Original Article
, Volume: 14( 1)Isolation and Characterization of Steroid from N-Hexane Extract of the Stem Bark of Indigofera arrecta
- *Corresponding Author:
- Akabuogu EP, Nigerian Institute of Leather and Science Technology, Samaru-Zaria, Nigeria, Tel: 08034828195; E-mail: beresunify16@gmail.com
Received Date: September 25, 2017; Accepted Date: October 25, 2017; Published Date: October 26, 2017
Citation: Akabuogu EP, Ndukwe GI, Okeh Q, et al. Isolation and Characterization of Steroid from N-Hexane Extract of the Stem Bark of Indigofera Arrecta. Org Chem Ind J. 2017;14(1):120
Abstract
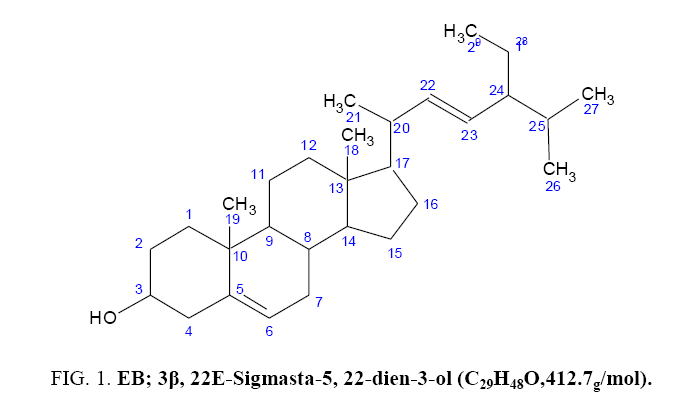
To isolate, characterize and determine the antimicrobial activity of compounds present in the n-Hexane fraction of the stem bark of Indigofera arrecta. Indigofera arrecta was collected, dried and pulverized. The pulverized plant material was exhaustively extracted with methanol, partitioned with different solvents. Based on the most active extract on the microorganisms, the n-Hexane extract was subjected to chromatographic techniques which yielded EB. EB was characterized using IR and NMR spectroscopic techniques. The structure of the isolated compound was established to be 3β, 22E-Sigmasta-5, 22-dien-3-ol (stigmasterol) using spectroscopic analysis. The isolated compound showed significant antimicrobial activity on some selected microorganisms.
Keywords
Indigofera Arrecta; Stigmasterol; 3β; 22E-Sigmasta-5; 22-dien-3-ol
Introduction
Plant is being used extensively in our country as herbal medicine [1], it is necessary to have knowledge of the constituents of the plant of our native species. Numerous applications in traditional medicine have been reported: leaves and roots are used externally to treat itching and in an infusion or decoction as an antispasmodic, sedative, stomachic, febrifuge, vermifuge, abortive, diuretic and purgative, [2] Example to treat gum infections, snakebites, gonorrhea, epilepsy and jaundice; the fruits and seeds are used to treat ophthalmia. In Ghana an aqueous extract of leaves from immature shoots is administered orally to patients with diabetes mellitus [3]. Medicines for the management of peptic ulcer and methods of its preparation and use have been patented. [4]. Previous phytochemical investigations resulted in the isolation of ursolic acid, corosolic acid, 24-hydroxy corosolic acid, maslinic acid and 3b, 7b, 24-trihydroxy-urs-12-en-28-oic acid [5].
Indigofera is a very large genus comprising approximately 700 species and is distributed throughout the tropics and subtropics of Africa, Asia and the Americas. Africa and the southern Himalayas are richest in species. Over 300 species have been recorded for tropical Africa [6]. For indigo production, several Indigofera species are used but there are 3 closely related major ones: Indigofera Arrecta, Indigofera tinctoria L., which probably originates from tropical Asia but is now distributed pantropically and Indigofera suffruticosa Mill., originating from tropical America and now locally cultivated elsewhere in the tropics, including Africa and Madagascar but not in tropical East Africa [7]. The origin and identity of Indigofera plants cultivated for dye production is often obscure as a result of introduction, selection and the close affinity of species. Indigofera Arrecta is sometimes difficult to separate from Indigofera tinctoria (Worldagroforestry, 2016).
