Original Article
, Volume: 14( 4)Microbial Synthesis of Silver Nanoparticles by Pseudomonas Sp.
- *Correspondence:
- Zaynitdinova Lyudmila, Institute of Microbiology, Academy of Sciences, 100128, Tashkent, A.Kodiry, 7b, Uzbekistan, E-Mail: zajn-lyudmila@yandex.ru
Received: July 30, 2018; Accepted: August 08, 2018; Published: August 12, 2018
Citation: Zaynitdinova L, Vokhidova N, Rashidova S, et al. Microbial Synthesis of Silver Nanoparticles by Pseudomonas Sp. Biotechnol Ind J. 2018;14(4):169.
Abstract
The aim of the study was to check the ability of microorganisms of Pseudomonas genus to form silver nanoparticles. Among the studied strains, Pseudomonas stutzeri showed the highest level of activity. Maximal amount of extracellular nanoparticles formation was observed after addition of 100 mg/l of silver ions. It is shown that the shape and size of the formed nanoparticles depends on the initial concentration of Ag+. Addition of the silver ions (in concentration 25-100 mg/l) to the bacterial culture medium leads to the appearance of the nanoparticles with the sizes varying from 20 nm to 300 nm which then form agglomerates, their sizes vary from 150 nm to 500 nm.
Keywords
Microorganisms, Pseudomonas, Silver nanoparticles, Microbial synthesis of nanoparticles.
Introduction
In recent years, the synthesis of metallic nanoparticles is carried out by both, physical and chemical methods. At the same time, the proper use of the biological systems potential, from higher plants to microorganisms, creates powerful capabilities for obtaining specific nanomaterials, which are used in various industries and particularly in biotechnology [1,2]. At present, silver nanoparticles (AgNPs) based materials are widely used in medicine, chemical catalysis, agro-industrial complex and other industries [3,4]. Microbial synthesis of nanoparticles is an environmentally safe method and, therefore, has significant advantages over other methods, as far asvarying of the synthesis conditions make it is possible to regulate the size of NP [5,6]. Microbial synthesis of metal nanoparticles can occur both intracellularly and extracellularly [7,8]. Extracellular synthesis of nanoparticles occurs quite often, and at the same time, it is practically feasible and effective. These qualities contribute to the large-scale production of silver nanoparticles, which increases the possibility of NP derived nanomaterials application. The large number of such studies provide the information about variety of microorganisms participating in these processes [2,9]. Thereby, the ability of the Bacillus sp. to form silver nanoparticles has been shown [10]. Studies, with the use of bacterial culture mediums supernatants, showed that Pseudomonas proteolytica and other bacteria of the genushave the ability to efficiently form extracellular nanoparticles [11]. Application of the representatives of Pseudomonas genus in the formation of nanoparticles of various metals has been shown in numerous studies [12-14]. Long and continuous interactions of microorganisms with various metals provide a high probability of obtaining nanomaterials with increased bioactive properties. Therefore, in areas exposed to anthropological influence, the possibility of presence of microorganisms capable of synthesizing nanoparticles, among bacterial isolates is quite high.
It should be mentioned that the screening of the understudied microorganisms on their ability to produce metallic nanoparticles is a topical task in the modern microbiology. Reducing reactions of silver ions carried out by different microorganisms are the effective methods of nanoparticle production [15].
Materials and Methods
In connection with the above mentioned, the process of biosynthesis of silver nanoparticles by various strains of the Pseudomonas sp. was studied. All studied strains of microorganisms were obtaned from the laboratory collection of industrially important microorganisms at the Institute of Microbiology, Academy of Sciences of Republic of Uzbekistan. All bacteria were isolated from mixed populations of soil microorganisms exposed for a long-term to the factors of chemical production and from soils contaminated with various xenobiotics. The choice of microorganisms (objects) was determined by their resistance to various contaminants, including heavy metals, and also by their ability to absorb silver, because it is believed that the ability to form metal nanoparticles is a protective function of microorganisms [16].
