Review
, Volume: 17( 1)Micelles Supported Organic Reactions in Water: A Review
- *Correspondence:
- Hardesh K Maurya
Department of Chemistry, Hygia Institute of Pharmacy
Uttar Pradesh, India
Tel: 9451407702
E-mail: hardesh11@yahoo.co.in
Received: July 07, 2022, Manuscript No. tsoc-22-68761; Editor assigned: July 11, 2022, PreQC No. tsoc-22-68761 (PQ); Reviewed: July 25, 2022, QC No. tsoc-22-68761; Revised: October 06, 2022, Manuscript No. tsoc-22-68761 (R); Published: October 14, 2022, DOI: 10.37532/0974-7516.22.16.001
Citation: Hardesh K Maurya. Micelles Supported Organic Reactions in Water: A Review. Org Chem Ind J. 2022;16(9):001
Abstract
Water has emerged as a versatile solvent for organic chemistry in recent years due to inexpensive and environmental benign solvents and plays a distinguished role in reactivity. Due to the insolubility of organic compounds in water, surfactants are utilized for solubilization of organic reactants in aqueous micelles. Recently, a variety of reactions have been explored in aqueous micelles. This review has been covering these types of selected reactions.
Keywords
Surfactant; Micelles; Water assisted; Organic synthesis; Aqueous micelles
Introduction
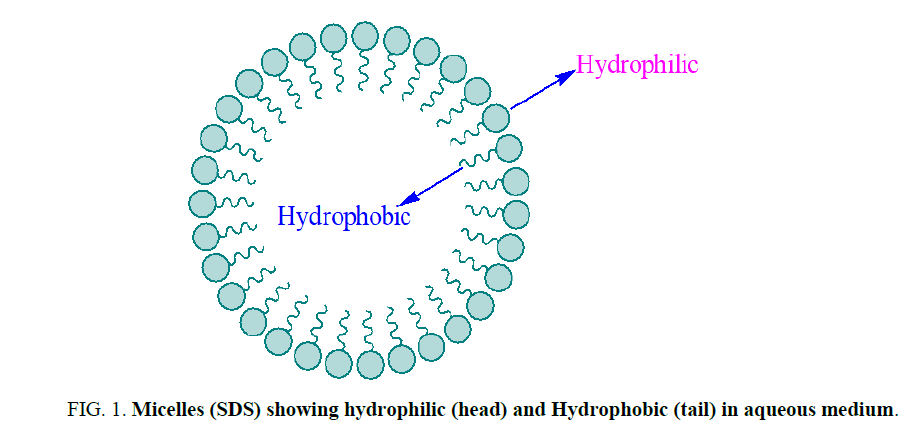
Surfactants are generally organic compounds which are amphiphilic in nature and contain both hydrophobic groups (tails) and hydrophilic groups (heads) (Figure 1) [1]. Due to amphiphilic nature, surfactants were diffuse in water and adsorbed at interfaces between water and air [2]. In which water insoluble hydrophobic group of a micelle may extend out of the bulk water phase into the air, while the water soluble head group remains in the water phase [3]. In organic reactions tail of micelles stick with reagent and help to solubilize in aqueous medium. For different reaction, a variety of surfactant are synthesized for batter efficacy [4,5] (Figure 1).
Literature Review
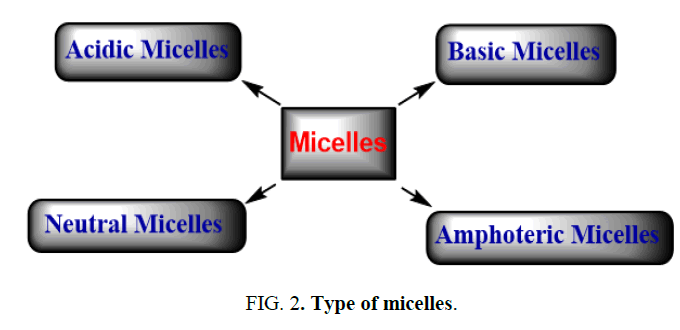
Surfactant classification according to the composition of their head: Surfactants are classified in four groups: a) Acidic micelles, b) Basic micelles, c) Neutral micelles, d) Amphoteric micelles (Figure 2).
Surfactants are classified according to the polar head group. A non-ionic surfactant has no charge [6]. The head of an ionic surfactant carries a net positive or negative charge. If the charge is negative, the surfactant is called anionic; if the charge is positive, it is called cationic [7]. If a surfactant contains a head with two oppositely charged groups, it is called amphoteric.
Role of micelles in organic reactions: Solutions of enormously surface-active materials frequently show infrequent physical properties. In dilute solution, the surfactant acts as a normal solute. With increasing concentration, unexpected changes in numerous physical properties such as turbidity, osmotic pressure, surface tension and electrical conductance take place [8]. The conductance of ionic surfactant solutions remains relatively high, which shows that ionic separation is still in force. This apparently anomalous behavior can be explained in terms of prearranged aggregates or micelles of the surfactant ions in which the hydrophobic hydrocarbon chains (tail) are orientated towards the interior of the micelle, leaving the hydrophilic groups (head) in interaction with the aqueous medium [9]. Micelles are formed at the Critical Micelle Concentration (CMC) and distinguished as a modulation point when physicochemical properties (such as surface tension) act as a function of concentration. The reason for micelle formation is the accomplishment of a smallest free energy state [10,11]. The main driving force for the formation of micelles is the enhancement of entropy that arises when the hydrophobic regions of the surfactant are not interacting with water and the well ordered structure of the water molecules around this region of the molecule is misplaced. Most micelles are spherical and contain between 60 and 100 surfactant molecules.
