Research
, Volume: 19( 8)Melatonin Effect on the Bisphenol-A-Induced Cytotoxicity and Genetic Toxicity in Colon Cancer Cell Line, Normal Gingival Cell Line, and Bone Marrow Stem Cell Line
- *Correspondence:
- Mohammad Shokrzadeh Department of Toxicology and Pharmacology, Mazandaran University of Medical Sciences, Iran, E-mail: ebrahimirouya@gmail.com
Received: August 20, 2021; Accepted: September 2, 2021; Published: September 21, 2021
Citation: Shokrzadeh M, Ebrahimi R, Barghi NG. Melatonin Effect on the Bisphenol-A-Induced Cytotoxicity and Genetic Toxicity in Colon Cancer Cell Line, Normal Gingival Cell Line and Bone Marrow Stem Cell Line. Int J Chem Sci. 2021;19(8):414.
Abstract
The most common path of Human exposure to Bisphenol-A-(BPA) is by oral intake. It involves genotoxicity, oxidative stress, endocrine disruption, mutagenicity, and carcinogenicity in both in vitro and in vivo models. Melatonin has known as a freeradical scavenger and powerful antioxidant agent. This study was initiated to investigate the role of melatonin on viability and genetic disorders of Normal Human Gingival Fibroblasts (HGF), Colon cancer (MKN45), and Bone marrow stem cell lines exposed to BPA. For this purpose, MTT and Comet assays were performed to evaluate the cytotoxicity and genotoxicity properties of BPA and Melatonin role. The results of the present study showed that BPA exposure resulted in increased oxidative stress parameters including MDA, ROS, and decreased GSH content. The current study demonstrated the cytotoxicity and genotoxicity effects of BPA and the protective role of melatonin for preventing cytotoxicity and DNA damage induced by BPA.
Keywords
Bisphenol A; Melatonin, MTT; Comet; Cytotoxicity; Genotoxicity
Introduction
Bisphenol-A (BPA. 2,2-bis (4-hydroxyphenyl) propane) a synthetic chemical, as a monomer, plasticizer, and phenolic compound that has been used widely in the production of polycarbonate plastic and epoxy resins in the manufacturing of consumer products such as food cans, beverage containers, baby feeding bottles, medical tubing’s, toys, water pipes, dental sealants, eyeglass lenses, several paper products and adhesives, protective coatings, powder paints, automotive lenses, protective window glazing, building materials, compact disks, optical lenses, thermal paper, paper coatings, for encapsulation of electrical and electronic parts [1-7]. BPA is a xenoestrogen, that able to trigger distinctive estrogen-signaling pathways with potential consequences on human health. Furthermore, an irreparable health effects of BPA relate to an early exposure during gestational and neonatal periods [8]. The prevalent use of BPA in the manufacturing of consumer products led to its detection in samples from dietary products, human fluids, water, dust, sewage, and indoor, outdoor air, and environmental media [6,9]. BPA is released by food and beverage containers especially when heated, so human exposure to it has been widespread and continuous through the food chain [1,2,7]. The major responsible matter for exposure to BPA in more than 90% of overall human at all age groups is through the ingestion of contaminated food, therefore tissues from the upper gastrointestinal tract are exposed to non-conjugated BPA (active form) [10], and the most common way of Human BPA exposure has been supposed to be by oral intake, but exposure may occur through inhalation during production in the workplace. [7]. Consider to its prevalent detection in human life, EPA put the BPA at the third-highest Toxicological Priority Index (ToxPi) among the 309 environmental chemicals using the ToxCast program [9]. This matter resulted in the imposition of a ban on the use of BPA in child care products including feeding bottles in European Union, the United States and Canada, and it has also been banned in thermal receipt papers in Japan [3,9]. Demand for this chemical agent has steadily been growing in the different industry over the recent years; so there is a rising concern about BPA due to its Toxicological properties including genotoxicity, oxidative