Original Article
, Volume: 12( 11)Ionizing Irradiations Impact on Structural Parameters of Chromium (III) - Silicates Base Mica
- *Correspondence:
- Khaled ME , Materials Science Unit, Chemistry Department, Faculty of Science, Tanta University, Tanta 31725, Egypt, Tel: 20 40 3344352; E-mail: khaledelsabawy@yahoo.com
Received: October 25, 2016; Accepted: November 03, 2016; Published: November 07, 2016
Citation: Khaled ME, Morsy MAS. Ionizing Irradiations Impact on Structural Parameters of Chromium (III) - Silicates Base Mica. Environ Sci Ind J. 2016;12(11):126.
Abstract
New family of free-fluoride synthetic clay was synthesized via solution route. All starting solutions were made by applying carbon tetra-chloride (CCl4) as a solvent. The selected sample of synthetic free fluoride–Na-4-mica was having the general formula (Na4Mg6M4Si4O22.nH2O) where M = Cr3+ was exposure to two different ɤ-irradiation doses 1st dose = 1.5 MR/h and 2nd dose = 3 MR/h at 25 cm distance. Structural parameters such as lattice constants, volume and phase quality were monitoring carefully by using both of XRD and SEM evaluating grain size of the mica bulk. Structural investigations proved that Cr-clay exhibits monoclinic phase accompanied with structure quality in comparison with both of Bi-and Al-clays.
Keywords
Synthesis; Mica clay; Free fluoride; XRD; SEM; Structural parameters
Introduction
Recently many studies were carried out to investigate the effects of nuclear irradiations such as gamma irradiations [1,2] on the physico-chemical parameters (degree of crystallinity, specific surface area, cation exchange capacity and main layers charge) "swelling micas." This group of synthetic clays, of which Na-4-mica is only one, were originally developed expressly for water treatment. They expand as they absorb metal ions, then collapse, sealing the metals inside. Na-4-mica is formed by combining kaolinite, a soft clay mineral used in the ceramics industry, with magnesium oxide in sodium fluoride at a temperature of 890ºC. The resulting product has natural mica's sheet like structure and brittle composition, but the space between the layers.
Waste streams encountered in mining operations, and various chemical processing industries, contain heavy metals which are non-biodegradable, toxic priority pollutants. Due to their tendency to accumulate in living organisms, causing various diseases and disorders, the treatment methods for metal-bearing effluents are essential for environmental and human health protection. Among numerous commonly used techniques for water purification, adsorption technologies have gained the most attention because of their low cost and easy operation [3-17].
In recent years, an intensive research was conducted focusing on the selection and/or production of low-cost adsorbents with good metal-binding capacities, which could be utilized as an alternative to the most widely used adsorbent in wastewater treatment-activated carbon. Natural materials of both organic and inorganic nature (such as chitosan, zeolites, minerals, etc.) and certain waste products from industrial operations (such as fly ash, coal and oxides) are classified as low-cost adsorbents because they are economical and locally available [18-29]. Na-4-mica has much the same composition as natural mica, containing aluminum, silicon, and magnesium. But natural mica also contains potassium ions, which sit in hexagonal holes in the mineral's layers, superimposed upon one another, bonding the sheets tightly together. This "closed" structure makes natural mica a poor ion exchange medium [27,30].
The major goal in the present article is to investigate the effect gamma irradiation doses on Structural and micro-structural properties of Na4Mg6Cr4Si4O22.nH2O mica clay sample.
Experimental
Samples preparation
The selected samples of synthetic free fluoride–Na-4-mica which having the general formula (Na4Mg6M4Si4O22.nH2O) where M = Cr3+ was synthesized by applying solution route and sintering procedure using the molar ratios of Na2O.2SiO2.2H2O, MgCO3 and Cr2O3 each of highly pure chemical grade purity. The mixture were ground carefully then disolved in few drops of concentrated nitric acid forming nitrate extract which diluted by water/carbon tetra-chloride 50% volume.
Mixture I was for sodium silicates solution and mixture II was for rest of component (Mg+ Cr) nitrates according to chemical formula desired. Mixture I was diluted by CCl4 to be 100 ml then pH was adjusted to be 8.5 concentrated solution of ammonia was added carefully till heavy white precipitate from Metals hydroxide is obtained and the pH must be higher than 8. The precursor is filtered and washed by 2.5% ammonium nitrate solution. Mixture II of (Mg+ Cr) was passing through the same treatment but in present of ethylene glycol as complexing agent to produce gelatinous precipitate of metals cations hydroxide precursor.
