defined the term nanotechnology as follows: The processing, separation, consolidation and deformation of materials by one atom or one molecule is referred to as “nanotechnology”. K Eric Drexler is credited with popularising it. One nanometer is one billionth of a metre 109.
Branches of nanotechnology
Nano engineering: It is the branch nanotechnology practice on the nanoscale.
Green nanotechnology: It is the branch of nanotechnology that enhances the environmental sustainability of processes. It entails creating green nano products and then putting them to use in support of long term sustainability.
Wet nanotechnology: This includes pharmaceuticals and bioscience. Wet nanotechnology refers to working up with large masses from small ones.
Manufacturing nanoparticles
The nanoparticles are synthesized by 2 approaches top down approach and bottom up approach.
Top down approach: In this approach, the bulk of the material is broken down to nano particles by use of recent technologies like precision engineering. In this technology uses sensors and diamond or cubic boron nitride for the control of size along with numerical system. The top down approach also uses the technology of lithography which exposes the surface to light, ions or electrons, followed by material deposition over it to form the desired substance [5].
Milling: The method is in use since years for converting a bulk of material into nano sized particles. This is done by means of mechanical grinding and transfer of energy. It should be noted that nanoparticles created using these methods are polydisperse, and extra surfactants and solvents are frequently necessary to prevent agglomeration and excessive temperature rises due to the high energy input. Another limitation of the process is the longer milling durations necessary for the manufacture of tiny particles (10 nm-100 nm), which results in contaminants that are difficult to separate from the product.
High pressure homogenization: High pressure homogenization is an energy intensive operation and producing smaller and narrower particle size distributions necessitates multiple homogenization cycles. However, because there are currently commercially available high pressure homogenizers appropriate for large batch sizes, this process does not require the use of an organic solvent and scale-up is generally acknowledged as practical [6].
Ultrasonication: During the production process, this approach uses less shear stress. However, the created nanoparticles have a wider particle size distribution, and the equipment poses a risk of metal contamination.
Bottom-up approach: It refers to the assembling of nanostructures at the atomic level by means of various physical and chemical methods. Chemical synthesis is a way of creating rough materials that can be utilized as building blocks for more sophisticated ordered materials or directly in products in their bulk disordered form. Self-assembly is a bottom-up strategy in which chemical physical interactions between atoms or molecules cause them to organize themselves into organized nanostructures. Positional assembly is the sole method that allows single atoms, molecules, or clusters to be individually positioned [7].
Nanoprecipitation: The spontaneous production of nanoparticles when a water miscible organic solvent is mixed with an aqueous phase is the basis of nanoprecipitation. When the organic solvent containing the chemical diffuses into the aqueous media in which it is insoluble, nanoparticles form naturally. At the laboratory scale, the nanoprecipitation process is highly efficient, requires no energy input, sonication or extremely high temperatures, and the most widely used organic solvents are non-toxic. During scale-up, however, control over particle size and polydispersity index loses dramatically. Furthermore, the purification stage, which removes the organic solvent as well as the free active ingredient, necessitates lengthy downstream processing durations. In order to achieve smaller particles with a narrow size distribution, high pressure homogenization and wet milling procedures have been combined with nanoprecipitation steps, according to reports [8].
Salting out: This approach is particularly well suited to the formulation of polymeric nanoparticles. An aqueous phase having high concentrations of salts or electrolytes is combined with a polymer dissolved in a water miscible organic solvent. The salting out method is efficient and simple to scale up. However, the substantial washing required for purification necessitates lengthy downstream processing.
Super critical fluid technology: One of the most widely used ways for nanopharmaceutical production is Supercritical Fluid (SCF) technology. The utilization of mild temperature conditions and the absence of the need for an organic solvent provide benefits over other processes, and huge scales can be obtained using commercially available setups. The disadvantage is that carbon dioxide is a poor solvent and cost reliability [9].