stress, endocrine disruption, mutagenicity, and carcinogenicity that have been figured out in vitro and in vivo model [11]. Recent years, BPA has noticed a great deal of public and researcher's attention because of its potential association with variety of toxicity and adverse health effects that makes scientific society work on prevention and protection paths of BPA adverse effects. [1,3,11]. BPA exposer cause to increase the MDA concentration and decrease the GSH content that have been known as an indication of increased generation of ROS, which led to lipid peroxidation [1]. Experiments on cultured cells and laboratory animals indicated the accumulation feature of BPA and its involvement on several vital organ functions, including the testis, brain, heart, liver, and pancreas. The findings also demonstrate relation of the oxidative stress and mitochondrial dysfunction to the adverse effect of BPA [12,13]. Melatonin (N-acetyl-5-methoxytryptamine) has been known as an indoleamine which is mainly released by the pineal gland [4,5]. Being free radical scavenger is one of its important feature that has significant antioxidant activity [5,11]. The most toxic free radical and the hydroxyl radical promptly are scavenged by this agent and it directly scavenges the peroxynitrite anion, nitric oxide, singlet oxygen, and the peroxyl radical. Melatonin provokes the mRNA amount of antioxidant enzymes like superoxide dismutase. Another antioxidant enzymes including Glutathione peroxidase and glutathione reductase are also agitated by melatonin. Other activities that Melatonin is involved, are including inhibition of the pro-oxidative enzyme, nitric oxide synthase, chelating transition metal ions, prevention of the cellular membranes deterioration, and reduction of lipid peroxidation [11]. The protection feature of vitamin C and N-acetylcysteine against BPA-induced toxicity has been reported [11]. Hens in this study, we chose melatonin as a protective agent regarding to its potential antioxidant activity. The objective of this study was to investigate the melatonin protective role against BPA-induced cytotoxicity and genetic toxicity in three cell lines including Colon cancer cell line, normal gingival cell line, and bone marrow stem cell line.
Materials and Methods
Chemicals
The following chemicals were purchased from Merck-KGaA Chemical Company: BPA, Melatonin, Methanol, MTT dye, Potassium chloride (KCl), Hydrochloric acid (HCl); DMSO, phenazine methosulfate (PMS), Penicillin/streptomycin, fetal bovine serum, L-glutamine, and phosphate-buffered saline (PBS), DMEM culture medium were purchased from Sigma- Aldrich Chemical Company. All chemical reagents were obtained from the highest and purest grade available.
Cell culture
Three cell lines including Normal Human Gingival Fibroblasts Cell Line (HGF), Colon Cancer Cell Line (MKN45), and Bone Marrow Stem Cell line (MSC) used in this study were obtained from the Pasteur Institute of Iran cell bank in Tehran. These cells were grown at 37°C and 5% CO2 in DMEM medium.
Concentrations of the tested Melatonin and BPA for determination of cytotoxicity, genotoxicity and oxidative stress parameters
Melatonin and BPA were prepared in four concentrations respectively including 50 μM, 100 μM, 200 μM, 400 μM and 0.5 μg/ml, 5 μg/ml, 50 μg/ml, 100 μg/ml. 50 μg/ml of BPA considered as a positive control group and cell culture medium was considered as a negative control group. The cell viability was studied after 24 and 72 h exposure of mentioned cell lines to BPA at concentrations 0.5 μg/ml, 5 μg/ml, 50 μg/ml, and 100 μg/ml. Genotoxicity of BPA was determined by the Comet assay after 24 and 72 h exposure to 0.5 μg/ml, 5 μg/ml, 50 μg/ml, and 100 μg/ml concentrations. Cisplatin as a positive control is used for evaluating the BPA-exposed cell viability. The melatonin role on BPA-induced cytotoxicity and genotoxicity evaluated at 50 μM, 100 μM, 200 μM, 400 μM concentrations; 50 μg/ml of BPA was used to cytotoxicity and genotoxicity induction. Experiments were performed in triplicate and repeated three times.
