Original Article
, Volume: 13( 2)Morphological and Anatomical Study of Cypsela in Two Species of Tribe Mutiseae, Family Compositae
- *Correspondence:
- Paul S, Department of Life Sciences, Central University of Tamil Nadu, Tamil Nadu, India, Tel: +919836595839; E-mail: souravpaul.136@gmail.com
Received: July 25, 2017; Accepted: September 28, 2017; Published: October 03, 2017
Citation: Paul S. Morphological and Anatomical Study of Cypsela in Two Species of Tribe mutiseae, Family Compositae. Nat Prod Ind J. 2017;13(2):111.
Abstract
Detailed studies on the mature cypselas of 2 species belonging to 2 genera (Gerbera jamessonii and Crepis lyrata) in the tribe Mutisieae have revealed the morphological and anatomical differences. At the species level although some similarities existing the basic structure. Morphological features of the apical part, surface hairs, location of vascular trace, structure of carpopodium and pappus bristles. Of cypselas are taxonomically significant. Anatomically, testal features are more important than the pericarp in the tribe Mutisieae and have potential value for characterization of taxa. On the basis of thickening of the cell walls in testal epidermis, the studied species can be grouped into 3 categories. On the other hand, the structure of endosperm cannot be treated as significant taxonomic parameter. An artificial key is provided on morphological features of cypselas for the identification of species.
Keywords
Mutisieae; Asteraceae; Morpho-anatomical features.
Introduction
Floristically, the tribe Mutisieae usually has unique bi-labiate flowers and lack of stylopodium in cypsela. Karisetal have also shown that the tribe Mutiseae (s.l.) are devoid of sweeping hairs on the styles which exist in rest of the tribes of Asteraceae. Current classification of the tribe Mutisieae begins with the system of Bentham [1]. Cabrera has divided the tribe into 4 subtribes, i.e., Barnadesiinae, Gochnatiinae, Mutisiinae and Nassauviinae. Very recently the subtribe Barnadesiinae has been recognized as a distinct subfamily Bamadesioideae, on the basis of absence of the chloroplast DNA inversion discovered by Jansen and Palmer". Moreover, Bremer' has recognized the tribe Mutisieae (s.s) as a provisional unclassified group which constitutes the phylogenetically basal complex of the family Asteraceae and known to be a Paraphyletic group [2].
Thus, the tribe Mutisieae draws more attention to the taxonomists for better understanding of its phytogeny. Cypselar anatomical features have been successfully employed in the classification of taxa in the Asteraceae, since the work of Lavialle, particularly in the tribe Mutisieae, whereas gross morphological features of cypselas have been incorporated in different system of classifications [3,4]. In recent years, cypselar characters have been successfully used for the identification of plants in different genera and tribes of the family Asteraceae [5]. Many authors have been – attracted and fascinated by different aspects of this tribe along with cypselar morphological features. Grau" has added detailed investigation on the anatomy of fruits [6]. The present paper deals with both morphological and anatomical features of cypselas of 2 species belong to 2 genera of the tribe Mutisieae. The aim of the present study is twofold: to describe the cypselar morpho-anatomical features in detail; and to prepare an artificial key based on these observations.
Materials and Methods
Some fully mature cypselas of each species were selected from the mass of each sample. These were boiled for few minutes with water by adding few drops of glycerol. Then all specimens were preserved in FAA solution for study. After that, 3 cypselas were immersed within the 5% NaOH solution for few days, depending upon the amount of mechanical tissue of cypselas [7]. Different parts of cypselas were stained in 0.5% aqueous safranin solution and different parts of cypselas were studied with the help of light compound microscope. Cross-section from each cypsela was taken from the middle part [8].