Solvent evaporation: For the creation of polymeric nanoparticles, the solvent evaporation process is extensively used, in which the polymer and drug are dissolved in a common volatile organic solvent. This solvent system is emulsified in a non-solvent aqueous system, which commonly contains surfactants. The organic solvent is evaporated using high temperature, vacuum or stirring after emulsification. Small, uniformly distributed particles are produced by the solvent evaporation process. Due to the low boiling temperatures of the solvents utilized in the manufacturing process, relatively minor thermal stress is imparted to the nanoparticles. The solvents employed in the manufacturing process necessitate explosion proof rooms and equipment, which is a major disadvantage of this technology.
Nanodiagnostics
In clinical diagnostics nanotechnology serves as a tool for earlier detection of a disease. It is so accurate that it can detect even a minor alteration in a cell leading to malignancy. The following are materials used in diagnostic approaches.
Nanoscale cantilevers: Uses elastic beams to attach cancer linked molecules.
Cantilever array sensors: These are used for mass detection and are ultrasensitive.
Nanopores: Improves DNA sequencing by permitting passage of strand of DNA via small holes present within it.
Nanotubes: Used to locate and detect the affected gene. These are rods primarily made of carbon.
Quantum dots: They bind to proteins in malignant cells to localize the tumor. They have the property to glow brightly in UV light.
Multiplexing modality: Ability to sense variety of large biomolecules at the same time.
The major handout of nanotechnology in the field of dentistry is its role in the diagnosis of oral squamous cell carcinoma. The potential to image these lesions plays a pivotal role in overall cancer management. Structural imaging techniques include Computer Tomography (CT), Molecular Resonance Imaging (MRI) and ultrasound, which are all common clinical imaging modalities. As a result, using endogenous contrast, they can help with the detection of anatomical patterns as well as the providing of basic information about tumour location, size and spread. These imaging modalities, however, become less reliable in distinguishing between benign and malignant tumours when tumours and metastases are smaller than 5 mm. Gold Nanoparticles serve as a contrast agent (GNPs) [10].
GNPs can be synthesized by 2 approaches: The citrate reduction of aqueous HAuCl4 by the Turkevich method and the Brust-Schiffrin two phase synthesis. These methods use NaBH4 as a reducing agent and a mercapto containing binding agent. To increase the potency of the nanoparticles they are modified either by direct thiolmodification of the targeting ligand or through the attachment of a targeting ligand to GNPs that have been modified with a coating material such as polymer, lipid and DNA.
Nanoparticles as biochips and salivary biomarkers
With the evolution of nanotechnology there comes the emergence of biochips. Biochips are micro devices on which consists of the mini version of test sites called microarrays are arranged. Early research in this area related to dental diagnosis began in 2002 when the national institute of dental and facial sciences in the United States began collaborative research activities in the field of saliva diagnosis. The national institute of dental and craniofacial research develops microfluidic and Microelectromechanical Systems (MEMS) for saliva diagnosis with the aim of identifying technically viable systems and supporting progress towards commercialization [11]. This work has led to the development of saliva diagnostic techniques using MEMS and nanoelectromechanical system biosensors. These devices show excellent sensitivity and specificity for detecting analytes down to the single molecule level. Another project in the field of biochips is based on the work of Weigum, et al. Researches are being carried out to develop cytology on chip technology which detects malignant and premalignant cells with intense accuracy. They aimed at designing a sensor for OSCC diagnostics. On an integrated microfluidic platform initially a pressure controlled flow drives the oral cytology suspension to the sensor and the cells that are comparatively larger than the membrane pore are retained on the surface. The captured cells are stained with a fluorescent dye along with an immune reagent to distinguish the nucleus and the cytoplasm. Also the uses of cancer biomarkers such as epidermal growth factor were used to characterize OSCC and their aggressive phenotypes. Finally, the stained cells are subjected to a 3D fluorescence microscopy scan of the membrane surface. This is followed by automatic image analysis using open source software. The advantage of this approach is that the diagnosis can be done quickly.
