Original Article
, Volume: 15( 4)Investigations on Some Divalent Transition Metal Complexes of 10-Membered Tellurium Containing N2S2 Donor Macrocycles
- *Correspondence:
- Garg S, Department of Chemistry, Maharshi Dayanand University, Rohtak, Haryana, India, Tel: 9896091443; E-mail: sapanagarg1511@gmail.com
Received: September 18, 2017; Accepted: October 10, 2017; Published: October 16, 2017
Citation: Kumari S, Verma KK, Garg S. Investigations on Some Divalent Transition Metal Complexes of 10-Membered Tellurium Containing N2S2 Donor Macrocycles. Int J Chem Sci. 2017;15(4):207
Abstract
A new series of complexes has been synthesized by template condensation of 2-aminoethanthiol and diaryltellurium dichlorides, R2TeCl2 (R=p-hydroxyphenyl and 3-methyl-4-hydroxyphenyl) in methanolic medium in the presence of divalent metal chlorides forming complexes of the type [ML1Cl2] and [ML2Cl2] where [M=Mn (II), Co (II) and Cu (II); L1 and L2=10-membered tellurium containing dithiadiaza macrocyclic ligands]. On the basis of elemental analyses, conductance, magnetic moment measurements, 1HNMR, Infrared and electronic absorption spectroscopy their structures have been elucidated. Based on these studies, a six coordinate octahedral geometry for these complexes have been proposed. These metal complexes have also been screened for their antimicrobial activity against some pathogenic bacteria and fungi.
Keywords
2-Aminoethanethiol; Antimicrobial activity; Octahedral geometry; Tellurium containing dithiadiaza macrocycles
Introduction
Coordination chemistry of organotellurium ligands containing hard donors such as nitrogen and oxygen along with soft tellurium is quite interesting as such ligand framework can provide insight into a competitive coordination behaviour between the hard and soft donors towards the metal center [1,2]. Some macrocyclic complexes have received special attention due to their mixed hard-soft donor character and versatile coordination behavior [3] and also due to their pharmacological properties [4].
The macrocyclic complexes have various applications such as dyes and pigments [5], biological activity [4,6,7], precursors in MOCVD processes [8-10], models for biologically important proteins and enzymes [11-16]. Template synthetic route for transition metal complexes of dithiadiaza 16 and tellurium containing tetraaza [17-21] macrocycles have been reported. Due to the growing interest in macrocyclic metal complexes and in continuation of our earlier research work [22,23] we herein report the synthesis, characterization and antimicrobial activity of some 10-membered tellurium containing dithiadiaza macrocyclic complexes of divalent cobalt, manganese and copper.
Materials and Methods
The chemicals used for synthesis and recrystallization were of reagent grade. The solvents, phenol and o-cresol were purified and dried by standard methods [24,25] before use. All the preparations were carried out under an atmosphere of dry nitrogen as the compounds are sensitive to moisture and air.
Preparation of diaryltellurium dichlorides
Bis (3-methyl-4-hydroxyphenyl) and bis (p-hydroxyphenyl) tellurium dichlorides were prepared by reactions of tellurium tetrachloride with o-cresol [26] and phenol [27] respectively, as reported in the literature.