Methods
One kilogram of the dried powdered stem bark was macerated in methanol until complete extraction, the solvent were removed in vacuo to yield methanol extracts of 200 g. The methanol extract was partitioned into n-hexane, ethyl acetate and chloroform extracts. A portion of each of the three extracts was subjected to preliminary phytochemical screening using standard methods.
Test for steroids/terpenes
Liebermann-Buchard test: 1 ml of anhydrous acetic acid was added to 1 ml chloroform and cooled to 0°C then one drop of concentrated sulphuric acid was added to the cooled mixture followed by the extract. The solution was observed for blue, green, red or orange color that changes with time [8-16].
Salkowski test: A little quantity of the extract was dissolved in 1 ml chloroform and to it 1 ml of concentrated sulfuric acid was added down the test tube to form two phases. Formation of red or yellow coloration was taken as an indication for the presence of sterols.
Thin layer chromatography was carried out on TLC aluminum sheet silica gel 60 PF254 pre coated with layer thickness of 0.2 mm.
Spots were applied manually using capillary tube; plates were dried using air blower and developed at room temperature using a Shandon chromatotank.
Spots on TLC plates were visualized under UV light (254 and 366 nm) and spraying with 10% sulphuric acid, followed by heating at 110°C for 5-10 min.
The n-Hexane extract of Indigofera Arrecta was subjected to thin layer chromatography using pre-coated aluminum TLC plate and Hexane: Ethyl acetate (1:1) as solvent system to determine the profile of this extract. Two prominent spots were observed after visualization.
7 g of the n-Hexane extract was chromatographed over silica gel packed column of dimension 75 by 3.5 cm, the column was eluted continuously using neat n-Hexane and then n-Hexane: Ethyl acetate mixtures as solvent systems. Eighty four fractions, 100 ml each were collected. The fractions were pooled together based on their TLC profile to give four major fractions (F1, F2, F5 and P1) and the column was finally washed with methanol. Fraction 1 which showed one major spot and some minor impurities washed with methanol, pure white crystal which shows a single spot when spotted and was labeled as compound EB. Compound EB was isolated as a white crystal. EB was subjected to spectroscopic analysis to elucidate its chemical structure Figure 1.
Results
The n-hexane extract of Indigofera Arrecta was subjected to thin layer chromatography using pre coated silica aluminum TLC plate and Hexane: Ethyl acetate (7:3) as solvent system to determine the profile of this extract. Three prominent spots were observed after visualization.
Seven grams of the n-hexane extract was chromatographed over silica gel packed column of dimension 75 by 3.5 cm, the column was eluted continuously using neat n-Hexane and then n-Hexane: Ethyl acetate mixtures as solvent systems, 90 fractions and 100 ml each were collected. The fractions were pooled together based on their TLC profile to give four major fractions and the column was finally washed with methanol to give the fifth major fraction.
Fraction four which showed one major spot and some minor spots were subject to washing using absolute n-hexane to remove some non-polar dirt or impurities. The fraction was further subjected to preparatory TLC using four plates of precoated aluminium sheets 20+20, the areas that contain the single spot were all scratched from the plates and were pooled together and labeled as compound EB (40.1 mg). EB was isolated as a white crystalline solid with the melting point range to be 141°C-145°C, which showed positive to steroids and triterpenes test of Salkowski and Liebermann-Buchard and was obtained from the chromatographic separation of n-Hexane extract. EB was subjected to spectroscopic analysis to elucidate its chemical structure. The structure of EB was analysed using 1HNMR, 13CNMR, Distortion less Enhancement polarization Transfer (DEPT).
Discussion
There are forty eight signals identified from the 1HNMR of compound EB, the signals ranges from 0.6 to 5.2. Signals from 0.6 to 0.1.45 may be due to the saturated hydrogens, the signals from 1.5 to 2.35 may be due to allylic hydrogen, signals at 3.5 is due to hydrogen attached to oxygen (OH), while the signals at 5.2 is due to olefinic hydrogen.
The 13CNMR spectrum show s twenty nine signals, the chemical value of the signals ranges from 11.9 to 140.8. The signals indicated that sample EB has twenty nine carbon atoms which can either be CH3, CH2, CH or C.