Nano based molecular imaging
Magnetic resonance imaging: With the advent of nanotechnology, various types of nanoparticles have been applied as specific MRI contrast agents for cancer screening. These nano-contrast agents have the capacity to exhibit better MRI contrast by recognizing cell surface markers. The most commonly studied nanoparticles are Superparamagnetic Iron Oxide (SPIO) and Ultra-Small Superparamagnetic Iron Oxide (USPIOs) nanoparticles [12].
Optical coherence tomography: Optical Coherence Tomography (OCT) produces cross sectional images of subsurface tissues by direct simulation of ultrasound. This is suitable for early oral cancer detection and to look for dysplastic changes. Gold nanoparticles are promising OCT contrast agents. They are biocompatible, easy to synthesize and can provide resonances that helps to avoid predominant tissues absorption.
Nanotechnology in biomarker detection: Scrutinising tumor markers such as Tumor Necrosis Factor-alpha (TNF-α), Vascular Endothelial Growth Factor (VEGF), EGF and Interleukin-6 (IL-6) holds a promising future for early cancer detection and diagnosis. Single biomarker detection method has also been incorporated for detecting oral cancer. As revealed by a conducted study, gold protein chip method detects TNF-α via Total Internal Reflection Fluorescence Microscopy (TIRFM).
Cancer therapy: Cancer therapy involves destroying tumour cells with atomic oxygen, which is generated by a laser. Molecular oxygen is very cytotoxic and it destroys tumour cells effectively. Dye used to produce atomic oxygen is occupied by cancer cells, and it only destroys the tumour cells that are directly exposed to the laser radiation. Normal cells are not affected. To avoid adverse effects on normal cells, porous nanoparticles are used to encapsulate hydrophobic dye molecules, preventing them from diffusing to other parts of the body [13].
Stem cells and nano-engineering: Nanotechnology offers a huge background for regenerative medicine. Nanomaterials and nanofibers are used for the controlled delivery of DNA molecules at target area and to make up the tissue for modification. Stem cells are revolutionising the era of regenerative medicine and their role in cancer therapy is immense. With the help of existing nanotechnological techniques haematopoietic stem cells, embryonic stem cells and mesenchymal stem cells can be bio-synthesized. Studies on surface of rat mesenchymal stem cells with nanotubes made of Titanium dioxide prove to be responsible for stem cell induction, differentiation and its migration. Biosynthesis of stem cells in combination with imaging techniques is found to be effective in the treatment of several abnormalities and disorders. Nanomaterials are put into play for labelling of stem cells, gene delivery, cellular differentiation and also in organ transplantation (Table 1).
| Technologies | Materials | Forms |
|---|---|---|
| Biologic | Peptides, lipids, vesicles, nanotube, rings |
Nucleic acids, nanoparticles |
| Polymeric | Poly (lactic acid), poly (glycolic acid), poly (alkylcyanoacrylate), poly (3-hydroxybutanoic acid), poly (organophosphazene), poly (ethylene glycol), poly (caprolactone), poly (ethylene oxide), poly (amidoamine), poly (l- glutamic acid), poly (ethyleneimine), poly(propylene imine | Vesicles, spheres, nanoparticles, micelles, dendrimers |
| Silicon based | Silicon, silicon dioxide | Porous, nanoparticles, nanoneedles |
| Carbon based metallic | Carbon, gold, silver, palladium, platinum | Nanotubes, fullerness nanoparticles, nanoshells |
TABLE 1. Drug delivery technologies.
Micelles: Micelles are amphipathic surface active molecules made of lipids and amphiphilic molecules. To improve the availability of drug inside the tissue the solubility of hydrophobic drugs is enhanced by its own property. Its diameter range from 10 nm-100 nm. These are used as drug delivery agents, imaging agents, contrast agents and therapeutic agents.
Polymeric micelles have a wide range of potential applications in biomedicine. Polymeric micelles, for example, have been proposed for intra parenteral drug delivery applications, particularly for poor water solubility medicines or as carriers for protein/peptide therapies. Micelles have a number of advantages as drug carriers: They physically entrap sparingly soluble medications and carry them to the intended site of action at concentrations that can surpass their inherent water solubility, hence increasing bioavailability. The drug’s stability is also improved by including micelles.