Organic reaction in micelles: Surfactant allows the catalytic organic reactions, commonly used to assemble organic structures such as drug molecules to be run in water and carrying out reactions in water rather than organic solvents because water is a potentially cleaner and cheaper reaction medium. However, organic substrates are typically too nonpolar to dissolve in water; Lipshutz has identified a surfactant that delivers a nonpolar side to organic molecules within the water [12]. Surfactants are structures with a polar head and a nonpolar tail in water; they self-assemble into spheres called micelles, with all the non-polar ends facing inwards. Organic molecules accumulate at high concentrations within the non-polar micelle core where they will react if a catalyst is also present.
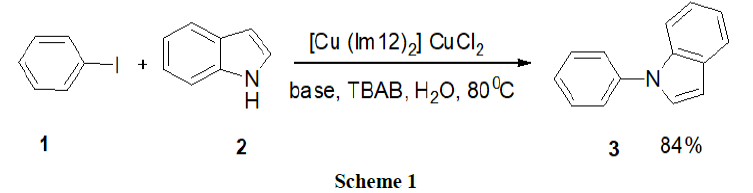
Reaction in basic micelles: Heidarizadesh, et al. have examined an efficient surfactant (TBAB)/copper based ionic liquid promoter as a reusable system for the N-arylation of indole (2), pyrazole and imidazole by a variety of aryl halides (1). The products were obtained in good to excellent yields by a convenient one pot procedure. The reaction was carried out in water and did not require the use of arylboronic acid as the active aryl source, palladium as the catalyst, or a strong base (Scheme 1).
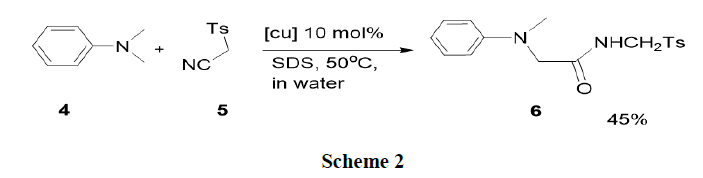
Xie, et al. have synthesised the direct assembly of α-amino amides from N-alkyl amines (4) and isocyanides (5) through oxidative Ugi-type reactions in aqueous conditions in the presence of a Cu (I)-TBHP-surfactant (tert-butyl hydroxide-surfactant) catalysis system. Various N-alkyl amines and isocyanides have been tolerated in this reaction and furnish α-amino amides (6) in moderate yields (Scheme 2) [13-20].
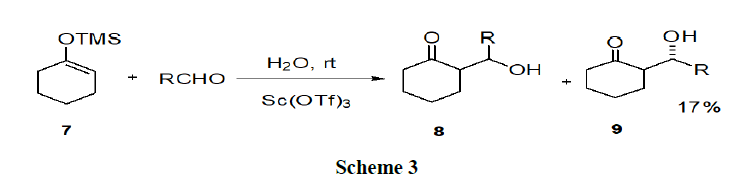
Tianet, et al. have reported the different effects of aromatic and aliphatic surfactants and they investigated the role of aromatic and aliphatic anionic surfactants in the Sc(OTf)3-catalyzed Mukaiyam aaldol reaction in water (Scheme 3).
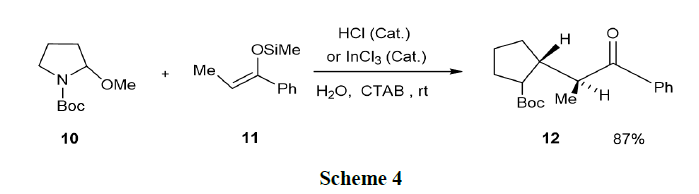
Camilo and Pilli have reported the acid-catalyzed addition of 1, 3-dicarbonyl compounds and activated olefins (silyl enol ether and ethyl vinyl ether, 11) to N-Boc-2-methoxypyrrolidine and N-Boc-2-methoxypiperidine in SDS/water medium. Good yields of the corresponding 2-substituted N-Bocpyrrolidines (12) were generally observed from (10) (Scheme 4).
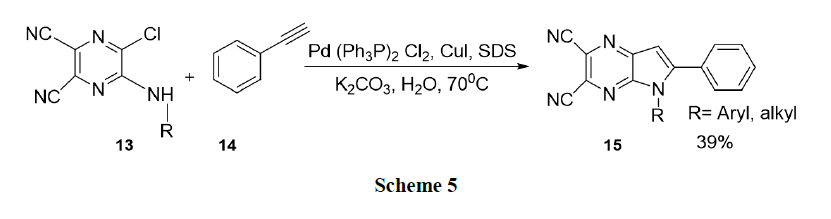
Keivanloo, et al. reported a highly efficient one-pot synthesis of 5, 6-disubstituted-5H-pyrrolo-(2,3-b) pyrazine-2,3-dicarbonitriles (15). The reaction of 5-(alkyl/arylamino)-6-chloropyrazine-2,3-dicarbonitriles(13) with phenyl acetylene(14) catalysed by Pd-Cu, in the presence of SDS as the surfactant in water, leads to the desired products in good to high yields (Scheme 5).