The subject of the research was silver nanoparticles obtaining, for this purpose solution of silver ions AgNO3 in distilled water was prepared. The equivalent of AgNO3 is 169.89, therefore, 1 liter of 0.1N solution of silver nitrate should contain 16.989 g. of the reagent. To prepare the solution, 17 g of the reagent is dissolved in 1 liter of distilled water. The titer of the prepared solution is conducted after 7-10 days by chemically pure sodium chloride. The final concentration of the test solution was 100 mg/l of Ag+.
The experiments were carried out by adding a solution of the silver salt to the culture liquid. Mixtures of silver ions and cells were incubated on a shaker at 150 rpm and 28°C for 3 days. The formation of silver nanoparticles was confirmed visually: solutions discolored to the characteristic yellow and brown colors. Cultivation of microorganisms was carried out on the nutrient broth meat-peptone medium (BMP).
UV spectroscopic studies were carried out using the SPECORD 210 spectrophotometer in the range 190-1000 nm. Accuracy of UV photometry with potassium dichromate was in accordance with Ph. Eur. ± 0.01. The morphology of films of nanostructured systems was studied by AFM Agilent 5500 (USA) atomic force microscope, at room temperature. The silicon cantilevers with a stiffness of 9.5 N/m with a frequency of 145 kHz were used in the work. The maximum scanning area on the AFM in X, Y is 15 × 15 μm2, Z-1 μm.
Result and Discussion
Various representatives of the Pseudomonas sp. were analyzed (TABLE 1). It should be noted that representatives of this genus, along with representatives of the Bacillus sp., under stress conditions retain the structures of the microbiocenosis and, according to the literature, are active producers of silver nanoparticles [13,14,16].
| No. | Microorganisms | Color | Intensity change of the color | |||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 2 days | 3 days | 5 days | 7 days | 14 days | |||
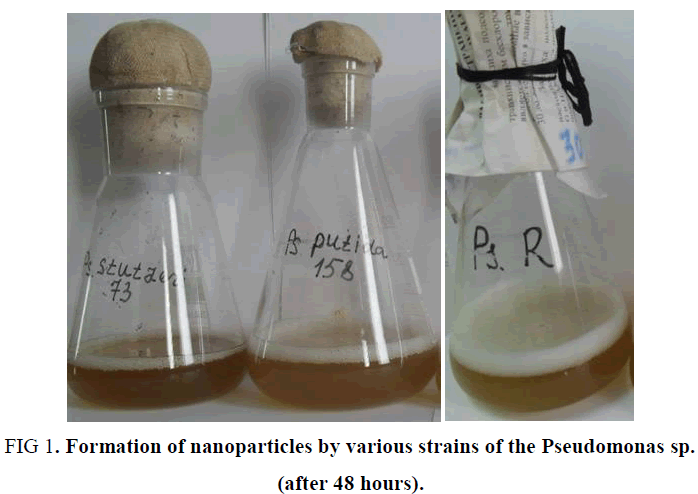
| 1 | Pseudomonas stutzeri | Brown | + | 3+ | 3+- | 3+ | 3+ | 3+ |
| 2 | Pseudomonas putida | Brown | + | 2+ | 2+ | 2+ | + | + |
| 3 | Pseudomonas sp. R | Light yellow | + | + | + | + | + | + |
TABLE 1. Screening of the р. Pseudomonas microorganisms on their ability of nanoparticles synthesis
In the study cultures of Pseudomonas sp., isolated from sierozem soils, as well as from the highly oil-contaminated soils of Mingbulok deposit (Namangan region) were used. As experiments have shown, among the studied strains, Pseudomonas stutzeri showed the highest level of activity. This strain has a high synthesizing ability and, in addition, the nanoparticles synthesized by this strain showed stability up to 2 weeks or more (FIG. 1). The reason for this, possibly, is the formation of smaller nanoparticles, as well as the synthesis of substances that envelop nanoparticles, preventing their aggregation and thus stabilizing them.