Cytotoxicity assay
MTT assay is one of the popular methods in evaluating cell viability. This assay is based on the reduction of MTT, a yellow water-soluble tetrazolium dye primarily, by mitochondrial dehydrogenases to purple-colored formazan crystals. The formazan product is dissolved in DMSO and then analyzed spectrophotometrically using an ELISA reader device in 570 nm and 630 nm [14-17].
Analyses of the induction of genetic toxicity by comet assay
DNA strand break induction was assayed by the comet assay as described by von Bardeleben et al., 2002. Comet assay is used to evaluate two dependent variables: Global DNA damage and oxidative damage a particular type of DNA damage caused by the oxidation of nucleotides. Comets were visualized by microscopy and quantified by determination of the ‘Olive tail moment’ (OTM) using computer-based software (Komet 4.02, Kinetics Imaging, UK). Fifty cells were analyzed per measurement for calculation of the mean value [7,10,18].
Biochemical determinations
The protocol of Buege and Aust's (1978) was used to estimate lipid peroxidation. The lipid peroxidation product, malondialdehyde (MDA), was measured by the thiobarbituric acid assay, which is based on the MDA reaction with thiobarbituric acid to give thiobarbituric acid reactive substances (TBARS), a red species that absorbs at 530 nm. The MDA levels were reported in the values of moles/mg protein [9,11,19,20]. GSH was estimated with minor variations to the protocol by Jollow et al. (1974). The supernatant was mixed with potassium phosphate buffer, 0.1 M, pH 7.4, and the reaction was initiated by the addition of DTNB, and the absorbance was measured at 412 nm. GSH levels were reported in the values of moles/mg protein. The calculations were made using the molar extinction coefficient of 1.36×104 M−1 cm−1 [9,21]. DCFH- DA was used to assay the production of intracellular reactive oxygen species (ROS). DCFH-DA is a non-fluorescent, non- polar, and electrically neutral compound and can easily enter the cell by passing through the membrane. The intracellular ROS oxidizes the DCFH to a highly fluorescent DCF. The treated PBMCs were incubated with 20 μM DCFH-DA for 1h at 37°C. The fluorescence to determine the increase in ROS was recorded at the emission wavelength of 530 nm using 485 nm as excitation wavelength [9,22].
Statistical analysis
Data were presented as the mean ± SD (standard deviation). The statistical analyses were performed by one-way ANOVA followed by the Tukey test. SPSS 17 software was used for statistical analysis. Statistical significance was considered as P<0.05.
Results
Cell viability
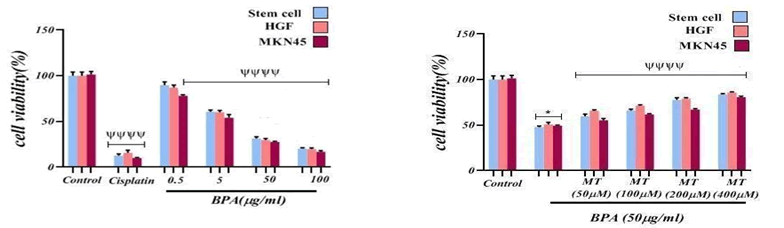
The effects of BPA on the viability of HGF, MKN45, and MSC cells were evaluated using the MTT assay. After 24 h exposure, BPA caused a decrease in cell viability on the mentioned cell lines. As it is obvious in the FIG. 1, there was a significant difference (P<0.00001) in the cytotoxicity effect of BPA on cells in comparison with the control group. MTT assay was also used to evaluate the Melatonin role on cytotoxicity caused by BPA on mentioned cell lines. As it can be observed in FIG. 1, the result of the investigation showed that Melatonin significantly (p<0.0001) at all concentrations (50, 100, 200, 400 μM) caused to enhance cell viability compared to the BPA group. All cell lines were exposed to 50 μg/ml BPA and different Melatonin concentrations to study the Melatonin effect.