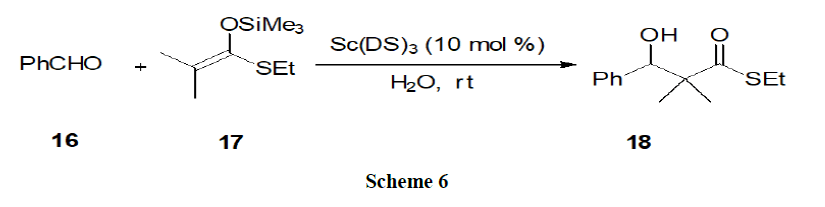
Reaction in acidic micelles: Shiri, et al. showed that the reactivity of scandiumtris (dodecyl sulphate) in aldol reaction of aldehyde (16) with silylenolates (17) enhanced remarkably in the presence of Brønsted acid (Scheme 6).
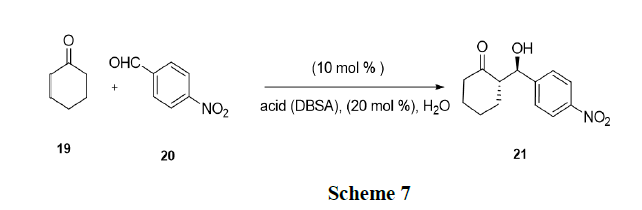
Luo, et al. have reported that simple mixing of chiral amines and surfactant Brønsted acids such as p-Dodecyl Benzene Sulfonic Acid (DBSA) led to highly effective and selective organocatalysts in water. In situ generated catalyst catalysed highly stereo selective desymmetrization of prochiralketones (19) via direct aldol reactions in water using micelle as reaction media [21-22]. The current strategy was also applied in asymmetric Michael addition leading to a catalytic system with good activity and stereo selectivity (Scheme 7).
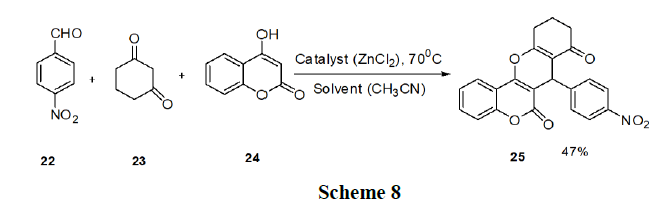
Pradhan, et al. have reported the synthesis of chromeno (4,3-b) chromene (25) derivatives. These procedures have restrictions such as high reaction temperature, prolonged reaction time, and low yields. Thus, they developed a green three component protocol for the synthesis of chromeno (4,3-b) chromene derivatives (25) from 4-hydroxycoumarin (24), aldehyde (22), 1,3-diketocompound (23) in the presence of a ‘Lewis acid-surfactant-combined catalyst’, Fe(DS)3 in aqueous media scheme 8.
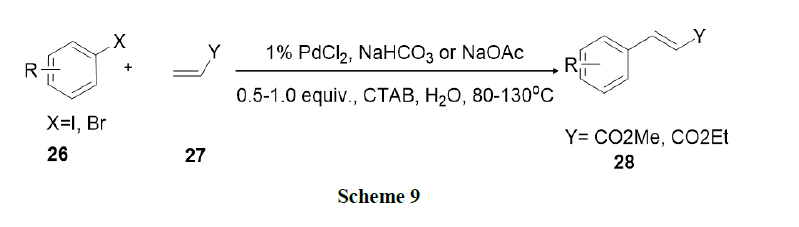
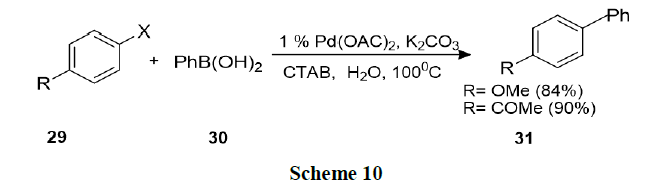
Bhattacharya, et al. explored surfactant mediated Heck and Suzuki coupling using ligand free Pd-catalysts in water. This procedure involved nano metric palladium colloids and found that the procedure was operationally simple, environmentally benign, and synthetically as efficient as conventional procedures in organic solvents (Schemes 9 and 10).
Kitanosono, et al. have discussed the utility of electrophilic palladium (II) species for Ce-H bond functionalization of indoles (32) and pyrroles (33) in water. The system displayed attractive features that are significant for both precious metal catalysis and micellar catalysis (Scheme 11).
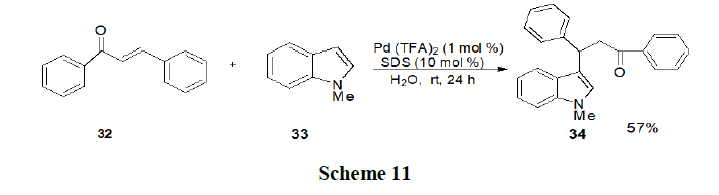
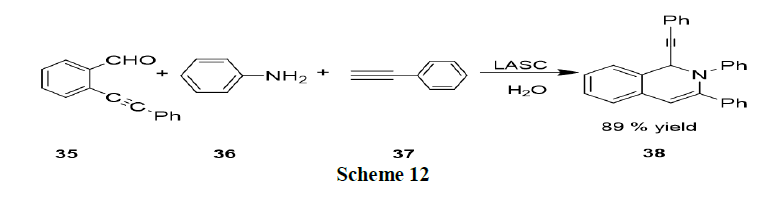
Qiuping and Wu have reported the Lewis Acid-Surfactant Combined catalyst (LASC) to catalyze the three component reactions of 2-alkynylbenzaldehyde (35), amines (36) and nucleophiles (alkyne, nitro methane, or diethyl phosphate, 37) in water under ultrasonic conditions afforded the corresponding 1,2-dihydroisoquinoline derivatives in good yields (Scheme 12).