Preparation of metal complexes with 10-membered tellurium dithiadiaza macrocycles (Te2N2 S2M system)
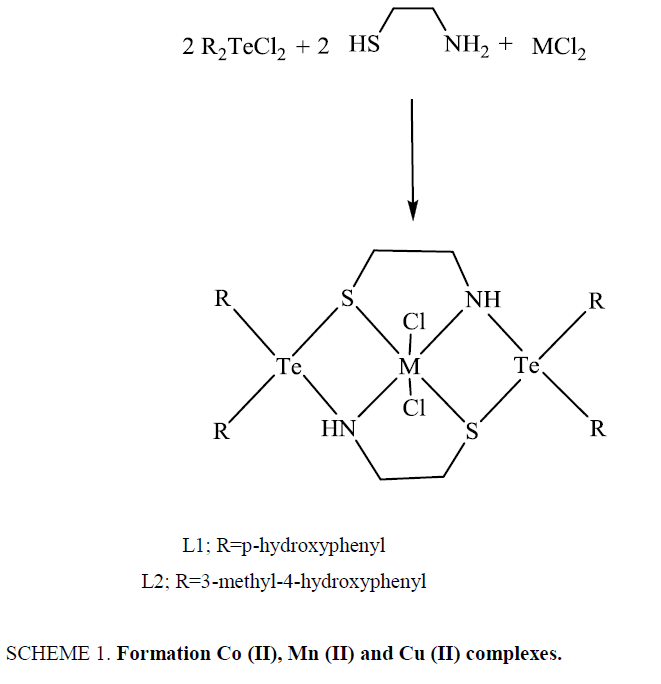
The metal complexes were synthesized by template condensation of diaryltellurium dichlorides and 2-aminoethanthiol in presence of divalent metal chlorides in 2:2:1 molar ratios, as per the procedure given below:
A hot saturated solution (4.0 mmol) of diaryltellurium dichloride in dry methanol was added dropwise with constant stirring to a hot solution (4.0 mmol) of 2-aminoethanthiol in methanol taken in a round bottom flask. An immediate change in colour was observed. The contents were refluxed for 4-5 h, followed by the addition of hot saturated methanolic solution of divalent metal chlorides (2.0 mmol). Again, a change in colour was observed. The mixture was then further refluxed for about 7-8 h. This was kept in refrigerator after concentrating to about one third of original volume to obtain the crystalline coloured product. This was filtered, washed with petroleum ether and dried in vacuum desiccator over P4O10.
Physical studies
Conductance was measured in acetonitrile at 25 ± 2°C using a dip type conductivity cell (cell constant=0.997) on a microprocessor based conductivity bridge type MICROSIL. Electronic spectra were recorded on a UV-VIS-NIR Spectrophotometer; model UV-3600 Plus (SHIMADZU) in BaSO4 at 25 ± 2°C. Magnetic mass susceptibility data were obtained from NPL, New Delhi on a Gouy’s balance (model Johnson Matthey Alfa products) using glycerin as a calibrant. IR (4000 cm-1-400 cm-1) spectra were recorded on Brucker (Alpha), software; OPUS 7.2.139.1294 Spectrometer.
1H NMR Spectra were obtained from SAIF, Panjab University, Chandigarh on BRUKER AVANCE II 400 NMR spectrometer in DMSO-d6 using TMS as reference. C, H, N analyses for these metal complexes were obtained from SAIF, Panjab University, Chandigarh on a Microprocessor based Thermo Scientific (FLASH 2000) CHN Elemental Analyser. Chlorine and tellurium contents were determined volumetrically [28] while cobalt and manganese gravimetrically [28]. Atomic absorption spectrophotometer (ECIL Model No.4129) was used to estimate copper.
Antimicrobial screening
The antifungal and antibacterial activity of the synthesized macrocyclic metal complexes and their precursors were tested against fungal strains: Candida albicans (MTCC 227), Aspergillus niger (MTCC 8189) and Aspergillus fumigatus (ITCC 4517); Gram +ve bacteria: Staphylococcus aureus (MTCC 2901), Bacillus subtilis (MTCC 2063) and Bacillus cereus (MTCC 7350) and Gram -ve bacteria: Escherichia coli (MTCC 1652) and Salmonella typhi (ATCC 15499) using tube dilution method [29] The test and standard compounds were both serially diluted in double strength nutrient broth I.P for bacteria and Sabouraud Dextrose Broth –I.P for fungi [30]. Fluconazole (antifungal) and Cefadroxil (antibacterial) were taken as standard drugs.
Results and Discussion
When tellurium tetrachloride is heated with arene, R-H (o-cresol and phenol) gives corresponding diaryltellurium dichlorides [26,27] as per the equation:
2 R-H + TeCl2 → R2 TeCl2 + 2 HCl
These diaryltellurium dichlorides when heated with 2-aminoethanthiol in presence of CoCl2.6H2O/CuCl2/MnCl2.4H2O in 2:2:1 molar ratios yield the desired complexes as shown in Scheme 1.