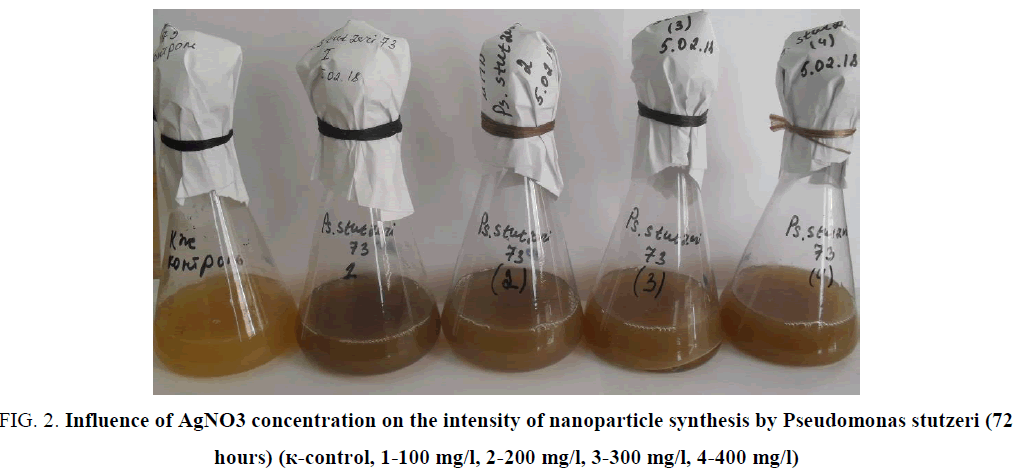
Intensity of nanoparticles synthesisin the experiment was dependent on initial concentration of bacteria and on the concentration of the silver solution. Experiments showed, that application of AgNO3 in concentration of 100 mg/l had maximal effect on synthesizing activity of bacteria (FIG. 2).
FIG. 2. Influence of AgNO3 concentration on the intensity of nanoparticle synthesis by Pseudomonas stutzeri (72 hours) (к-control, 1-100 mg/l, 2-200 mg/l, 3-300 mg/l, 4-400 mg/l)
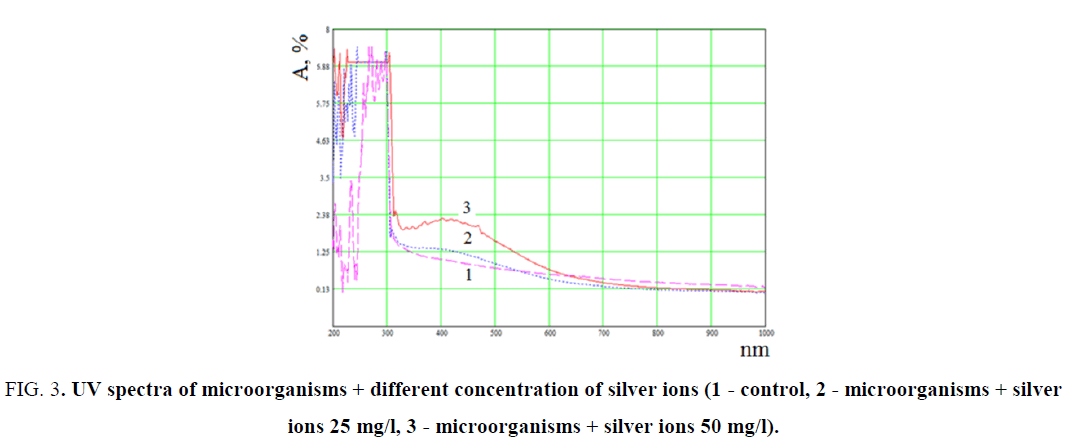
The observed color change was additionally confirmed by spectral analysis by UV-Vis (FIG. 3).
FIG. 3. UV spectra of microorganisms + different concentration of silver ions (1 - control, 2 - microorganisms + silver ions 25 mg/l, 3 - microorganisms + silver ions 50 mg/l).