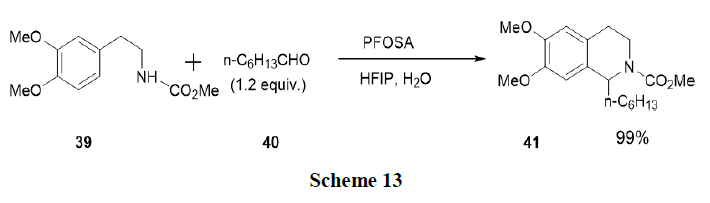
Saito, et al. have reported the formation of tetrahydroisoquinolines (41) and isochromans via Pictet-Spengler reaction using Brønsted acid surfactant combined catalyst in aqueous media and observed that the reaction accelerates with various aldehydes (40) after the addition of Hexafluro-2-Propanol (HFIP) in aqueous media. Perfluorooctansulfonamide (PFOSA) in 10 v/v% HFIP-water system was applied to the oxa-Pictet-Spengler reactions of β-aryl ethyl alcohols with aldehydes and contributed to the synthesis of tetrahydroisoquinolines and isochromans (Scheme 13).
Discussion
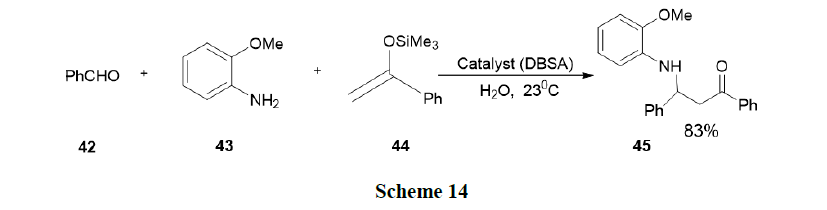
Manabe, et al. have reported Mannich type reactions of silylenolates with imines generated in situ from aldehydes and amines in water. Various catalysts were tested for the reaction of Benzaldehyde (42), o-anisidine (43) and 1-phenyl-1-(trimethylsiloxy) ethane (44) in water. The reaction in the presence of SDS alone afforded the desired β-amino ketone in a very low yield. This result suggested that a combination of a Bronsted acid and an anionic surfactant leads to an effective catalyst for this Mannich-type reaction. They were tested with p-dodecylbenzenesulfonic acid (DBSA), which was expected to behave both as a Bronsted acid and as a surfactant. Indeed, DBSA (10 mol %) was found to be a good catalyst for the reaction and even better than a surfactant-type Lewis acid, Sc(DS)3 (Scheme 14).
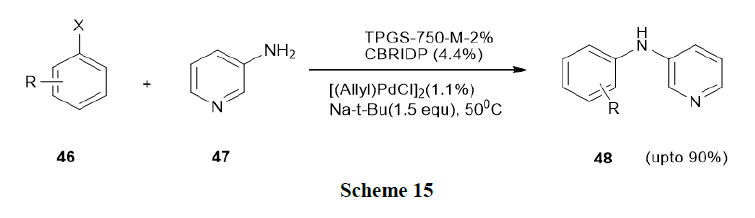
Salem, et al. have reported the scope and limitations of the Buchwald Hart wig reaction in a micellar medium and which was earlier represented by Lipshutz’s catalyst system ((allyl)PdCl)2, t-BuONa, TPGS-750-M-2% (polyoxyethanyl-α-tocopheryl succinate) in water and cBRIDPby reacting various (hetero)aryl halides with diverse nitrogen coupling partners. Thus, this study utilized both water-soluble amines, such as dimethylamine (47) and specific examples of fairly water-soluble polar solid organic substrates/reagents with relatively high melting points (Scheme 15).
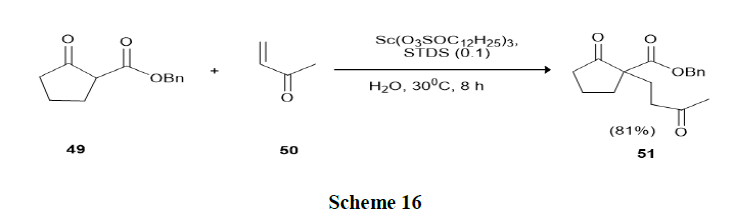
Mori, et al. have discussed the Michael reactions in water using Lewis Acid-Surfactant-Combined catalysts (LASCs) in the presence of a catalytic amount of Scandium Tris Dodecylsulfate (STDS), the reactions of various keto-esters (49) with enones (50) proceeded smoothly in water without any organic solvents and afford the Michael adducts in high yields and catalytic activity in aqueous medium was found to be higher than that in organic solvents (Scheme 16).