The analytical data for these complexes are presented in Table 1. These newly synthesized metal complexes are colored, crystalline solids which are stable in dry air and soluble in polar donor organic solvents.
| Complex | Empirical formula (FW) |
Colour (Yield, %) |
M.P. °C | Analysis found (calculated), % | ?M at ca.10-3 M Scm2 mol-1 in DMSO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Cl | Te | M | |||||
| [CoL1Cl2] | C28H30Cl2N2O4S2Te2Co (907.72) | Green (76) | 140-142 | 36.88 (37.05) |
3.51 (3.33) |
2.88 (3.09) |
7.31 (7.81) |
27.88 (28.11) |
6.12 (6.49) |
37.7 |
| [MnL1Cl2] | C28H30Cl2N2O4S2Te2Mn (903.72) | Light yellow (80) |
120-122 | 37.01 (37.21) |
3.04 (3.35) |
2.89 (3.10) |
7.50 (7.85) |
28.01 (28.24) |
5.88 (6.08) |
26.4 |
| [CuL1Cl2] | C28H30Cl2N2O4S2Te2Cu (927.37) | Blue (68) | 152-154 | 37.79 (37.56) |
3.33 (3.59) |
2.88 (3.02) |
7.42 (7.65) |
27.96 (27.52) |
6.66 (6.85) |
92.9 |
| [CoL2Cl2] | C32H38Cl2N2O4S2Te2Co (963.82) | Dark green (70) |
142-144 | 39.54 (39.88) |
3.79 (3.97) |
2.75 (2.91) |
7.01 (7.36) |
26.17 (26.48) |
5.87 (6.11) |
41.3 |
| [MnL2Cl2] | C32H38Cl2N2O4S2Te2Mn (959.83) | Brown (81) | 135-137 | 39.80 (40.04) |
4.12 (3.99) |
2.68 (2.92) |
7.12 (7.39) |
26.78 (26.59) |
5.44 (5.72) |
29.2 |
| [CuL2Cl2] | C32H38Cl2N2O4S2Te2Cu (968.44) | Light green (74) |
110-112 | 39.34 (39.69) |
3.76 (3.96) |
2.61 (2.89) |
7.14 (7.32) |
26.10 (26.35) |
6.25 (6.56) |
53.6 |
Table 1: Analytical data and physical properties for metal complexes.
Conductance measurement
The molar conductance values for Co (II) and Mn (II) complexes in DMSO at ca. 10-3 M are lower than the ranges reported [31] for 1:1 type electrolytes. These values reflect the partial dissociation of these complexes in this solvent. The molar conductance for CuL2Cl2 is close to those of 1:1 electrolytes and for CuL1Cl2 is higher than those of 1:1 type electrolytes. These two complexes probably ionize into [CuLCl.DMSO]+ and Cl− ions due to the solvation process, since DMSO is known to be a good donor. So, on the basis of conductance studies, these complexes in general may be represented as [MLCl2].
Magnetic moment and electronic spectra
The electronic absorption and magnetic moment data for the complexes are presented in Table 2. Co (II) complexes show magnetic moment 4.65-4.81 B.M. corresponding to three unpaired electrons. Electronic spectrum shows bands at 9398 cm–1–9890 cm–1, 14992 cm–1–15649 cm–1 and 32362 cm–1–32530 cm–1, which may be assigned to the transitions from 4T1g→ 4T2g (F), 4T1g →4A2g (F) and 4T1g (F)→ 4T1g (P) and CT, respectively. The electronic spectral pattern suggests octahedral geometry18,19,32-34 of Co (II) complexes.