Liposomes: These are spherical structures measuring 30 nm to several microns. They are lipid bilayered. They are used to introduce hydrophilic therapeutic agents within the aqueous phase. Being versatile they can be altered with polymers, antibodies and/or proteins, facilitating integration of macromolecular drugs, including nucleic acids and crystalline metals, into the liposomes.
Dendrimers: These are macromolecules with branching repetitive units consisting of functional groups in the exterior. The functional groups can be positively or negatively charged or neutral. These can also use to transform the structure and properties of a material. Thus they serve as promising systems for biomedical uses, imaging and drug delivery. Dendrimers transporting various materials and their branches can do multiple tasks at once, including detecting diseased cells, diagnosing diseased states (including cell death), delivering drugs, characterizing location, and reporting therapy occurrences.
Dendrimers functional groups have been linked to a variety of biologically active compounds, including medicines, antibodies, sugar moieties and lipids. In dendrimer-drug conjugates (prodrugs), the drug is attached to the dendrimer by a covalent connection, either directly or via a linker/spacer. A chemical or enzymatic cleavage of a hydrolytically labile link releases drug from a prodrug. Drug conjugation to Polyamido Amines (PAMAM) dendrimers as drug delivery systems has been studied in several studies. Conjugation of drugs to Polyamido Amines (PAMAM) dendrimers as drug delivery systems.
Nanotubes: Surface functionalized Carbon Nanotubes (CNTs) can be absorbed by mammalian cells and used as a vaccine delivery system when bound to peptides. PHSNPs (Porous Hollow Silica Nanoparticles) are made in a suspension with sacrificial nanoscale templates like calcium carbonate. Porous silicon embedded with platinum acts as an anticancer agent and is one of the therapies being researched for use using silicon based delivery methods.
Carbon nanotubes consist of a rolled up sheet form of single layer of carbon atoms but appears cylindrical. They are composed of several concentrically interlinked nano tubes. Due to their increased surface area, they possess high loading capacities and thus used as drug carriers. These are also used as imaging contrast agents and biological sensors due to their unique optical, mechanical and electronic properties.
In 2004, a new class of carbon nanomaterials measuring about 10 nm or less was discovered and was named as Carbon dots (C-dots). C-dots exhibit properties such as low toxicity and other properties rendering it towards bioimaging, biosensor and drug delivery.
Metallic nanoparticles: Iron oxide and gold nanoparticles are examples of metallic nanoparticles. A magnetic core and hydrophilic polymers, such as dextran or PEG, make up iron oxide nanoparticles. Gold nanoparticles, on the other hand, are made up of a gold atom core surrounded by negative reactive groups on the surface that can be functionalized by adding a monolayer of surface moieties as ligands. Metallic nanoparticles have been utilized as contrast agents in imaging, laser based treatment, optical biosensors and drug delivery vehicles.
Quantum dots: Quantum Dots (QDs) are fluorescent semiconductor nanocrystals with a diameter of 1 nm to 100 nm that have showed promise in a variety of biomedical applications, including drug administration and cellular imaging.
Nanocapsules: They have a polymeric wall with a liquid inner core in which the drug is encased, whereas nanospheres have a solid polymeric matrix in which the medication can be spread.
Hydrogels: A hydrogel is a mixture of hydrophilic polymeric chains and water that can expand and release pharmaceuticals through the spaces in their mesh for dissolution and disintegration. Because their polymeric chains can interact tightly with saliva glycoproteins, generating a mucoadhesion phenomena, hydrogels are appealing for oral delivery.
Liquid Crystals (LC): Liquid Crystals systems alter the medication release profile and reduce drug toxicity, resulting in increased clinical efficiency. LCs could be a potential cancer therapeutic method, including for oral cancer.
Lipid nanoparticles and solid polymeric nanoparticles: These are nanoscale solid structures made of natural or synthetic polymers, lipids with high melting points, or proteins. Drugs might be integrated into the matrix or affixed to the surface of the matrix.