The Mixture I + Mixture II precursors were forwarded to muffle furnace and calcinations process was performed at 880°C under a compressed air atmosphere for 10 hrs then reground and pressed into pellets (thickness 0.2 cm and diameter 1.2 cm ) under 10 Ton /cm2. Sintering was carried out under air stream at 1000°C for 10 hrs. The samples were slowly cooled down (20°C /hr) till 500°C and annealed there for 3 hrs under air stream. The furnace is shut off and cooled slowly down to room temperature. Finally the materials are kept in vacuum desiccator over silica gel dryer.
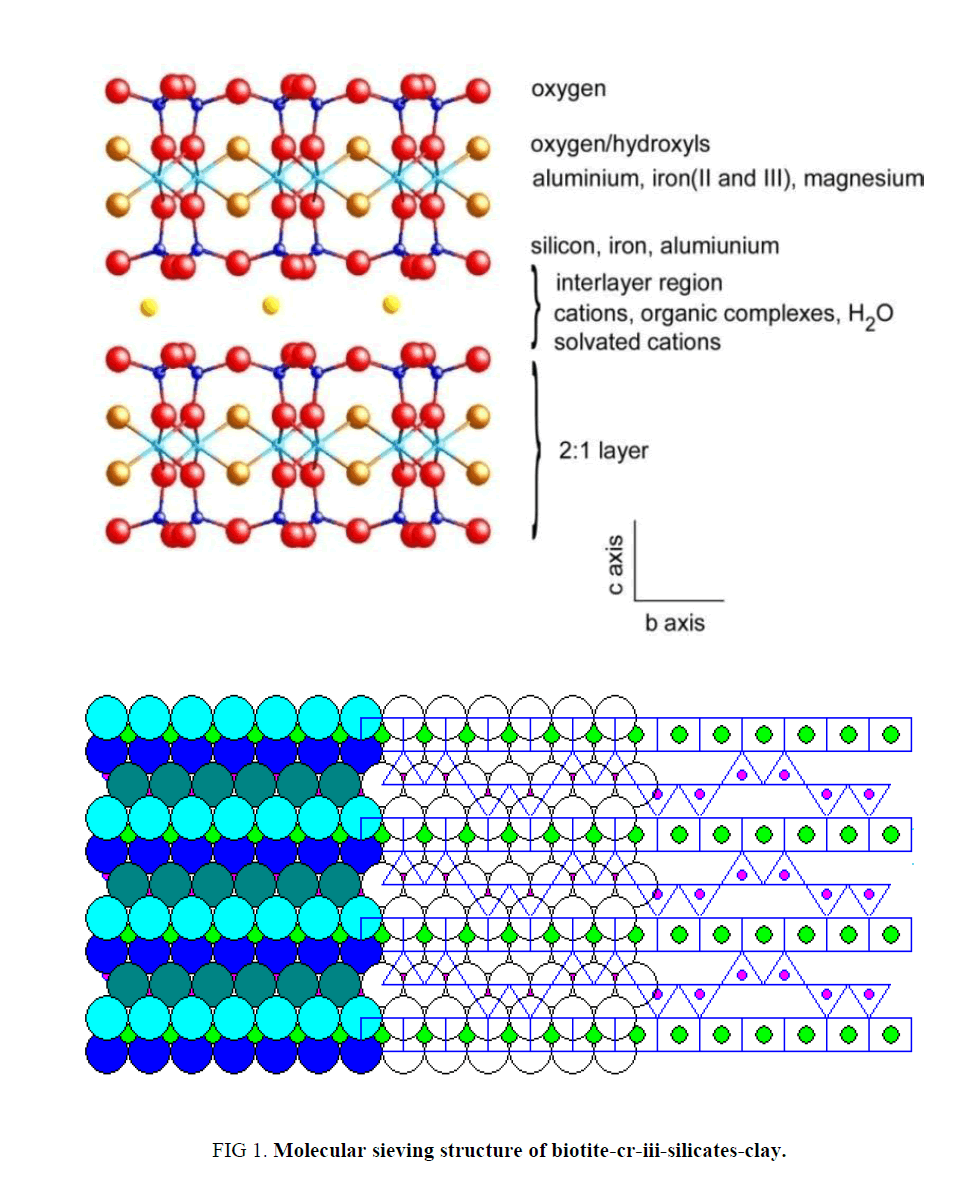
The sample was named as Clay I = (Na4Mg6Al4Si4O22.nH2O ), Clay II = (Na4Mg6Bi4Si4O22.nH2O ) and Clay III = Na4Mg6Cr4Si4O22.nH2O. As described in Figure. 1 tetrahedral units of silicate are the backbone structure of mica clay indicating that each unit cell surrounded by 4-Na-atoms that can be replaced if it is applied as cations exchanger or molecular sieving material.