Figure 1: The cytotoxicity effect of BPA and Melatonin effect on BPA-induced cytotoxicity on Stem cell, HGF and MKN45 lines. ψψψψ p<0.0001: Significant to control group.
Comet assay
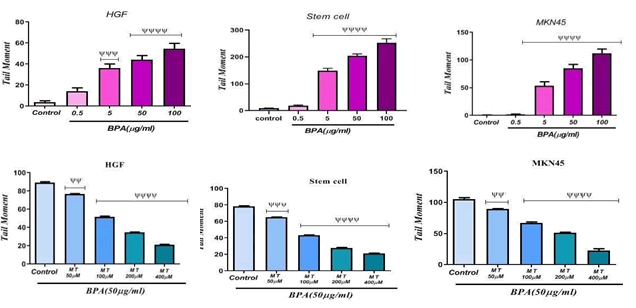
Comet assay is a sensitive method for detecting DNA strand breakage at the level of an individual cell; comet assay was run on HGF, MKN45, and MSC cells exposed to various concentrations of BPA and Melatonin. FIG. 2 shows the tail moment obtained through the comet assay on MKN45 cells. There was a significant increase in the tail moment suggesting DNA damage or fragmentation. The DNA damage was found to be significant at four concentrations including 0.5, 5, 50, and 100 μg/ml of BPA with p<0.0001 on MKN45 cells. Therefore, BPA was genotoxic. On close observation of the results in FIG. 2, we also found that BPA was able to increase significantly (p<0.0001) genotoxic effect on the Stem cell line. An increase in the tail moment was observed at concentrations more than 5 μg/ml of BPA on MSC cells. As it is obvious in FIG. 2, there was a significant increase in the tail moment at concentrations 5, 50, 100 μg/ml of BPA on HGF cells which indicates the genotoxic effect of BPA. Comet assay was also used to investigate the influence of Melatonin on BPA-induced genotoxicity at four concentrations. As it can be figured out in FIG. 2, the results showed that it could significantly (p<0.0001) decrease the Tail moment on Stem cell line at 100, 200 and 400 μM concentrations of melatonin. A significant (p<0.001) decrease in the Tail moment was also observed in the group that was exposed to 50 μM of Melatonin. With an increase in the concentration of Melatonin, its genotoxicity decreasing effect has been increased. As it is illustrated in FIG. 2, a significant (p<0.0001) decrease was found in the genotoxicity of BPA on the HGF and MKN45 cell lines that were exposed to 100, 200, and 400 μM concentrations of Melatonin in comparison to the control group. Data also showed that Melatonin on mentioned cell lines at 50 μM concentration caused to decrease significantly (p<0.01) genotoxicity of BPA compared to the control.
Figure 2: The genotoxicity effect of BPA and Melatonin effect on BPA-induced genotoxicity on HGF, Stem cell and MKN45 lines. ψψψψp<0.0001: Significant to control group. ψψψp<0.001: Significant to control group. ψψ p<0.01: Significant to control group.