| Complex | Band position (cm-1) | Assignments | Observed magnetic moment (B.M.) |
|---|---|---|---|
| [CoL1Cl2] | 9890 14992 32530 |
4T1g → 4T2g (F) 4T1g → 4A2g (F) 4T1g(F) → 4T1g (P) and CT |
4.65 |
| [MnL1Cl2] | 24390 35299 |
6A1g → 4A1g (G) 6A1g → 4A2g (F) and CT |
5.62 |
| [CuL1Cl2] | 12500 26666 33333 |
2B1g→ 2A1g 2B1g→ 2B2g CT |
2.16 |
| [CoL2Cl2] | 9398 15649 32362 |
4T1g→ 4T2g (F) 4T1g→ 4A2g (F) 4T1g(F)→ 4T1g (P) and CT |
4.81 |
| [MnL2Cl2] | 24096 34965 |
6A1g → 4A1g (G) 6A1g → 4A2g (F) and CT |
5.82 |
| [CuL2Cl2] | 12562-32467 | 2B1g → 2A1g 2B1g → 2B2g CT |
1.80 |
Table 2: Magnetic moment and electronic spectral data for metal complexes.
The Mn (II) complexes show magnetic moment corresponding to five unpaired electrons (5.62-5.82 B.M.) at room temperature close to the spin only value of 5.92 B. M. Electronic spectra exhibit two weak absorption bands as shown in Table 3, which are characteristics of octahedral geometry [18,19,21,32-34].
| Complex | vN-H | N-H def. | N-H out of plane bending | vC-N | vC-S | vM-N | vM-S | vM-Cl | vTe-N |
|---|---|---|---|---|---|---|---|---|---|
| [CoL1Cl2] | 3200 m | 1625 m | 826 s | 1172 s | 756 w | 472 w | 338 w | 302 w | 404 w |
| [MnL1Cl2] | 3162* s | 1629 s | 826 s | 1172 s | 755 m | 510 w | 328 w | 328 m | 436 w |
| [CuL1Cl2] | 3121 s b | 1615 s | 825 s | 1173 s | 758 m | 510 w | 325 w | 331 w | 418 w |
| [CoL2Cl2] | 3173 s | 1622 m | 811 s | 1175 m | 759 m | 475 w | 349 w | 314 w | 410 w |
| [MnL2Cl2] | 3100 s | 1633 s | 811 s | 1176 m | 763 m | 516 w | 339 w | 329 w | 434 w |
| [CuL2Cl2] | 3120 m | 1651 s | 812 s | 1175 m | 759 m | 460 w | 321 w | 321 w | 434 w |
m: Medium, b: Broad, s: Strong, w: Weak, *Mixed with nO-H
Table 3: Important IR Data (cm-1) for metal complexes.
Cu (II) complexes show magnetic moment in the range 1.80-2.16 B.M. at room temperature corresponding to one unpaired electron. In these complexes, bands in the region 12500 cm-1-12562 cm-1, 26666 cm-1 and 32467 cm-1-33333 cm-1 may be assigned to 2B1g→2A1g, 2B1g→2B2g and CT bands respectively, corresponding to a distorted octahedral or tetragonal geometry [18,19,21,32,35-39].
Infrared spectra
The important IR data along with their assignments are presented in Table 4. The metal complexes under study did not show any band corresponding to free amino group; instead a new single sharp band appeared in the region 3100 cm-1-3200 cm-1 (sometimes mixed with O-H) assignable to vN-H vibration [19,20,40,41] provide an evidence for the skeleton of the macrocyclic moiety. This was further supported by appearance of medium to strong intensity band at 1615 cm-1–1651 cm-1 and 811 cm-1-826 cm-1 attributed to N-H deformations coupled with N-H out of plane bending vibrations41. Medium to weak intensity bands at ~1175 cm-1 and 460 cm-1-516 cm-1 may be assigned to C-N stretching vibration19,42,43 and M-N stretching16-18,44,45 respectively.