Experimental data indicate that for the systems of microorganisms + silver ions, absorption is observed in the wavelength range from 200 nm to 600 nm, which is associated with the formation of silver clusters and nanoparticles. It can be seen that in the sample with 25 mg/l of AgNO3, characteristic absorption bands of clusters are observed at 275 nm and nanoparticles at 400 nm. These bands correspond to formed silver NPs, which sizes for clusters are 2-3 nm, and for nanoparticles 5-20 nm (FIG. 3).
The samples with concentration 50 mg/l of silver ionshad the same characteristic absorption bands at 275 nm for clusters and 410 nm for nanoparticles. However, the intensity increased and the absorption wavelength of the nanoparticles shifted to 410 nm, which indicates, that the size of formed NPs has increased [17].
It is known that silver nanoparticles have characteristic absorption bands in the UV region, because of its surface plasmon excitation. For the Pseudomonas stutzeri culture, the absorption band is observed at 450 nm and that is an indicator of the formation of silver nanoparticles (FIG. 3, line 3). Thus, a change in the initial color and of the absorption band in the UV region confirms the reduction of silver ions to nanoparticles in the presence of microorganisms of the Pseudomonas stutzeri species.
The obtained silver NP were stabilized by methylcellulose (MC) and shaped as films by dry forming technique (TABLE 2). In order to prevent the agglomeration of NP, to the solution of the formed polymer there was added small amounts (1-12.7 ÷ 16,0) of silver NP (in liquid form).
| No. | V of the solution, ml, рН=8,68 |
V of the solution, ml MC-0,9% рН=6,8 |
Culture:MC, volume ratio |
|---|---|---|---|