Reactive oxygen species
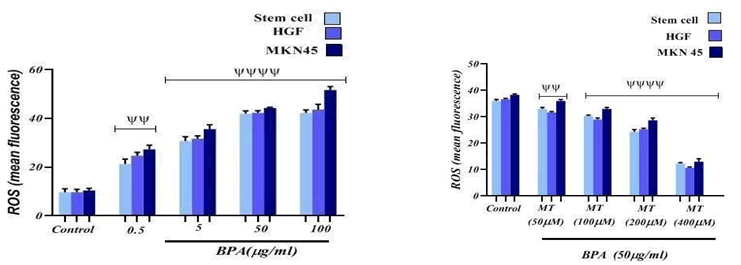
The reactive oxygen species production increase more than their intracellular threshold concentration due to external stimuli which resulted in occurring Oxidative stress. To understand whether the cytotoxicity of BPA was due to induction of oxidative stress in HGF, MKN45, and MSC cells upon their exposure, intracellular reactive oxygen species detection was carried out using DCFH-DA as a probe. According to the data which is expressed in FIG. 3, it was figured out that ROS content in the cell lines exposed to BPA at concentrations 5, 50, and 100 μg/ml has been significantly (p<0.0001) elevated compared to the control group which is indicated the cytotoxic effect of it. BPA also caused to increase significantly (p<0.01) the amount of ROS at 0.5 μg/ml concentration in comparison with the control group. we conclude that BPA can exert their cytotoxicity through oxidative stress. FIG. 3 also shows the decrease in the percentage fluorescence intensity of DCF upon treatment with Melatonin on cells exposed to BPA. It demonstrated a steady decrease in intensity and a significant (p<0.0001) decrease at different concentrations of Melatonin including 100, 200, and 400 μM compared to the control group which is just exposed to BPA. Melatonin also decreased significantly (p<0.01) ROS amount at 50 μM concentration in comparison with the control group, which proves its protective effect against BPA-induced cytotoxicity.
Figure 3: The effect of BPA and Melatonin effect on ROS content on Stem cell line, HGF and MKN45 cell lines. ψψψψ p<0.0001: Significant to control group. p<0.01: Significant to control group
Estimation of GSH and lipid peroxidation
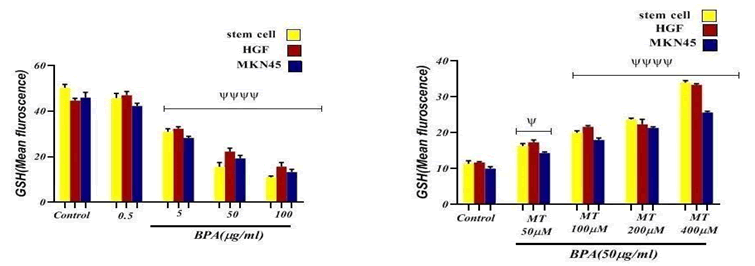
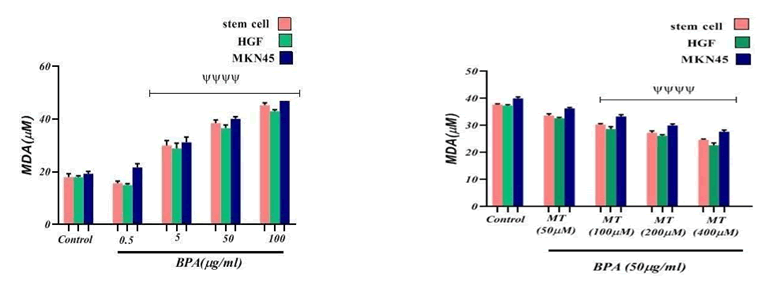
Glutathione has been known as an important free radical scavenger which is mainly involved in the detoxification process including suppressing the ROS; a decrease in GSH content and an increase in MDA levels indicate an oxidative stress induction. The major point concerning ROS is its reaction with vital macromolecules like lipids that resulted in their oxidation to MDA leading to damage to membrane lipids [8]. To confirm whether the BPA was able to induce oxidative stress, estimation of GSH and MDA levels were carried out. FIG. 4 and 5 show the effect of BPA on the status of GSH and MDA content of BPA-exposed cell lines. There was a significant depletion in GSH content and a rise in MDA level in the BPA-exposed cells at 5, 50, and 100 μg/ml concentrations (p<0.0001). These data suggested that BPA-induced ROS- mediated oxidative stress was responsible for the mitigation of GSH content and an increase in the levels of MDA [8].
Figure 4: The effect of BPA and Melatonin effect on GSH content on Stem cell line, HGF and MKN45 cell lines. ψψψψ p<0.0001: Significant to control group. ψp<0.05: Significant to control group.