Samples irradiation
The sample was cut as three-dimensional rectangle and exposure to perpendicular gamma-irradiations source by two different doses 1st dose = 1.5 MR/h at 25 cm distance and 2nd dose = 3 MR/h at 25 cm distance by using Cs137 as gama-ray irradiations source.
Phase identification
The X-ray diffraction (XRD) measurements were carried out at room temperature on the fine ground samples using Cu-Kα radiation source, Ni-filter and a computerized STOE diffractometer/Germany with two theta step scan technique. Scanning Electron Microscopy (SEM) measurements were carried out at different sectors in the prepared samples by using a computerized SEM camera with elemental analyzer unit (PHILIPS-XL 30 ESEM /USA).
Results and Discussion
Phase identification
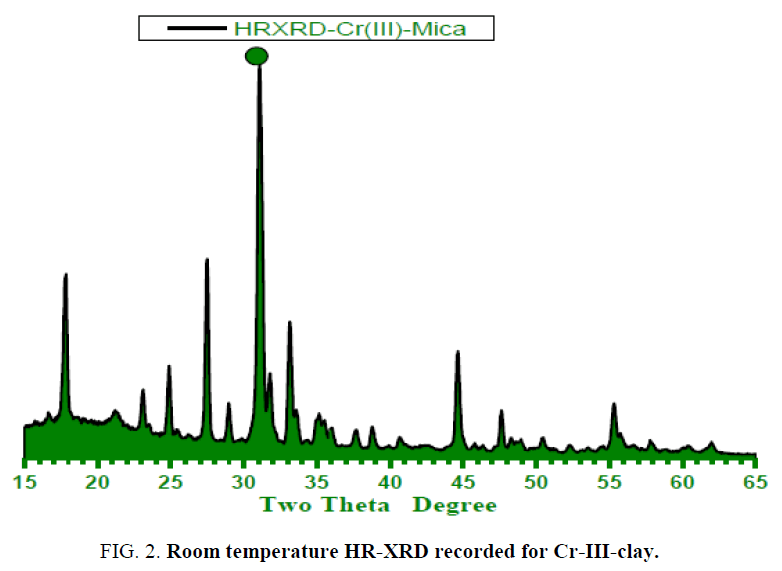
Figure. 2 and 3a-c display the high-resolution X-ray powder diffraction pattern recorded for synthetic free fluoride–Na-4-mica samples which are having the general formula (Na4Mg6M4Si4O22.nH2O) where M = Cr3+. The analysis of the corresponding 2θ values and the interplanar spacings (A°) were carried out using computerized program and indicated that, the X-ray crystalline structure mainly belongs to a monoclinic phase Na4Mg6M4Si4O22.nH2O in major besides few peaks of unreacted starting oxides as secondary phase in minor. The lattice parameters of the unit cell were refined using the least squares sub-routine of a standard computer program these refined lattice parameters were found typically to those reported in Hamdaouia et al.; Khraisheh et al. literatures. These unit cell parameters are in good agreement with those of the reported ones for Na4Mg6M4Si4O22.nH2O structure [3].