| 1 | 2,2 | 28 | 12,7 |
| 2 | 2,2 | 30 | 13,6 |
| 3 | 2,2 | 32 | 14,5 |
| 4 | 2,2 | 35 | 16,0 |
TABLE 2. Stabilization of silver NP (synthesized by bacteria Pseudomonas stutzeri) by methylcellulose solutions.
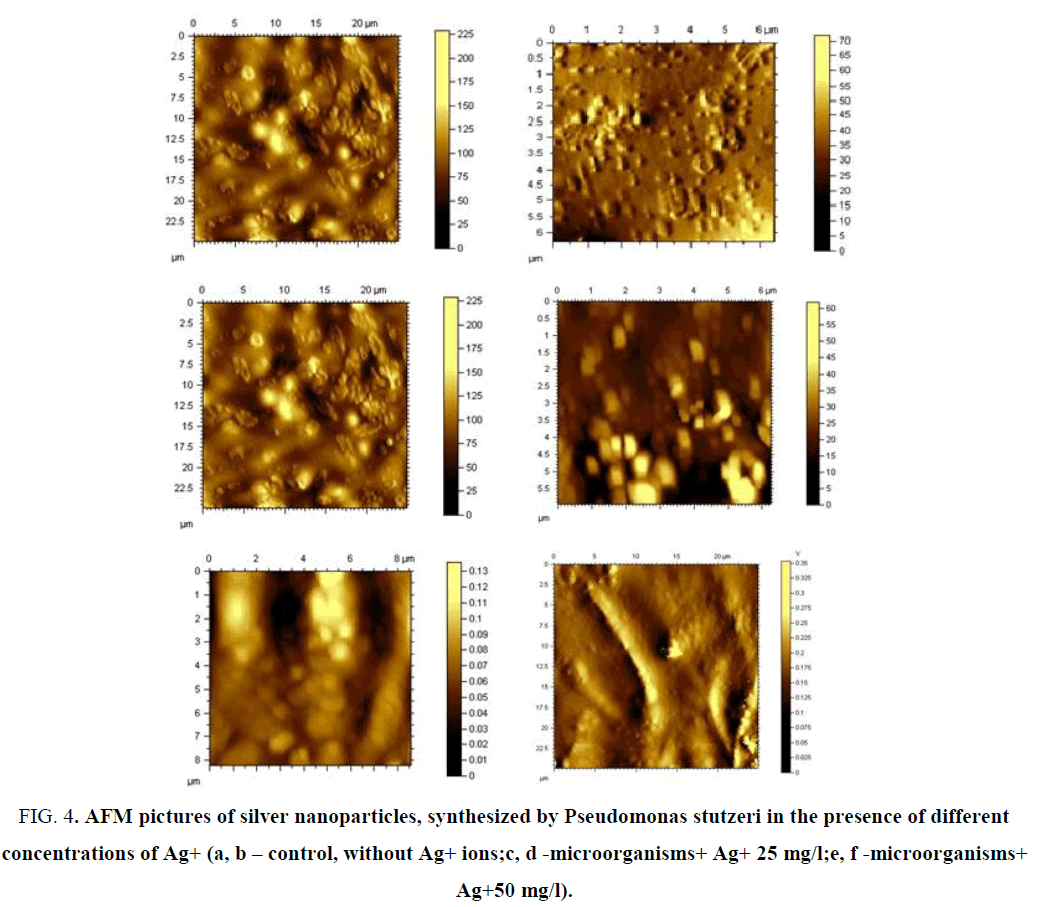
The films were formed at atmospheric pressure and at 22°C. The morphology of the films has been studied by the methods of electron microscopy. Atomic force microscopy (AFM) studies showed that in the absence of silver ions in a nutrient medium, microorganisms form spherical particles, whose growth starts from the center in the radial direction, similar to the spherilitic structure of polymers (FIG. 4).
FIG. 4. AFM pictures of silver nanoparticles, synthesized by Pseudomonas stutzeri in the presence of different concentrations of Ag+ (а, b – control, without Ag+ ions;c, d -microorganisms+ Ag+ 25 mg/l;e, f -microorganisms+ Ag+50 mg/l).
It is shown that the initial concentration of Ag+ ions have a noticeable effect on the shape and size of the formed nanoparticles. Addition of the silver ions (from 25 to 100 mg/l) to the bacterial culture medium, nanoparticles appear as agglomerates, their sizes vary from 150 nm to 500 nm. Increasing the concentration of Ag+ ions leads to the formation of dendritic structure of the system, because of the spherical form of synthesized NP. AFM studies of the sample (Solution 1, Ag+, 200 mg/l) show the presence of the cubic silver NP in the system, with wide range of sizes - from 20 to 300 nm. With an increase of silver ions content, the needle-shaped crystals are formed.
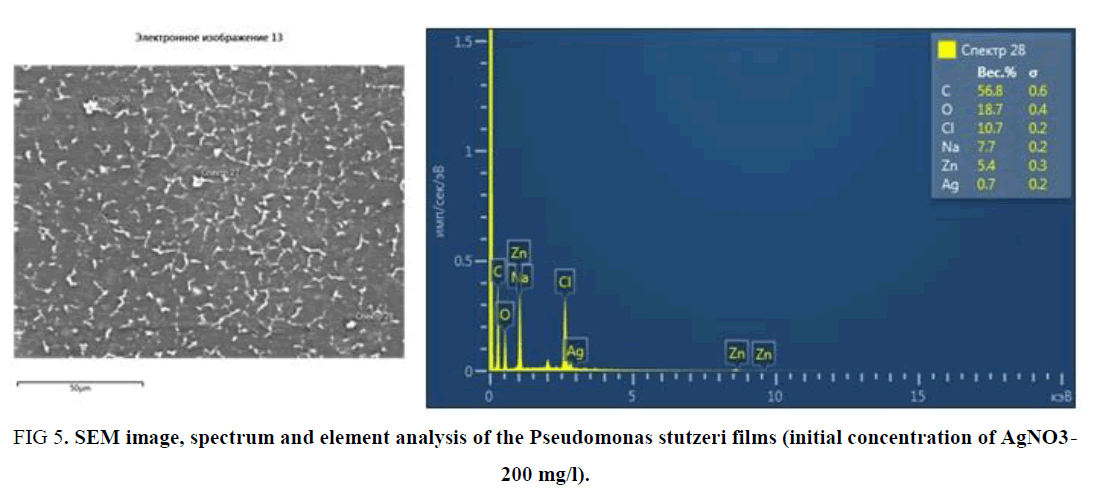
It should be noted that the obtained SEM data of the Pseudomonas stutzeri sample confirm the results of the AFM studies (FIG. 5).