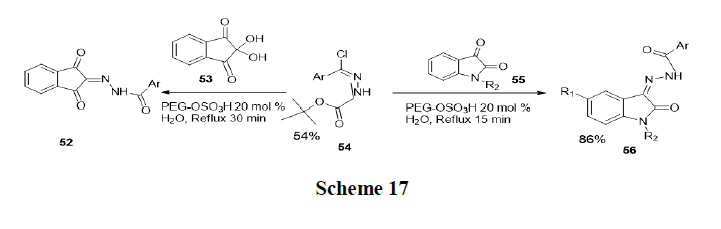
Debnnath, et al. were synthesized hydrazine derivatives of ninhydrinandisatins from condensation of ninhydrin and isatinderivatives with aryl hydrazides, initially a series of carbo-tert-butoxyhydrazones were synthesized from aromatic aldehydes and tert-butylcarbazate. Subsequently, compound treated with N-chlorosuccinamideto get NO-(chloroaryl-methylene)-tert-butylcarbazates. Then a mixture of ninhydrin (53) and NO-(chloro-aryl-methylene)-tertbutylcarbazates (54) was refluxed in the presence of PEG-OSO3H in water and produces product 52. Similarly, isatin (55) and NO-(chloro-aryl-methylene)-tert-butylcarbazates (54) were refluxed in the presence of PEG-OSO3H in water to get product 56 (Scheme 17).
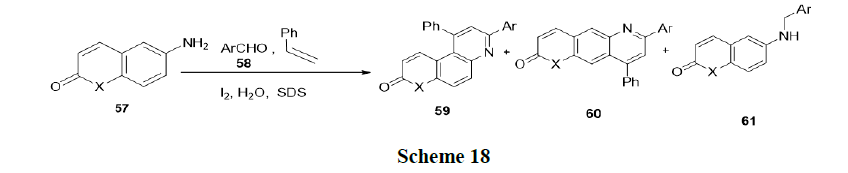
Reaction in neutral micelles: Ganguly and Sumata have reported the reaction of 6-aminocoumarins (57), benzaldehydes (58), and styrene in the presence of aqueous micellar (SDS) without any additional catalyst at room temperature and synthesized various Schiff’s base (Scheme 18).
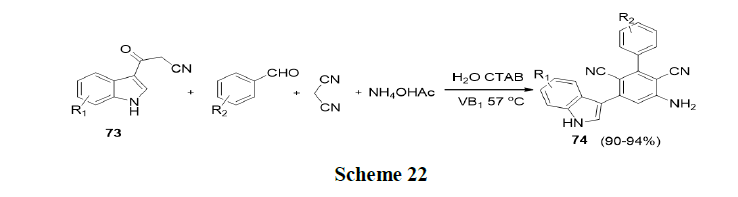
Peng, et al. have explored the aldol reaction of acetones with 4-nitrobenzaldehyde (63) in water (absence of surfactant) in anticipation of the aldol product, 4-hydroxy-4-(4-nitrophenyl)-2-butanone (64) in only 15%, yield. While in the presence of surfactant sodium dodecyl sulphate (20 mol% SDS), 87% yield of aldol product was obtained (Scheme 19).
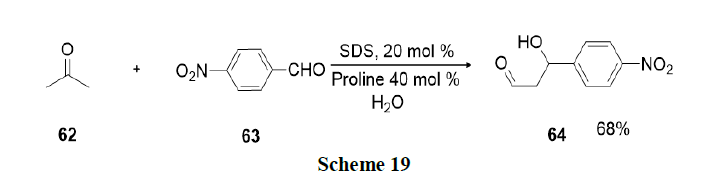
An environmentally benign and operationally simple methodology was developed by Pal and Shankar for the synthesis of 3-benzyl-3-(indol-3-yl)-2-phenyl-2,3-dihydroisoindolinones (68) via a multicomponent one-pot reaction involving 2-iodo-N-phenylbenzamides (65), terminal alkyne (66) and substituted indoles (67) in aqueous micellar medium (Scheme 20).
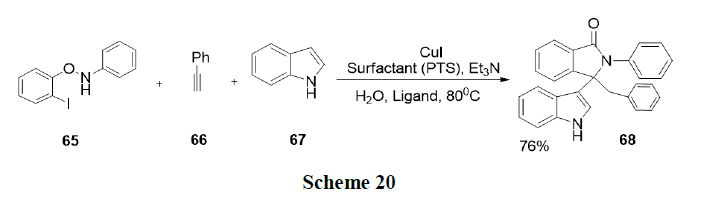
Sobhani and Parizi have described a new, one-pot convenient method for the synthesis of a variety of β-phosphonomalonates by a tandem Knoevenagel-phospha-Michael reaction of phosphate esters with aldehydes and malonitrile in an aqueous micellar solution of sodium stearate. This method presented several advantages, such as the use of cheap and environmentally benign reaction media, low loading of sodium stearate as catalyst and good yields (Scheme 21).
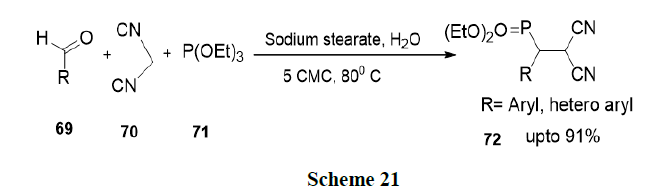
Fatma, et al. have reported thiamine hydrochloride accelerated one pot synthesis of 2-amino-6-(1H-indol-3-yl)-4-arylpyridine-3,5-dicarbonitriles (74) via four-component reaction of 3-cyanoacetyl indole (73), aromatic aldehydes, ammonium acetate and malononitrile in aqueous micellar conditions by a Knoevenagel condensation reaction followed by Michael-addition, which upon cyclization and dehydration yielded the corresponding product in excellent proportion (Scheme 22).