Figure 5: The effect of BPA and Melatonin effect on MDA level on Stem cell, MKN45 and HGF cell lines. ψψψψ p<0.0001: Significant to control group
Influence of Melatonin on the depletion in GSH content and rise in MDA level caused by BPA expressed in FIG. 4 and 5. It was found that GSH content has been significantly (p<0.0001) increased in cell lines exposed to 100, 200, and 400 μM concentrations of Melatonin compared to the control group. Melatonin at 50 μM concentration also caused to increase significantly (p<0.05) the GSH content in comparison with the control group. Results of Melatonin evaluation on MDA amount in cell lines exposed to different concentrations of Melatonin showed a significant (p<0.0001) decrease at 100, 200, and 400 μM concentrations in comparison with the control group. The Melatonin effect at 50 μM concentration wasn’t a significant difference compared to the control group.
Discussion
BPA as an endocrine disruptor is a monomer of polycarbonate plastic and a constituent of epoxy and polystyrene resins [11]. Human exposure to BPA is widespread due to various uses in consumer products [2]. BPA is released by food and beverage containers, so human exposure to it is prevalent and continuous through the food chain [1]. The factors involving in BPA toxicity are including lipid peroxidation and generation of free radicals causing oxidative stress [11]. Melatonin unique features including low toxicity and ability to cross biological barriers enable it to prevent oxidative damage to DNA, hence it has been known as a free-radical scavenger that able to neutralize diverse threatening factors [1,11,23]. This study was initiated to investigate Melatonin effects on the BPA-induced genotoxicity and cytotoxicity on MKN54, HGF, and stem cell lines. We used melatonin as a protective agent against BPA-induced genotoxicity and cytotoxicity considering Melatonin’s potential antioxidant activity and protective role.
Data obtained from the present study showed that BPA caused to diminish cell viability percentage which is due to its cytotoxicity effect. It is also figured out that Melatonin was able to increase the cell viability percentage of cell lines exposed to BPA. Results of the Comet assay showed that there was a significant increase in the tail moment suggesting DNA damage on cell lines exposed to BPA; on the other hand, an investigation of Melatonin’s influence on BPA-induced genotoxicity showed that Melatonin able to decrease the Tail moment significantly on cell lines exposed to BPA. Biochemical factors determinations illustrated that BPA disrupts the biochemical agent’s state as evidenced by elevated lipid peroxidation, Reactive Oxygen Species and decreased GSH content. In the current study, melatonin significantly modulated the biochemical factors state in the cell lines exposed to melatonin+BPA. This was verified by a decrease in the levels of MDA and ROS with an increase in levels of GSH in the melatonin+BPA-exposed cell lines compared to the BPA-exposed groups. An anti-oxidative and scavenging properties of melatonin enable it to protect DNA and lipids from oxidative agent like BPA [1].
In vitro study done by Shoeb Ikhlas et al. in 2019 approved our study results. They used the MTT test to assess the cytotoxicity by measuring the reduction in cell viability due to apoptosis or necrosis [9]. Our results were also in line with previous reports of Klara Hercog and colleagues in 2019. They showed the BPA genotoxic potential and its analogs such as BPS, BPF, and BPAF, as well as their mixtures, their effects on the expression of selected genes involved in xenobiotic metabolism, response to oxidative stress, and DNA damage on HepG2 cells [17].
Another study is consistent with our Cytotoxicity and Comet assay results were done by Carina Ramosa and colleagues on cytotoxic and genotoxic effects of environmentally relevant concentrations of BPA and interactions with doxorubicin. Comet assay results in their study demonstrate DNA damage as a consequence of Hep-2 cells exposure to BPA which is in agreement with our study [10].
The results of Biochemical factors determinations were consistent with previous reports of Mohamed A. El-Missiry and his colleagues on Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against BPA-induced apoptosis, that expressed Melatonin owns unique physical and chemical properties among several antioxidants enable it to cross easily the biological membranes and reach the cytosol, nucleus, and mitochondria [1].