Self-Emulsifying Drug Delivery Systems and nanoemulsions (SEDDS): Nanoemulsions are made up of two immiscible liquids, one of which is disseminated as droplets in the other. The oil and surfactants in an O/W emulsion are disseminated in an aqueous phase. SEDDS are made up of drugs that are dissolved in oils and stabilized by surfactants, which produce o/w micro or nano-emulsions in situ when exposed to water.
Microspheres: Microspheres are spherical particles with a diameter of a few micrometres that are comprised of polymers, lipids, or proteins.
Organic nanocarriers: Organic nanocarriers are said to be adaptable in terms of shape and the integration of diverse hydrophilic/hydrophobic medicines. They are biocompatible and have a higher medication loading capacity. Because of the great affinity for water hydrogels are frequently used in medication delivery applications. These hydrogel systems can be either neutral or ionic and they respond to physical or chemical stimuli. They can be made from either natural or synthetic polymers.
Inorganic nanocarriers: Used for drug conjugation of biomacromolecules.
The inorganic nanocarriers comprises of 2 components
• Core of inorganic components such as gold, quantum dots, silica or iron oxide.
• Shell of organic polymers such as metals.
Microdevices in drug delivery
Oral medication delivery formulations, such as solutions, emulsions, capsules and tablets, have been created for decades. Polymers have been used in conjunction with nanotechnology for a long time, resulting in the development of carriers for controlled medication release and targeting. This method has paved the path for several new drug delivery innovations, such as the utilization of liposomes and improved polymeric nanoparticles. pH responsive carriers have also been extensively used, albeit primarily for research purposes. The medicine is protected in the stomach before being released in a regulated manner in the intestine, where it is then absorbed. Although these formulation procedures for creating particles for oral medication delivery are often effective, they also result in polydispersity. Furthermore, they are not always enough for drug protection in the GI system and do not always give adequate oral bioavailability. There are still many unresolved concerns with the oral administration of various medications. Furthermore, obtaining satisfactory oral bioavailability using the aforementioned formulation techniques for peptides and proteins is frequently problematic. As a result, improved drug delivery systems that use microfabrication techniques and 3D printing, for example, have got a lot of attention.
Discussion
Design of the device
The devices used for drug delivery can be divided into three main classes:
• Micropatches
• Microwells
• Microcontainers
They are all based on the same idea of creating a structure with a medication embedded or encased within it. Microdevice manufacturing is dependent on the microfabrication techniques available and 3D printing is becoming a part of the techniques that can be utilized to make such devices.
Micropatches: Transdermal patches are inspired by Micropatches. They are flat, with a modest drug reservoir in micrometres and a low aspect ratio. Flat, thin and disc shaped devices have been shown to have the most contact surface with the GI tract. In the continuous flow of the gut, micropatches can have a lengthy contact time with the intestinal membrane, allowing the medicine to be delivered over a longer period of time than standard drug formulations.
Microwells and microcontainers: The microwells and microcontainers are comparable in design and size (100 m-300 m) to the patches, but they have significantly higher aspect ratios. The distinction between the wells and the containers is that the wells adhere to the surface layer, while the microcontainers are constructed on top of it. The microwells have been used as a proof of concept design for testing novel fabrication procedures in new materials, and they can also be used to evaluate new drug loading methods. Microcontainers are being used in a lot of promising in vivo studies. It is suitable to store the medicine within the device until it reaches the intestine, because of their mucoadhesive nature and increased retention time. Because of their design, the devices allow unidirectional drug release, preventing luminal drug loss.
Drug delivery alternatives: Drugs can be delivered in a variety of ways, including transdermal, transmucosal, ophthalmic, pulmonary and implantation, in addition to the regularly used oral and injectable methods. Biologics, polymers, silicon based materials, carbon based materials and metals are commonly used in the methods used to provide alternative medication delivery. These materials have microscale and more recently, nanoscale structures.