Specimens
1. Gerbera jamesonii Adlam SP- 106
2. Crepis lyrata Benth. and Hook.f. KAL- 1209
Observations
Cypselar morphology
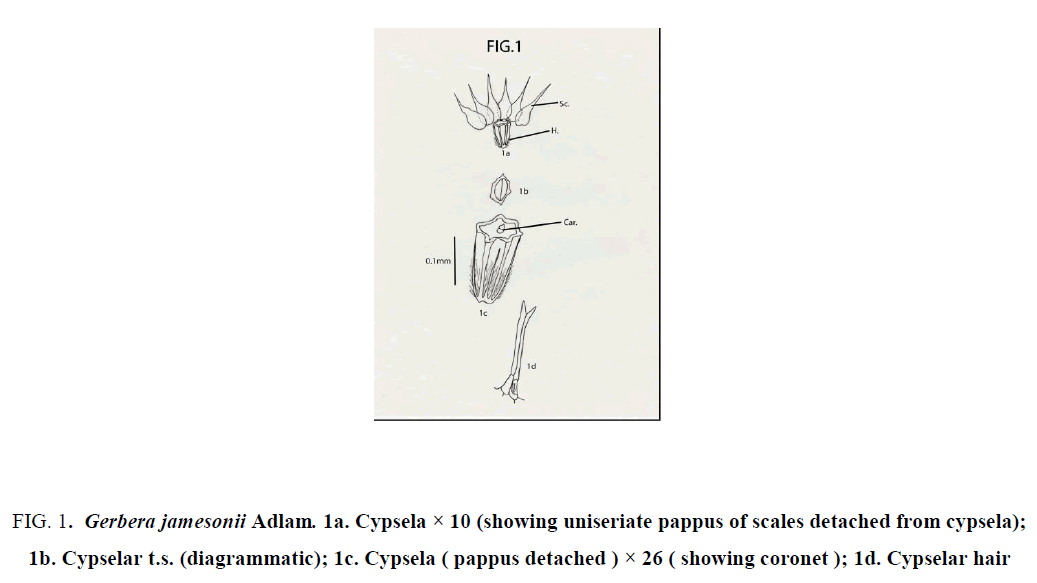
Gerbera jamesonii Adlam: Cypselas homomorphic; not beaked, but with coronet; 2.5 mm × 1.5 mm; round or almost oval in transaction; ash colored or yellowish brown; pubescent; hairs profuse, long, white, glossy, stiff, “ twin “ type with unequal arms; hairs restricted only at the lower half of cypsela; cypsela with 6 continuous longitudinal ridges; obconical with truncate apex and without carpopodium; pappus of 6 fabricately arranged, white, scarious scales; each scale 4-5 mm × 1mm, ovate with narrow long, apical projections; upper half of scale with dissected margin [9-12] (Figure 1).
Figure 1: Gerbera jamesonii Adlam. 1a. Cypsela × 10 (showing uniseriate pappus of scales detached from cypsela); 1b. Cypselar t.s. (diagrammatic); 1c. Cypsela ( pappus detached ) × 26 ( showing coronet ); 1d. Cypselar hair
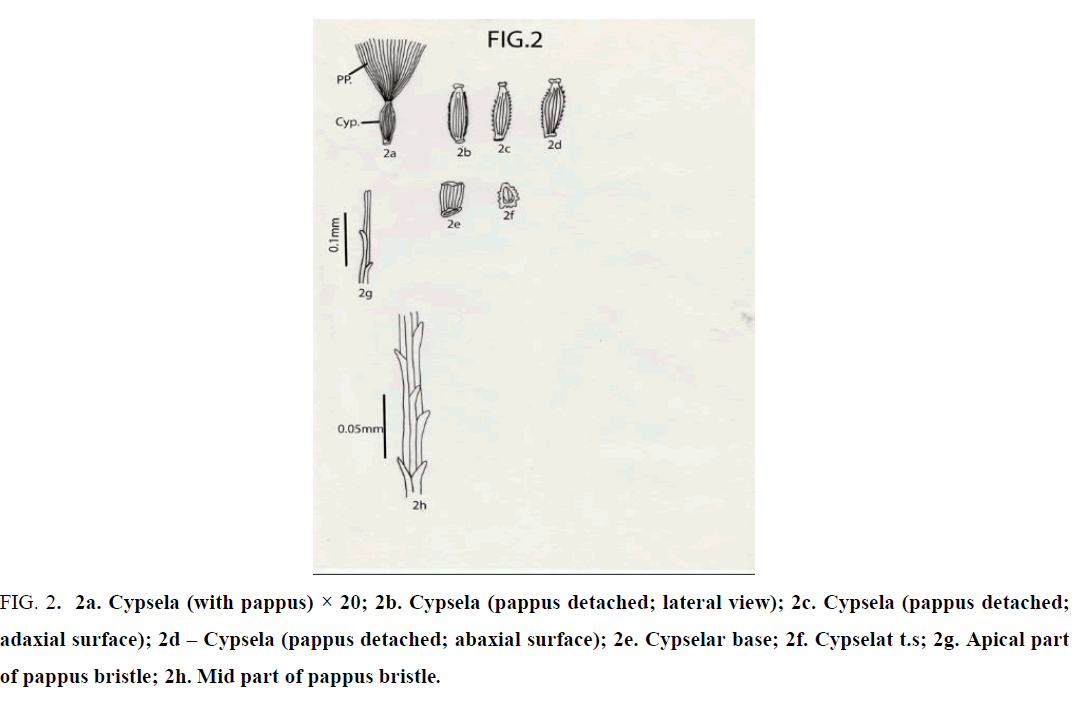
Crepis lyrata Benth. and Hook.f.: Cypselas homomorphic; 2 mm × 0.5 mm; beaked; with an apical cap and 10 narrow ridges; ridges both narrow and broad; cypsela nearly triangular in cross section; reddish brown or coffee colored; dorsiventrally compressed; cylindric; attenuated at both ends; base truncate without any carpopodium and slightly oblique; cypselar beak very short, narrow, with marginally small papillae; cypsela with pappus of many, penicillate, simple, unbranched, white, capillary hairy bristles; bristles almost equal in length ( 3 – 3.5 mm long ); apical cells of pappus bristle 2 in number, equal or unequal with pointed apex; barbs very short, pointed, overlapping and outwardly and upwardly directed [13-16] (Figure 2).