FIG 5. SEM image, spectrum and element analysis of the Pseudomonas stutzeri films (initial concentration of AgNO3- 200 mg/l).
Element analysis and the spectrum of the films show that there are 0.7% silver NP in the sample. From the SEM picture it can be seen that silver NP has the needle-like form (TABLE 3).
| Element | Weight % | Weight sigma % |
|---|---|---|
| C | 56.83 | 0.6 |
| O | 18.66 | 0.45 |
| Na | 7.75 | 0.21 |
| Cl | 10.67 | 0.19 |
| Zn | 5.41 | 0.34 |
| Ag | 0.7 | 0.17 |
| Total: | 100 |
TABLE 3. Element weight percentage.
Conclusion
Thus, we have investigated the possibility of silver NP synthesis in the presence of microorganisms of the Pseudomonas sp. genus with bioactive properties. It was found that under the chosen synthesis conditions, NPs are formed in the range of sizes from 20 to 300 nm which then form agglomerates, and their sizes vary from 150 nm to 500 nm. It was established that variation of the silver ions concentration in the initial system, effects to the size and shape of the produced nanoparticles.
References
- Pantidos N, Horsfall LE. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. Journal of Nanomedicine & Nanotechnology. 2014;5(5):1.
- Durán N, Marcato PD, Alves OL, De Souza GI, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of nanobiotechnology. 2005;3(1):8.
- Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids and Surfaces B: Biointerfaces. 2010;81(1):358-62.
- Christopher P, Xin H, Linic S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nature chemistry. 2011;3(6):467.
- Wei X, Luo M, Li W, Yang L, Liang X, Xu L, Kong P, Liu H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresource technology. 2012;103(1):273-8.
- Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Advances in colloid and interface science. 2010;156(1-2):1-3.
- Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi AA. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochemistry. 2007;42(5):919-23.
- Kalishwaralal K, Deepak V, Pandian SR, Kottaisamy M, BarathManiKanth S, Kartikeyan B, Gurunathan S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids and Surfaces B: Biointerfaces. 2010;77(2):257-62.
- Zaynitdinova L, Vokhidova N, Tashpulatov J, Kukanova S, Ashurov N, Juraeva R. Microorganisms-Producers of Nanoparticles of Silver. Journal of Nanoscience and Nanoengineering. 2017;3(1):1-5.
- Saravanan M, Vemu AK, Barik SK. Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids and Surfaces B: Biointerfaces. 2011;88(1):325-31.
- Saifuddin N, Wong CW, Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. Journal of Chemistry. 2009;6(1):61-70.
- Yadav A, Theivasanthi T, Paul PK, Upadhyay KC. Extracellular biosynthesis of silver nanoparticles from plant growth promoting rhizobacteria Pseudomonas sp. 2015.
- Baker S, Kumar KM, Santosh P, Rakshith D, Satish S. Extracellular synthesis of silver nanoparticles by novel Pseudomonas veronii AS41G inhabiting Annona squamosa L. and their bactericidal activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;136:1434-40.
- Jeevan P, Ramya K, Rena AE. Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas aeruginosa.
- Pomogailo AD, Kestelman VN. Metallopolymer nanocomposites. Springer Science & Business Media;2006.
- Khandel P, Shahi SK. Microbes mediated synthesis of metal nanoparticles: current status and future prospects. Int J Nanomater Biostruct. 2016;6(1):1-24.
- Kreibig U, Vollmer M. Springer Series in Material Science. Optical properties of metal clusters, Springer Verlag, Berlin. 1995.