The other study confirmed our results, which was performed by Anongporn Kobroob et all on Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidence from in Vivo and in Vitro Studies. They figured out that BPA exposure not only increased the oxidant molecule nitric oxide (NO) but also decreased the antioxidants glutathione (GSH) level and superoxide dismutase enzyme (SOD) in the kidney tissues. Consistent with the current study, BPA has been shown to stimulate oxidative injury in a variety of cells and organs both in vitro and in vivo experiments [12]. Results of Sameya Anjum and colleague’s studies on Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of the mouse also approved our findings. It was observed that BPA treatment significantly affected GSH as a vital agent in cell viability. It is expressed that Glutathione either alone or in conjugation with other proteins able to protect the cell against lipid peroxidation. The results expressed that melatonin as a potent antioxidant is able to ameliorate the BPA-induced oxidative stress. they also showed that the melatonin function as a free radical scavenger may relate to its electron-donating ability. It has been reported that Long-term melatonin intake led to increase the number of mitochondria in cells [11].
The results of the present study are consistent with the findings of Shoeb Ikhlas et all in 2019 study on evaluating the cytotoxicity of BPA analogs based on their oxidative and genotoxic potential using human peripheral blood cells. They suggested although BPA genotoxicity is conflicting in nature, some studies found that BPA is able to induce DNA damage in mouse cell lymphoma cells, MCF-7, cells and chicken DT40 cells while some reports have figured out its non-genotoxic function on Chinese hamster ovary cells, intestinal cell lines (LS174T), hepatoma cell lines (HepG2) and renal cell lines (ACHN) [9]. Our findings are also in agreement with prior findings showed DNA damage in the study of the effect of BPA and melatonin treatments on DNA damage in male germ cells that done by Hong-Juan Wu and colleagues in 2013. In their study, it was observed Significant increase in the comet parameters in the pachytene spermatocytes of rats exposed to BPA. Moreover, they suggested that metabolites of BPA may react with DNA especially when the glutathione level is low in the cell [7]. In the another study, the histomorphological, immunohistochemistry and TUNEL analyses of the cultured tests were performed by Teng Zhang and colleagues in order to investigate whether the addition of Melatonin to the culture medium would prevent the adverse effects of BPA on the testis, and the results showed that 10 μM Melatonin was able to show a protective function against BPA which is in agreement our study result about the melatonin protective effect on cell lines exposed to BPA [24]. Another study was done by Hyo-Jin Park and colleagues due to investigate the anti-oxidative effects of Melatonin against BPA-derived superoxide on oocyte maturation in pigs. In Their study the Melatonin protective effect was certified concerning superoxide production upon BPA exposure during oocyte maturation [25]. The other study that approved our results is related to Abdullah, Saleh M, and colleagues on evaluating BPA induced oxidative stress in human RBCs in vitro and its amelioration by melatonin. They figured out that BPA exposer of red blood cells caused an increase in oxidative stress, while melatonin decreased the BPA-induced oxidative stress in these cells [26].
Neslihan Coşkun Akçay and colleague’s findings related to study on the effects of melatonin on possible damage that will occur on adipocytokines and liver tissue by coadministration of fructose and bisphenol a (BPA), was in agreement with our data; they stated that co-administration of BPA and fructose caused an increase in lipid peroxidation level due to the increase of oxidative stress. On the other hand melatonin treatment induced antioxidant enzyme activity and reduced lipid peroxidation level [27].