Figure 2: 2a. Cypsela (with pappus) × 20; 2b. Cypsela (pappus detached; lateral view); 2c. Cypsela (pappus detached; adaxial surface); 2d – Cypsela (pappus detached; abaxial surface); 2e. Cypselar base; 2f. Cypselat t.s; 2g. Apical part of pappus bristle; 2h. Mid part of pappus bristle.
Cypselar Anatomy
Crepis lyrata Benth. and Hook. f
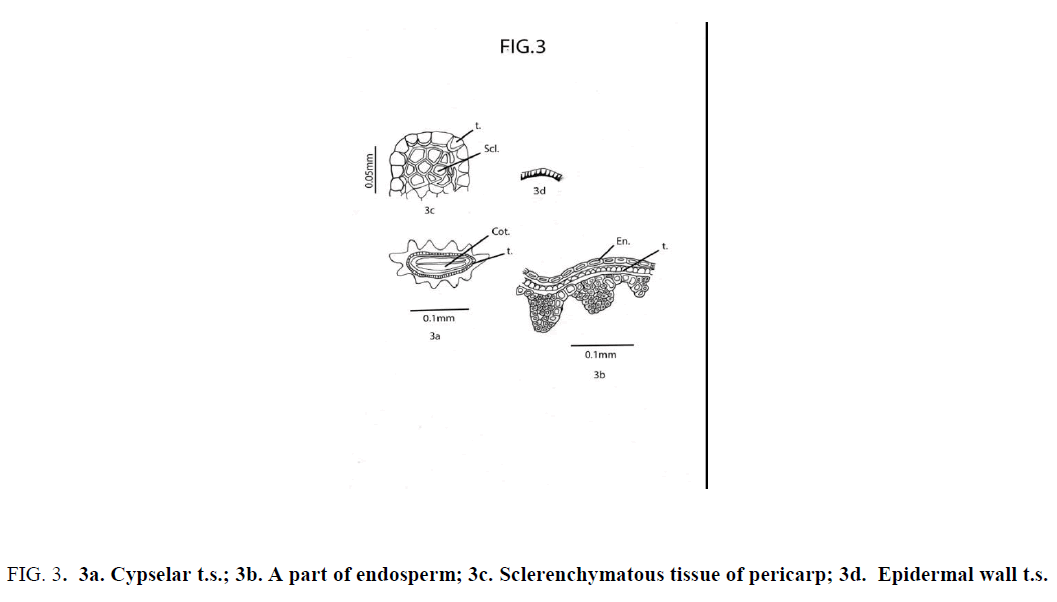
Cypsela dorsiventrally compressed; deeply 11-ribbed; cotyledon thin and plano-convex. Pericarp glabrous; sclerotic, undifferentiated, heterocellular and composed of thick-walled, compactly arranged cells of unequal size and shape; The cells in the furrow region of pericarp are comparatively large; cells at the middle of each rib comparatively small in size [17,18]. Testa uniseriate; composed of compactly and radially arranged rectangular cells having unevenly thickened wall; the walls toward the endosperms and the radial wall broad; testal cells tooth like in appearance [19,20]. At the two lateral sides of cypsela a group of polygonal sclerotic cells ( one below each ridge ) are embedded in parenchyma below the aforemid layer of testa [21]. Endosperm uniseriate (Figure 3).
Figure 3: 3a. Cypselar t.s.; 3b. A part of endosperm; 3c. Sclerenchymatous tissue of pericarp; 3d. Epidermal wall t.s.
Results and Discussion
In the systematic review of Mutisieae by Cabrera in 1977 only a few features of cypselar morphology has been considered in description of the tribe: “cypsela turbinate or obconic, truncate, attenuate or rostrate at the apex, glabrous or hairy with 2- armed hairs, pappus usually of one or more series of bristles, rough or plumose; seldom formed of palae or absent” [22-24].