Barriers to oral drug delivery: The harsh acidic pH of the stomach (pH 1-2) and enzymes such as pepsin, which breakdown and destroy some active pharmacological components, are the first biological obstacles that medications face after administering orally. As a result, it is critical to safeguard children against these adverse circumstances. Various pH sensitive hydrogels have been recorded in the literature to deal with the acidic and enzymatic environment (for example, pH sensitive hydrogel for insulin administration). The pH then varies in the duodenum and small intestine and by controlling the pKa of the delivery system; unintended release of the active component can be avoided. Poly (Lactic-co-Glycolic) Acid (PLGA) nanoparticles, Poly Acrylamide grafted Guar Gum (PAAm-g-GG) co-polymer particles and Carboxymethyl Chitosan (CMC) have all been shown to prevent undesired drug release following oral administration.
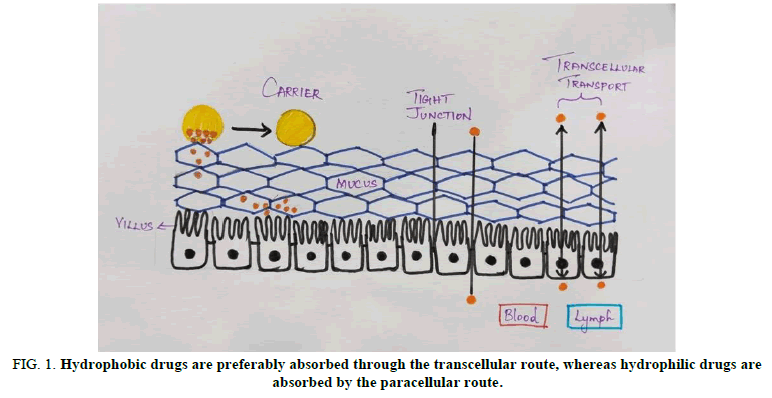
The presence of tight connections between epithelial cells is the next extracellular biological barrier that oral delivery methods must overcome. The size, shape and chemistry of the delivery systems have a significant impact on their ability to pass this barrier. Hydrophobic medications are absorbed primarily through the transcellular route, whereas hydrophilic pharmaceuticals are taken mostly through the paracellular route. Receptor mediated endocytosis is another mechanism implicated in intestinal drug transport. Intestinal membrane lectin, for example, could bind to a variety of drug loaded polymeric matrices, resulting in delayed drug diffusion. Furthermore, a large number of studies have found a link between size dependent receptor mediated endocytosis and size dependent receptor mediated phagocytosis. Particles larger than 1 m are said to be internalised by phagocytosis. Particles larger than 1 m are said to be internalised by phagocytosis. Pinocytosis, on the other hand, is linked to fluid internalisation, in which tiny particles are ingested by receptor mediated processes.
The mucus layer, which prevents the cargo from interacting with the epithelial layer, is the next barrier. Goblet cells secrete mucus, which is predominantly made up of mucins (e.g., MUC1, MUC2, MUC13, MUC16). The mucus layer in the stomach is thick under homeostatic conditions and acts as a physical barrier to protect the epithelium from injury, which can trigger innate and adaptive immune responses. Figure 1 represents that hydrophobic drugs are preferably absorbed through the transcellular route, whereas hydrophilic drugs are absorbed by the paracellular route.
Others include disturbance in bioavailability due to the changes in Ph, enzymatic digestion, poor absorption of the macromolecules by the intestine.
In comparison to other techniques to correct the physiological barrier nanotechnology emerges with its uniqueness to set right the challenges in oral route of administration of the drug to the utmost level.
Surface properties of nanoparticles: The water dispersibility, drug entrapment and even their interaction with biological systems are all influenced by the nanoparticles surface chemical characteristics. As previously stated, the surface charge of nanoparticles guides their inter nanoparticle interactions, which plays a significant role in their stability and dispersibility. Furthermore, the surface chemistry of nanoparticles determines their interaction with biological systems, as well as their biocompatibility, biodistribution and clearance. Chemical changes made in the surface characteristics of nanoparticles, such as nature of the particles, molecular weight and polarity has modified these interventions in certain studies.