The DEPT experiment also revealed the presence of C atoms to be twenty nine in the compound just like the 13CNMR, but this experiment distinguishes the carbon atom into C, CH, CH2 and CH3. From the DEPT it can be seen that we have four quaternary carbons at 138.3 for C5, 37.3 at C10, 42.3 at C13 and 140.8 at C24 respectively. Nine methine carbon atoms (CHs) were observed at 71.8 for C3, 129.8 for C6, 31.9 for C8, 51.2 for C9, 56.9 for C14, 56.8 for C17, 39.7 for C20, 36.5 for C25 and 121.7 for C28. The carbon atoms identified to be CH2 i.e methylene Carbons are C1 at 38.8, C2 at 29.7, C4 at 45.8, C7 at 31.7, C11 at 23.1, C12 at 42.2, C15 at 26.1, C16 at 29.2, C22 at 33.9 and C23 at 36. While the remaining six carbon atoms were all methyl carbons CH3 at 11.9 for C18, 19.8 for C21, 18.8 for C21, 21.2 for C26, 21.1 for C27 and 12.3 for C29 respectively. Therefore, the DEPT experiment revealed the presence of six CH3, ten CH2, nine CH and four quartenary carbon Table 1 and Table 2.
| Position | 13C(ppm) | Lit |
|---|---|---|
| C1 | 38.80 | 37.26 |
| C2 | 29.70 | 31.68 |
| C3 | 71.80 | 71.82 |
| C3 | 71.80 | 71.82 |
| C4 | 45.80 | 42.32 |
| C5 | 138.30 | 140.76 |
| C6 | 129.30 | 121.72 |
| C7 | 31.70 | 31.90 |
| C8 | 31.93 | 31.90 |
| C9 | 51.20 | 51.24 |
| C10 | 37.30 | 36.52 |
| C11 | 23.10 | 21.09 |
| C12 | 42.20 | 39.69 |
| C13 | 42.30 | 42.32 |
| C14 | 56.90 | 56.87 |
| C15 | 26.10 | 24.37 |
| C16 | 29.20 | 28.92 |
| C17 | 56.80 | 55.96 |
| C18 | 11.90 | 12.05 |
| C19 | 19.80 | 21.22 |
| C20 | 40.48 | 40.49 |
| C21 | 21.23 | 21.22 |
| C22 | 138.31 | 138.30 |
| C23 | 129.31 | 129.28 |
| C24 | 50.17 | 50.17 |
| C25 | 31.66 | 31.68 |
| C26 | 21.23 | 21.22 |
| C 27 | 19.40 | 19.40 |
| C28 | 25.40 | 25.41 |
| C29 | 12.30 | 12.25 |
Table 1: Comparison of 13C NMR data of EB with literature data.
| Carbon | Δ(ppm) | CHn |
|---|---|---|
| 1 | 38.80 | CH2 |
| 2 | 29.70 | CH2 |
| 3 | 71.80 | CH |
| 4 | 45.80 | CH2 |
| 5 | 138.30 | C |
| 6 | 129.30 | CH |
| 7 | 31.70 | CH2 |
| 8 | 31.90 | CH |
| 9 | 51.20 | CH |
| 10 | 37.30 | C |
| 11 | 23.10 | CH2 |
| 12 | 42.20 | CH2 |
| 13 | 42.30 | C |
| 14 | 56.90 | CH |
| 15 | 26.10 | CH2 |
| 16 | 29.20 | CH2 |
| 17 | 56.80 | CH |
| 18 | 11.90 | CH3 |
| 19 | 19.80 | CH3 |
| 20 | 39.70 | CH |
| 21 | 18.80 | CH3 |
| 22 | 33.90 | CH2 |
| 23 | 36.10 | CH2 |
| 24 | 140.80 | C |
| 25 | 36.50 | CH |
| 26 | 21.20 | CH3 |
| 27 | 21.10 | CH3 |
| 28 | 121.70 | CH |
| 29 | 12.30 | CH3 |
Table 2: 13C NMR assignment of sample EB.