As subtribal character the pappus has not been favoured much by Cabrera (l.c.). Practically no cypselar and pappus characters have been considered in characterization of subtribes, through Cabrera in 1977 recognized four subtribes within the tribe.
The cypselas of G. jamessonii are quite different morphologically from other species of Gerbera studied. In G. jamessonii the cypselas are devoid of carpopodia [25], but with a prominent coronet; they are obconic, prominently ribbed, 6 continuous longitudinal ribs alternating with incomplete ribs (extended from base of cypsela but never merging at the apical end), ribs are connate at the base. Cypsela bears pappus of five to six unequals to almost equal scales [26].
Cypselas of G. jamessonii are yellowish brown or ash coloured. In Gerbera jamessonii typical long hairs are restricted only at the lower half of the cypselar surface.
Pericarp is usually ribbed with twin hairs, uniseriate, parenchymatous with tangentially elongated cells. Below each ridge compactly arranged sclerotic tissue is present just below the epidermis. Testa is usually uniseriate. Thickening is more on the basal tangential wall and less on radial wall. Non-cellular pellicle is present in both the cases. Endosperm is biseriate, cellular [27].
Conclusion
Based on the above observations, it can be concluded that the members of the tribe Mutiseae are with diverse macro as well as micromorphological features of cypselas. These characters are a mixture of both primitive and advanced features. However, their value as taxonomic criteria will be greatly increased in combination with other lines of evidence.
References
- Rodriguez P, Plana D, Fermin DJ, et al. New insights into the catalytic activity of gold nanoparticles for CO oxidation in electrochemical media. Chinese J Catal. 2014;311:182-9.
- Cortie M, Van der Lingen E. Catalytic gold nano-particles, Mater forum, Citeseer, 2002.
- Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv colloid interfac. 2010;156:01-13.
- Bora U, Sett A, Singh D. Nucleic acid based biosensors for clinical applications. Biosen J. 2013.
- Babu P, Saranya S, Sharma P, et al. Sonocatalytic synthesis of gold nanoparticles using ethnolic extract of Andrographis paniculata and functionalization with gelatin-polycaprolactone composites. Front Mater Sci. 2012;6:236-49.
- Patra S, Mukherjee S, Barui AK, et al. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng. 2015;53:298-309.
- Sheny DS, Philip D, Mathew J. Rapid green synthesis of palladium nanoparticles using the dried leaf of Anacardium occidentale, Spectrochim. Acta A. 2012;91:35-8.
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638-50.
- Mukherjee S, Sushma V, Patra S, et al. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nano Technol. 2012;23:455103.
- Das RK, Gogoi N, Bora U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioproc Biosynthesis. 2011;34:615-9.
- Agnihotri M, Joshi S, Kumar AR, et al. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589, Mat Let. 2009;63:1231-4.
- Gogoi N, Babu PJ, Mahanta C, et al. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C. 2015;46:463-9.
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4:01-6.
- Babu PJ, Das RK, Kumar A, et al. Microwave-mediated synthesis of gold nanoparticles using coconut water. Int J Green. Nanotech. 2011;3:13-21.
- Kirtikar KR, Basu BD. Indian medicinal plants. Indian Medicinal Plants. 1918:72.
- Evans WC, Trease and Evans' pharmacognosy, Elsevier Health Science. 2009.
- Kapoor L. Opium poppy: Botany, chemistry, and pharmacology. CRC Press. 1997.
- Barceloux DG. Medical toxicology of drug abuse: Synthesized chemicals and psychoactive plants. John Wiley Sons. 2012.
- A Century of International Drug Control, United Nations Publications, 2010.
- Robbers JE, Speedie MK, Tyler VE. Pharmacognosy and pharmacobiotechnology. Williams & Wilkins, USA. 1996.
- Chouvy PA. Afghanistan’s opium production in perspective. Chi Eur For Quart. 2006;4:21-4.
- Dewick P. Medicinal natural products. A biosynthetic approach. John Wiley & Sons Inc. New York, USA. 1997.
- Das RK., Sharma P, Nahar P, et al. Synthesis of gold nanoparticles using aqueous extract of Calotropis procera latex. Mat. Let. 2011;65:610-3.
- Vijayakumar R, Devi V, Adavallan K, et al. Green synthesis and characterization of gold nanoparticles using extract of anti-tumor potent Crocus sativus. Physica E Low Dimens Syst Nanostruct. 2011;44:665-71.
- Song JY, Jang HK, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process. Biochem. 2009;44:1133-8.
- Smitha S, Philip S, Gopchandran K. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta A. 2009;74:3.
- Kumar KP, Sharma PW. Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility. Process Biochem. 2007;46:10.