Potential risks of nanotechnology
Host mechanism: Nanoparticles can enter the body through a variety of ways, including inhalation, ingestion, skin absorption, and injection in surgical procedures. Nanoparticles’ great mobility may allow them to cross the blood brain barrier after they’ve entered the body. Nanoparticles seem to trigger the immune system by over activity of phagocytes. Inflammation and stress reactions may be triggered, weakening the body’s defences against future threats. Because of their vast surface area, they could disrupt physiological and biological processes in the body, such as enzyme regulatory mechanisms, by adsorbing onto the surface of the cells or fluids they come into contact with.
Effect on nature: High energy requirements for nanoparticle manufacturing could affect the environment by dispersing harmful and persistent nanoparticles. Low recycling potential, as well as low rates of recovery. There are no convincing signs of any other environmental consequences.
Marketing: Nanotechnologies could lead to a corporate takeover of these cutting edge technologies. By claiming patents on nanoscale inventions, large businesses are holding the market. More than 3,500 nano related patents have been granted to date, and the number is growing every year.
Conclusion
Nanotechnology is creating a breakthrough in the discipline of drug delivery. The upcoming phase of nano drug delivery systems is purely based on the large scale production of nano or micro materials. This becomes possible when the works of nano engineering and drug delivery science goes hand in hand with quite some knowledge of prevailing hazards which are to be considered. Thus nanotechnology opens up a pathway for the drug delivery systems in the near future.
References
- Poonia M, Ramalingam K, Goyal S, et al. Nanotechnology in oral cancer: A comprehensive review. J Oral Maxillofac Pathol. 2017;21(3):407-414.
[Crossref] [Google Scholar] [PubMed]
- Calixto G, Bernegossi J, Fonseca-Santos B, et al. Nanotechnology based drug delivery systems for treatment of oral cancer: A review. Int J Nanomed. 2014;9:3719-3735.
[Crossref] [Google Scholar] [PubMed]
- Masareddy RS, Sutar RS, Yellanki SK. Nano drug delivery systems-A review. Int J Pharm Sci Res. 2011;2(2):203.
- Sim S, Wong NK. Nanotechnology and its use in imaging and drug delivery. Biomed Rep. 2021;14(5):1-9.
[Crossref] [Google Scholar] [PubMed]
- Park K. Nanotechnology: What it can do for drug delivery. J Control Release. 2007;120(1-2):1-3.
[Crossref] [Google Scholar] [PubMed]
- Abou Neel EA, Bozec L, Perez RA, et al. Nanotechnology in dentistry: Prevention, diagnosis and therapy. Int J Nanomed. 2015;10:6371-6394.
[Crossref] [Google Scholar] [PubMed]
- Chen XJ, Zhang XQ, Liu Q, et al. Nanotechnology: A promising method for oral cancer detection and diagnosis. J Nanobiotec. 2018;16(1):52.
[Crossref] [Google Scholar] [PubMed]
- Bayda S, Adeel M, Tuccinardi T, et al. The history of nanoscience and nanotechnology: From chemical physical applications to nanomedicine. Molecules. 2019;25(1):112.
[Crossref] [Google Scholar] [PubMed]
- Zaib S, Iqbal J. Nanotechnology: Applications, techniques, approaches and the advancement in toxicology and environmental impact of engineered nanomaterials. Imp Appl Nanotechnol. 2019;8.
- Saini R, Saini S, Sharma S. Nanotechnology: The future medicine. J Cutan Aesthet Surg. 2010;3(1):32-33.
[Crossref] [Google Scholar] [PubMed]
- Rewatkar P, Kumeria T, Popat A. Size, shape and surface charge considerations of orally delivered nanomedicines. Edited by Joao Pedro Martins and Helder A. Santos. London, United Kingdom: Elsevier. 2020;143-176.
- Mazzoni C, Nielsen LH. Microdevices to successfully deliver orally administered drugs. Nanotechnol Oral Drug Deliv. 2020;285-315.
- Türeli NG, Tureli AE. Industrial perspectives and future of oral drug delivery. Nanotechnol Oral Drug Deliv. 2020;483-502.
[Crossref]