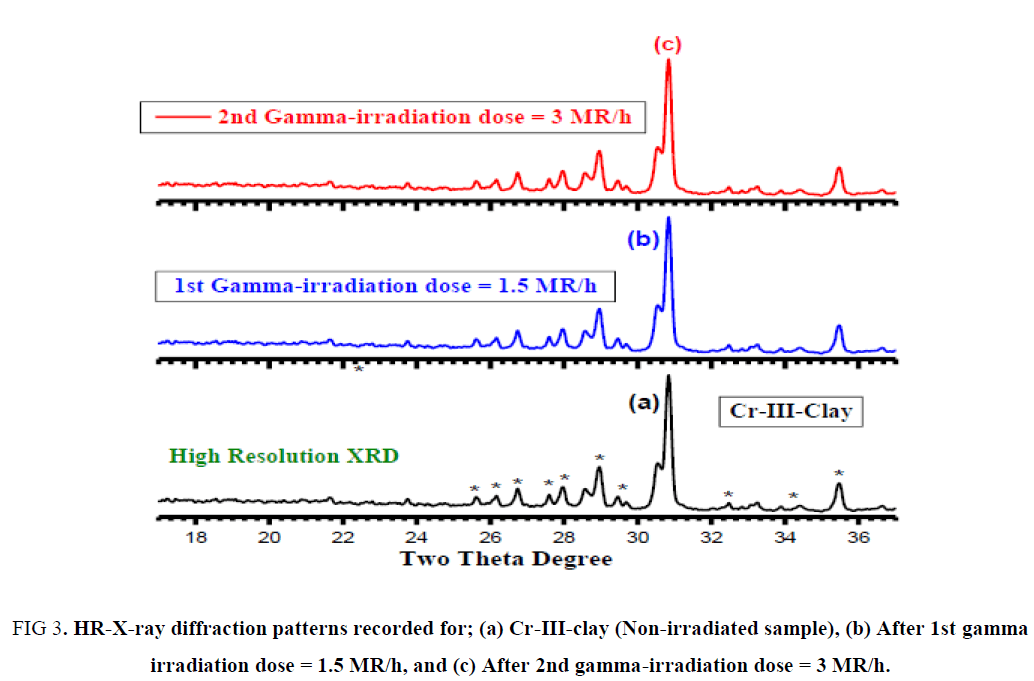
Figure 3: HR-X-ray diffraction patterns recorded for; (a) Cr-III-clay (Non-irradiated sample), (b) After 1st gamma irradiation dose = 1.5 MR/h, and (c) After 2nd gamma-irradiation dose = 3 MR/h.
It is obviously that; gamma irradiation doses have a negligible effect on the main crystalline structure of Na4Mg6Cr4Si4O22.nH2O as shown in Figure. 2b and 2c.
From Figure. 3a and 3b one can indicate that monoclinic phase of mica-clay Na4Mg6Cr4Si4O22.nH2O is the dominating phase by ratio exceeds than 90% (d100 = 1.12-1.13 nm) confirming that gamma-irradiation doses does not affect on the monoclinic biotite phase.
These results are in full agreement with those reported by Allard et al. [29] who are confirmed that radiations effects have negligible on both of structural and chemical properties of clay mineral.
Table 1 explain EDX-elemental analysis data recorded for Na4Mg6Cr4Si4O22.nH2O that prepared via solution route. It is clear that the atomic percentage recorded is approximately typical with the molar ratios of the prepared sample emphasizing the quality of preparation through solution technique.
| Cr-Clay-III | ||||||
|---|---|---|---|---|---|---|
| Element | Wt% | At% | K-Ratio | Z | A | F |
| OK | 37.81 | 57.25 | 0.1069 | 1.7334 | 0.1481 | 1.0804 |
| NaK | 18.21 | 35.13 | 0.0479 | 1.1733 | 0.7353 | 1.0131 |
| MgK | 12.22 | 16.48 | 0.2731 | 1.0746 | 0.9607 | 1.0813 |
| Cr L | 16.66 | 16.65 | 0.0513 | 0.5181 | 1.0718 | 1.1498 |
| Si L | 18.98 | 16.23 | 0.2364 | 0.2175 | 1.0501 | 1.1212 |
Table 1: EDX-elemental analysis data recorded for Cr-clay-III.
On the basis of molar ratio the allowed error in experimental procedures throughout solution rout is lesser than those reported in literatures for those synthesized by solid state route [20,21].
SE-microscopy measurements
Figure. 4 show the SEM-micrographs recorded Na4Mg6Cr4Si4O22.nH2O that prepared via solution route where M = Cr+++. The estimated average of grain size was calculated and found in between 2.37μm-3.63μm supporting the data reported in Khraisheh et al.
The EDX examinations for random spots in the same sample confirmed and are consistent with our XRD analysis for polycrystalline Na4Mg6M4Si4O22.nH2O that prepared via solution route, such that the differences in the molar ratios EDX estimated for the same sample is emphasized and an evidence for the existence of monoclinic-phase with good approximation to molar ratios (Table 1).
From Figure 4, it is so difficult to observe inhomogeneity within the micrograph due to that the powders used are very fine and the particle size estimated is too small.
This indicates that, the actual grain size in the material bulk is smaller than that detected on the surface morphology. Furthermore, particle size was estimated from both of XRD and SEM analyses and its average found to be in between 25-124 nm confirming that solution route synthesis increases the fraction ratio of nano-particles formation.
In our opinion this structure quality enhanced by polar solvent applied (carbon tetra chloride) that prevent coagulation of smaller grains with each other acting as grain size splitter enhancing nano-particle formation as occurred in our results.
The conclusive remarks inside this article can be summarized in the following points:
1. Solution technique applying organic solvent (Polar Solvent) as media matrix exhibits structure quality as preparation technique.