Wang, et al. synthesized a new type of Lewis Base Surfactant Combined Catalyst (LBSC), sodium stearate, which was applied as a catalyst in three-component one-pot reaction involving isatin (76), malononitrile (75) and 1,3-dicarbonyl (77) compounds to afford the corresponding spirooxindoles derivatives in good yields (91-97%) under aqueous micellar media (Scheme 23).
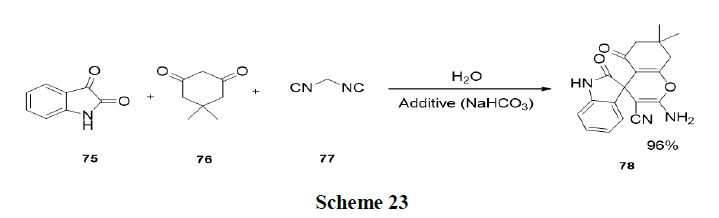
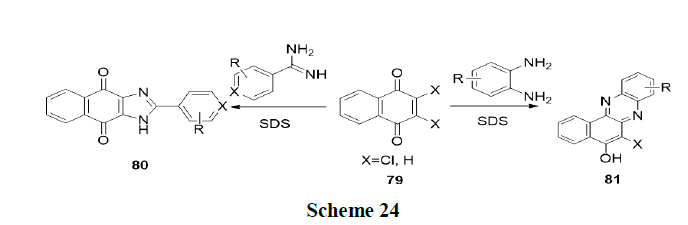
Tandon, et al. have reported one pot regio and chemo selective preparation of naphtha (2,3-d) imidazole’s (80) and benzo (a) phenazines (81) by nucleophilic substitution reaction of 1,4-naphthoquinones (79) with o-phenylenediamines and benzamidines respectively in water using base and micelles (SDS) as catalyst (Scheme 24).
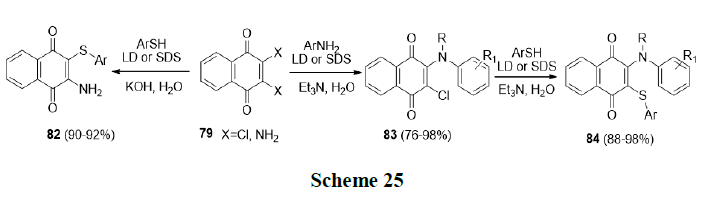
Tandon, et al. also synthesized various biologically active nitrogen and sulphur containing 1,4-naphthoquinone derivatives (82-84) in aqueous micelles using Laundry Detergent (LD) (Scheme 25).
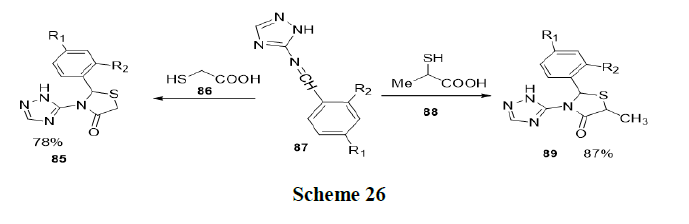
Singh, et al. has reported new environment friendly methodology for the efficient synthesis of biologically significant triazolethiazolidinone (85) hybrids in aqueous medium, using acetic acid as an organic catalyst in the presence of Acetyltrimethylammonium Bromide (CTAB) surfactant. The effect of several surfactants on the yield and completion time of the reaction was investigated and it was found that the use of CTAB at 60°C gave the best results (79-96% in 20 min-35 min) for the synthesis of the target compound (Scheme 26).
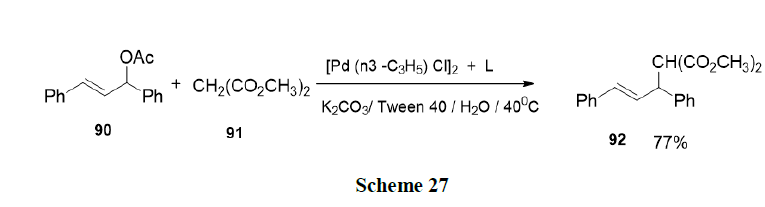
Rabeyrin, et al. investigated the alkylation of 1, 3-diphenyl-2-propenyl acetate (90) with dimethyl malonate (91) in the presence of K2CO3 and the palladium complex (Scheme 27).
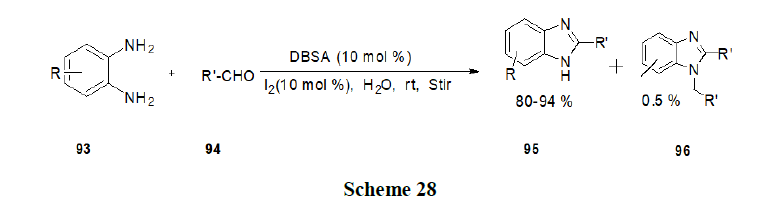
Kumar, et al. have developed an efficient synthetic method for the facile synthesis of 2-substituted benzimidazole (95) organized aqueous media in the presence of a surfactant (viz. DBSA) as catalyst and I2 as co catalyst. The method described has the advantages of operational simplicity, excellent yields, high chemo-selectivity, clean and green reaction profile (Scheme 28).