Conclusion
In conclusion, the present study provides convincing evidence to prove that BPA has a detrimental impact on the stem cell line, HGF, and MKN45 cells. The current study confirmed that BPA exposure resulted in increased oxidative stress factors including MDA, ROS, and decreased GSH content. BPA exposure also induced lipid peroxidation demonstrating the vulnerability of the mitochondrial membrane to it. BPA had clearly shown cytotoxicity in three cell lines. Investigation of certain oxidative stress parameters showed that it induced ROS overproduction and lipid peroxidation and also caused depletion of GSH levels. Further studies concerning their capability to induce DNA damage showed that it was. Therefore, we conclude that BPA was able to induce cytotoxicity via oxidative stress and genotoxicity. This study stated the melatonin protective effect on BPA-induced cytotoxicity and genotoxicity. It is showed that melatonin could abate BPA-induced ROS and MDA increase and relieve DNA damage. On other hand melatonin could compensate the GSH decrease that induced by BPA exposure.
In summary we emphasize the importance of continuing to investigate the beneficial qualities of melatonin due to its unique features including being very nontoxic, inexpensive, readily available and potentially protective pharmacologic agent for preventing cytotoxicity and DNA damage induced by toxic chemicals specially BPA.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- El-Missiry MA, Othman AI, Al-Abdan MA, et al. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J neurolog sci. 2014;347(1- 2):251-6.
- Yamazaki E, Yamashita N, Taniyasu S, et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicology and environmental safety. 2015;122:565- 72.
- Fu P, Kawamura K. Ubiquity of bisphenol A in the atmosphere. Environmental Pollution. 2010;158(10):3138-43.
- Wu G, Song D, Wei Q, et al. Melatonin mitigates bisphenol A-induced estradiol production and proliferation by porcine ovarian granulosa cells in vitro. Animal Reproduction Sci. 2018;192:91-8.
- Olukole SG, Ajani SO, Ola-Davies EO, et al. Melatonin ameliorates bisphenol A-induced perturbations of the prostate gland of adult Wistar rats. Biomedicine and Pharmacotherapy. 2018;105:73-82.
- Elmetwally MA, Halawa AA, Lenis YY, et al. Effects of Bisphenol-A on proliferation and expression of genes related to synthesis of polyamines, interferon tau and insulin-like growth factor 2 by ovine trophectoderm cells. Reproductive Toxicology. 2018;78:90-6.
- Wu H-J, Liu C, Duan W-X, et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2013;752(1-2):57-67.
- Moghadam ZA, Mirlohi M, Pourzamani H, et al. Exposure assessment of Bisphenol A intake from polymeric baby bottles in formula-fed infants aged less than one year. Toxicology Reports. 2015;2:1273-80.
- Ikhlas S, Usman A, Ahmad M. In vitro study to evaluate the cytotoxicity of BPA analogues based on their oxidative and genotoxic potential using human peripheral blood cells. Toxicology in Vitro. 2019.
- Ramos C, Ladeira C, Zeferino S, et al. Cytotoxic and genotoxic effects of environmental relevant concentrations of bisphenol A and interactions with doxorubicin. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2019;838:28-36.
- Anjum S, Rahman S, Kaur M, et al. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food and chemical toxicology. 2011;49(11):2849-54.
- Kobroob A, Peerapanyasut W, Chattipakorn N, et al. Damaging effects of bisphenol a on the kidney and the protection by melatonin: Emerging evidences from In Vivo and In Vitro Studies. Oxidative medicine and cellular longevity. 2018;2018.
- Akarca-Dizakar S, Erdogan D, Peker T, et al. Effects of co-administered melatonin, fructose and bisphenol A (BPA) on rat epididymis and sperm characteristics. Biotechnic and Histochemistry. 2019:1-9.
- Azarova AM, Lyu YL, Lin C-P, et al. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proceedings of the National Academy of Sciences. 2007;104(26):11014-9.
- Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27(1):123- 8.
- Shokrzadeh M, Eshghi Z, Dehpouri AA, et al. Cytotoxic Effects of Fe2O3 Nanoparticle on Cervical Cancer Cell Line (Hela) and Hepatocellular Carcinoma Cell Line (HepG2). Advanced Science, Engineering and Medicine. 2020;12(5):652-6.
- Hercog K, Maisanaba S, Filipic M, et al. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Science of the total environment. 2019;687:267-76.
- Damrot J, N