2. Synthetic free-fluoride Na4Mg6M4Si4O22.nH2O crystallize in monoclinic phase.
3. SE-micrographs confirmed that particles size was found in nano-scale ( 25-135 nm ).
4. Gamma-irradiations have a slight effect on both of structural and microstructural parameters.
5. HP-synthetic micas can applied as cations selectivity for water remediation.
References
- Reed DT, Scott DD, Weiner MF (1987) Academic press–Orlando 8: 325-338.
- Plotze M, Kahr G, Hermann R (2003) Alteration of clay minerals—gamma-irradiation effects on physicochemical properties. Applied Clay Science 23: 195-202.
- Komarneni S, Ravella R (2008) Novel clays: Solid-state synthesis, characterization and cation exchange selectivity. Current Applied Physics 8: 104-106.
- Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2006) The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J Hazard Mater 134: 130-139.
- Al-Degs Y, Khraisheh MA, Tutunji MF (2001) Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res 35: 3724-3728.
- Al-Ghouti MA, Khraisheh MAM, Tutuji M (2004) Flow injection potentiometric stripping analysis for study of adsorption of heavy metal ions onto modified diatomite. Chemical Engineering Journal 104: 83-91.
- Allen SJ, Koumanova B (2005) Decolourisation of Water/Wastewater Using Adsorption (Review). Journal of the University of Chemical Technology and Metallurgy 40: 175-192.
- Aytas S, Akyil S, Aslani MAA, Aytekin U (1999) Removal of uranium from aqueous solutions by diatomite (Kieselguhr). Journal of Radioanalytical and Nuclear Chemistry 240: 973-976.
- Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97: 219-243.
- Barrett EP, Youner LG, Halenda P (1951) The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. Journal of the American Chemical Society 73: 373-380.
- Bhattacharyya KG, Gupta SS (2007) Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: influence of acid activation. J Colloid Interface Sci 310: 411-424.
- Camilo C, Carmen G, Paula M (2005) Sorption characteristics of heavy metal ions by a natural zeolite. Journal of Chemical Technology & Biotechnology 80: 477-481.
- Charnock JM, England KER, Farquhar ML, Vaughan DJ (1995) A REFLEXAFS study of metal adsorption on a mica surface. Physica B Condensed Matter 208: 457-458.
- Dantas TN, Dantas Neto AA, Moura MC (2001) Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res 35: 2219-2224.
- Doula MK, Ioannou A (2003) The effect of electrolyte anion on Cu adsorption–desorption by clinoptilolite. Microporous and Mesoporous Materials 58: 115-130.
- Ekmekyapar F, Aslan A, Bayhan YK, Cakici A (2006) Biosorption of copper(II) by nonliving lichen biomass of Cladonia rangiformis Hoffm. Journal of Hazardous Materials 137: 293-298.
- Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280: 309-314.
- Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147: 381-394.
- Ho YS, Porter JF, McKay G (2002) Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water, Air, and Soil Pollution 141: 1-33.
- Kaya A, Oren AH (2005) Adsorption of zinc from aqueous solutions to bentonite. J Hazard Mater 125: 183-189
- Khraisheh MAM, Al-degs YS, Mcminn WAM (2004) Remediation of wastewater containing heavy metals using raw and modified diatomite. Chemical Engineering Journal 99: 177-184.
- Kubilay S, Gürkan R, Savran A, ahan T (2007) Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 13: 41-51.
- Lazarevi S, Jankovi astvan I, Jovanovi D, Milonji S, Janakovi DJ, et al. (2007) Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Applied Clay Science 37: 47-57.
- Lin SH, Juang RS (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 92: 315-326.
- Meunier N, Laroulandie J, Blais JF, Tyagi RD (2003) Cocoa shells for heavy metal removal from acidic solutions. Bioresour Technol 90: 255-263.
- Mishra T, Tiwari SK (2006) Studies on sorption properties of zeolite derived from Indian fly ash. J Hazard Mater 137: 299-303.
- Osmanlioglu AE (2007) Natural diatomite process for removal of radioactivity from liquid waste. Appl Radiat Isot 65: 17-20.
- Panayotova M, Velikov B (2002) Kinetics of heavy metal ions removal by use of natural zeolite. J Environ Sci Health A Tox Hazard Subst Environ Eng 37: 139-147.
- Allard T, Calas G (2009) Radiation effects on clay mineral properties. Applied Clay Science 43: 143-149.
- Madsen FT (1998) Clay mineralogical investigations related to nuclear waste disposal Clay Mineral J 33: 109-129.