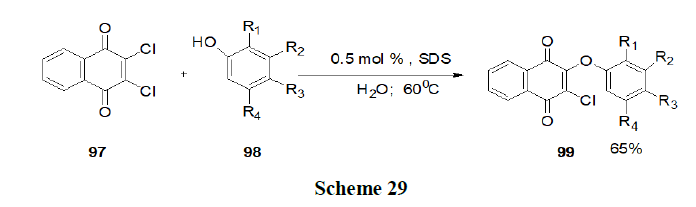
Tandon, et al. have synthesised the various oxygen containing 1,4-naphthoquinone derivatives by an economical, viable green methodology approach using water as solvent with or without surfactants such as Triton X-100, SDS, LD (laundry detergent) and TBAB, phase transfer catalyst and evaluated for their in vitro antifungal and antibacterial activity (Scheme 29).
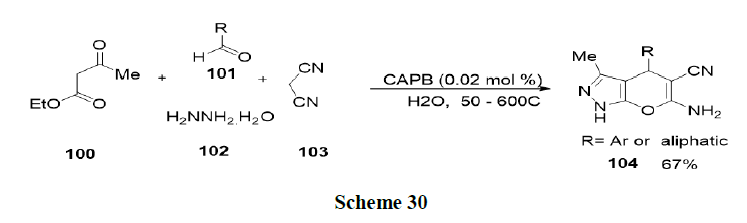
Reaction with other micelles: Tamaddon and Alizadeh have discussed the use of Cocamidopropylbetaine (CAPB) as a biodegradable surfactant which produces a new worm like micelle medium for rapid synthesis of dihydropyrano (2,3-c) pyrazole (104) via a four component reaction of aldehydes (101), ethyl acetoacetate (100), malononitrile (103) and hydrazine hydrate (102) at 50-60ºC and reported that CAPB, a zwitterionic surfactant was superior to anionic, cationic and non-ionic alternatives for accessing high yields of pure products without the use of any organic solvent. While the reaction medium was reusable, simple isolation of products, mild reaction conditions, low loading of CAPB at critical micelle concentration, and short reaction times were additional advantages of this green procedure (Scheme 30).
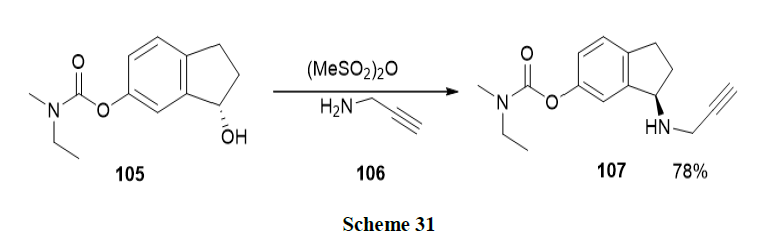
Luo, et al. developed the enantiopure multifunctional anti-Alzheimer drug, Ladostigil (107) by using Asymmetric Transfer Hydrogenation (ATH) as a key step catalysed by Ru-Cs-DPEN in an HCOONa-H2O surfactant system. Good chemical yields with high enantiomeric excess were obtained and the catalyst was recycled another five times (Scheme 31).
Yin, et al. have reported a series of α-hydroxy esters which were rapidly prepared (1.5 h) from keto ester via Asymmetric Transfer Hydrogenation (ATH) in water using surfactants. This green method, catalysed by a water soluble and recyclable Ru (II)-complex, gave moderate to high enantioselectivity (up to 99.7%) with DTAB as an additive and HCOONa as the hydrogen source (Scheme 32).

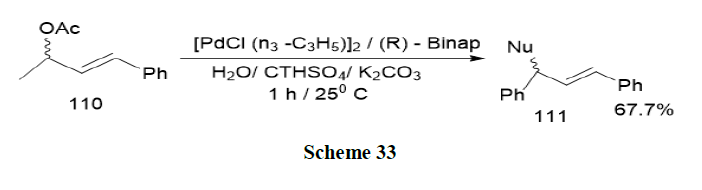
Rabeyrin and Sinou have reported the asymmetric palladium catalysed alkylation of 1,3-diphenyl-2-propenyl acetate (110) on carbon and nitrogen nucleophiles in water and in the presence of a surfactant, base and Binap as the chiral ligand. Enantioselectivities up to 91% were obtained using carbon nucleophiles and 93% using nitrogen nucleophiles, in the presence of CTHASO4 as surfactant. While the efficiency of the catalyst was higher in water in the presence of the surfactant in the case of carbon nucleophiles, no micellar effects were observed using the nitrogen nucleophiles (Scheme 33).
Conclusion
In conclusion, we have searched various literature related to micelle assisted organic reaction during the last five years. It was found that different types of micelles play an important role in various organic reactions. This review shows the applications of surfactant in organic reactions as a catalyst to activate the substrate molecules and as a surfactant to increase the concentration of organic reactants to form micelle particles in water. In this review, we have discussed various reactions performed using different types of surfactants such as SDS, CTAB, Triton-100, DTAC, DDBAC, OTAC, etc. In which, SDS has found the best surfactant.