Summary
The whole plant of Indigofera Arrecta were collected from Makurdi, Benue state having been properly identified at the Herberium unit of department of Biological science, A.B.U Zaria. The stem was subjected to air drying, pulverization and extraction. The crude extracts obtained were further subjected to preliminary phytochemical screening and then antimicrobial activity test. n-Hexane extract being the most active of all, was subjected to chromatographic separations where a compound named EB was obtained, which after undergoing some spectroscopic analysis revealed to be a steroid called Stigmasterol.
Conclusion
Indigofera Arrecta is a plant used in traditional medicine for the treatment of several diseases such as ulcer, sore treatments, epilepsy and as a chief source of blue dyes, etc. preliminary phytochemical screening revealed the presence of some secondary metabolites which are responsible for the observed antimicrobial activity seen against the tested microorganisms. So, the claim made by the local practitioners was verified to be true.
Acknowledgements
I give the greatest thanks and praise to God for his love, mercy and blessings throughout this research work. I appreciate and acknowledge the contributions made by my project supervisor Prof. G. I. Ndukwe for his fatherly and moral support, unending patience, advice, useful suggestions and supervision.
My sincere regards go to my husband Mr. Sunday Akabuogu for his financial, material and moral support. I am greatly indebted to Dr. Sani Muhammed Yahaya of Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Science for his assistance throughout the research. I would like to appreciate Dr. Habila for his assistance in running the NMR spectra analyses in South Africa and helping in elucidation of the structure of compound EB.
My heartfelt thanks goes to my bosom friends Queendalin, Okeh, B. Igiri of NILEST, Mr. Ochie Ochigbo, Mr. Danbata Mubarak, Mr. Nasiru, Mr. John Anya and all others who has been a source of inspiration during this research work.
I am eternally grateful to my beloved children for their love, support and prayers throughout the research work.
References
- RobensonMM, Zhang X. The world medicine situation.Traditional Medicine;Global Situation,Issues and Challenges, 3rd ed,WHO,Geneva; 2011.
- Akpuaka MU. Essentials of natural product. Mason Publishers, Enugu.Frontiers in Natural Product Chemistry. 2009:2-18.
- Lemmens RHMJ.IndigoferaarrectaHochst. Ex A.Rich. PROTA (Plant Resources of Tropical Africa/Resources végétales de lid’Afriquetropicale), Wageningen, Netherlands. 2015.
- Kumar P.Indigo plantations and science in colonial India.Cambridge University Press. 2012:334-42.
- Mazumder K, Siwu ERO, Nozaki S, et al. Ursolic acid derivatives from Bangladeshi medicinal plant, Sauraujaroxburghii: Isolation and cytotoxic activity against A431 and C6 glioma cell lines. Phytochemistry Letters. 2011;4(3):287-91.
- XinfenGao, Brian D.Schrire,abcd flora of China. 2009;10:137.
- Burkill HM. The useful plants of West Tropical AfricaRoyal Botanical Garden, Kew. 1995;4:85-90.
- Brunton LL, Lazo JS, Parker KL.Godman and Gilman pharmacological Basis of Therapeutics McGraw – Hill Medical Publishing Division, New York.J Med Chem. 2006:95.
- Cheesbrough M. District laboratory practice in Tropical countries. Cambridge UniversityPress, USA. 2002:40-45.
- Finar IL.Organic Chemistry, Stereochemistry and the Chemistry of Natural Products. Pearson Education PTC Ltd., Singapore. Journal of Chemical Education. 2005:442-880.
- Khan S, Khan H. New species of Pseudocercospora and Stenella from India. Dictionary of Chemistry. Academic Publishers. India.2006:134-11.
- Olokun AA. Chemistry in national health development research and development of food and medicines from natural sources for primary healthcare in Nigeria. 2000.C.S.N. Abstract, p: 62.
- Hornby AS. Oxford Advanced Learners Dictionary OxfordUniversity Press, Oxford. 2001:871.
- Dey S.Pharmaceuticals from plants. Indian Express Newspaper (Mumbai) Ltd. via Express Pharma.Optics Express.2006.
- Morah FNI. Chemistry and herbal medicine. A paper presented at the plenary session of the 30th International conference of the Chemical Society of Nigeria (CSN), Abuja.pp: 2007:1-8.
- Wikipedia the free Encyclopedia (2009). http://en.wikipedia.org/wiki/Carbon-13NMR. (retrieved 14/4/2009)