References
- Santelli M, Pons JM. Lewis Acids and Selectivity in Organic Synthesis, 1st Edition, New York, 1995.
- Cochran JC. Lewis Acid/Base Reaction Chemistry (Leach, Mark R.). J Chem Educ. 2001;12(2):166. [Crossref][Googlescholar]
- Corma A, Garcia H. Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem Rev. 2003;103(11):4307-4366. [Crossref][Googlescholar][Indexed]
- Li CJ, Chen L. Organic Chemistry in Water. Chem Soc Rev. 2006;35(1):68-82. [Crossref][Googlescholar][Indexed]
- Manabe K, Mori Y, Wakabayashi T, et al. Organic Synthesis Inside Particles in Water: Lewis Acid Surfactant Combined Catalysts for Organic Reactions in Water Using Colloidal Dispersions as Reaction Media. J Am Chem Soc. 2000;122(30): 7202-7207. [Crossref][Googlescholar]
- Andrade CK, Alves LM. Environmentally Benign Solvents in Organic Synthesis: Current Topics. Curr Org Chem. 2005;9 (2):195-218. [Crossref][Googlescholar]
- Chanda A, Fokin VV. Organic Synthesis “On Water”. Chem Rev.2009;109(2):725-748. [Crossref][Googlescholar][Indexed]
- Lindstrom UM. Stereo selective Organic Reactions in Water. Chem Rev. 2002;102(8):2751-2772. [Crossref][Googlescholar][Indexed]
- Lipshutz BH, Aguinaldo GT, Ghorai S, et al. Olefin Cross Metathesis Reactions at Room Temperature Using the Nonionic Amphiphile “PTS”: Just Add Water. Org Lett. 2008;10(7):1325-1328. [Crossref][Googlescholar][Indexed]
- Heidarizadeh F, Majdi-nasab A. A green, homogeneous and reusable surfactant/copper based ionic liquid for the N-arylation of indoles, pyrazoles and imidazoles.Tetrahedron Lett. 2015;56(46):6360-6363. [Crossref][Googlescholar]
- Xie C, Han L. Development of aqueous oxidative Ugi type reactions by copper catalyzed surfactant promoted C(sp3)-H direct functionalization in water. Tetrahedron Lett.2014;55(1):240-243. [Crossref][Googlescholar]
- Tian HY, Chen YJ, Wang D, et al. The effects of aromatic and aliphatic anionic surfactants on Sc(OTf)3-catalyzed Mukaiyama aldol reaction in water. Tetrahedron Lett. 2001;42(10):1803-1805. [Crossref][Googlescholar]
- Camilo NS, Pilli RA. Addition of carbon nucleophiles to cyclic N-acyliminium ions in SDS/water. Tetrahedron Lett. 2004;45(13):2821-2823. [Crossref][Googlescholar]
- Keivanloo A, Bakherad M, Nasr-Isfahani H, et al. Highly efficient synthesis of 5,6-disubstituted-5H-pyrrolo[2,3-b]pyrazine-2,3-dicarbonitriles through a one pot palladium-catalyzed coupling reaction/cyclization in water. Tetrahedron Lett. 2012;53(25):3126-3130. [Crossref][Googlescholar]
- Shiri M, Zolfigol MA. Surfactant type catalysts in organic reactions. Tetrahedron. 2009;65(3):587-589. [Crossref][Googlescholar]
- Luo S, Xu H, Li J, et al. Facile evolution of asymmetric organocatalysts in water assisted by surfactant Brønsted acids. Tetrahedron.2007;63(46):11307-11314. [Crossref][Googlescholar]
- Pradhan K, Paul S, Das AR, et al. Fe(DS)3, an efficient Lewis Acid Surfactant combined Catalyst (LASC) for the one pot synthesis of chromeno (4,3-b) chromene derivatives by assembling the basic building blocks. Tetrahedron Lett. 2013;54(24): 3105-3110. [Crossref][Googlescholar]
- Bhattacharya S, Srivastava A, Sengupta S, et al. Remarkably facile Heck and Suzuki reactions in water using a simple cationic surfactant and ligand-free palladium catalysts. Tetrahedron Lett. 2005;46(20):3557-3560. [Crossref][Googlescholar]
- Kitanosono T, Miyo M, Kobayashi S, et al. The combined use of cationic palladium (II) with a surfactant for the C–H functionalization of indoles and pyrroles in water. Tetrahedron. 2015;71(40):7739-7744. [Crossref][Googlescholar]
- Ye Y, Ding Q, Wu J, et al. Three component reaction of 2-alkynylbenzaldehyde, amine, and nucleophile using Lewis acid-surfactant combined catalyst in water. Tetrahedron. 2008;64(7):1378-1382. [Crossref][Googlescholar]
- Saito A, Takayama M, Yamazaki A, et al. Synthesis of tetrahydroisoquinolines and isochromans via Pictet–Spengler reactions catalyzed by Brønsted acid surfactant combined catalyst in aqueous media. Tetrahedron. 2007;63(19):4039-4047. [Crossref][Googlescholar]
- Manabe K, Mori Y, Kobayashi S, et al. Three component carbon–carbon bond-forming reactions catalyzed by a Brønsted acid–surfactant-combined catalyst in water. Tetrahedron.2001;57(13):2537-2544. [Crossref][Googlescholar]