| Complex | Chemical shift, δ ppm |
|---|---|
| [CoL1Cl2] | 1.65 (s, 2H, -NH-), 3.01 (t, 4H, -CH2-S-), 3.18 (t, 4H, -CH2-N-), 6.63d and 7.58d (16H, phenyl), 8.33 (s, 4H, OH) |
| [MnL1Cl2] | 1.99 (s, 2H, -NH-), 2.96* (t, 4H, -CH2-S-), 3.16* (t, 4H, -CH2-N-), 6.49d and 7.37d (12H, phenyl), 9.68 (s, 4H, OH) |
| [CuL1Cl2] | 2.10 (s, 2H, -NH-), 3.00 (t, 4H, -CH2-S-), 3.18 (t, 4H, -CH2-N-) 6.90d and7.76d (16H, phenyl), 8.11 (s, 4H, OH) |
| [CoL2Cl2] | 1.87 (s, 2H, -NH-), 2.79 (t, 4H, -CH2-S-), 2.93 (t, 4H, -CH2-N-) 3.27 (s, 12H, phenyl -CH3), 6.74d, 7.34d and 7.43s (12H, phenyl), 8.9 (s, 4H, OH) |
| [MnL2Cl2] | 1.78 (s, 2H, -NH-), 2.79* (t, 4H, -CH2-S-), 3.12* (t, 4H, -CH2-N-), 2.50* (s, 12H, phenyl -CH3), 6.64d, 7.20d and 7.33s (16H, phenyl), 8.02 (s, 4H, OH) |
| [CuL2Cl2] | 2.03 (s, 2H, -NH-), 3.00 (t, 4H, -CH2-S-), 3.13 (t, 4H, -CH2-N-) 3.41 (s, 12H, phenyl -CH3) 6.81d, 7.57d and 7.66s (12H, phenyl), 8.18 (s, 4H, OH) |
(s: Singlet, d: Doublet, t: Triplet, m: Multiplet); *Poorly resolved.
Table 4: 1H-NMR Spectral Data (δ ppm) for the metal complexes in DMSO-d6.
New weak intensity bands supporting the formation of tellurium containing macrocyclic ring appear at 404 cm-1-436 cm-1 due to Te-N vibrations [18-20,42-46]. Metal complexes show complete absence of the band at around 2400 cm-1 due to S-H vibrations. Two new medium intensity bands in the region 321 cm-1-349 cm-1 and 755 cm-1-763 cm-1 assigned to v(M-S) and v(C-S) vibrations respectively [16,44,47] are consistent with metal-sulfur co-ordination. Further, Nickel complexes show presence of medium to weak intensity bands in the region 302 cm-1-331 cm-1 assigned to v(M-Cl) vibration [16,44,48-50].
Proton magnetic resonance spectra
The proton chemical shifts for the metal complexes in DMSO–d6 are presented in Table 4. Phenyl protons in the metal complexes resonate at slightly upfield side (6.49-7.76 δ ppm) as compared to the parent diaryltellurium dichlorides [26,27] due to replacement of 2 Cl by 2 nitrogen atoms and hence increase in electron density at the tellurium atom. 2-aminoethanthiol, H2N-CH2-CH2-SH, exhibits proton chemical shifts at 1.45 (2H), 2.50 (1H), 2.75 (2H) and 2.87 (2H) δ ppm due to amino, thiol, methylene (adjacent to N) and methylene (adjacent to S) groups respectively [51,52].
A broad singlet in all the complexes at 1.65-2.10 δ ppm assignable to coordinated secondary amino group [16-18] confirms the formation of macrocyclic skeleton. Triplets in all the complexes at 2.95-3.07 and 3.12-3.19 δ ppm (slightly downfield side) may be assigned to (S-CH2; 4H) and (N-CH2; 4H) respectively, of the 2-aminoethanethiol moiety. All the above along with the absence of any band characteristic of NH2 or SH protons, support the proposed macrocyclic framework.
Further, the independence of chemical shifts of aryl protons on the metal ions, hints at non-participation of Te atoms of the macrocycle in coordination with the metal ions. Thus, proton NMR studies support the formation of 10-membered tellurium containing dithiadiaza macrocycles and their quadridentate behavior as predicted by IR studies.
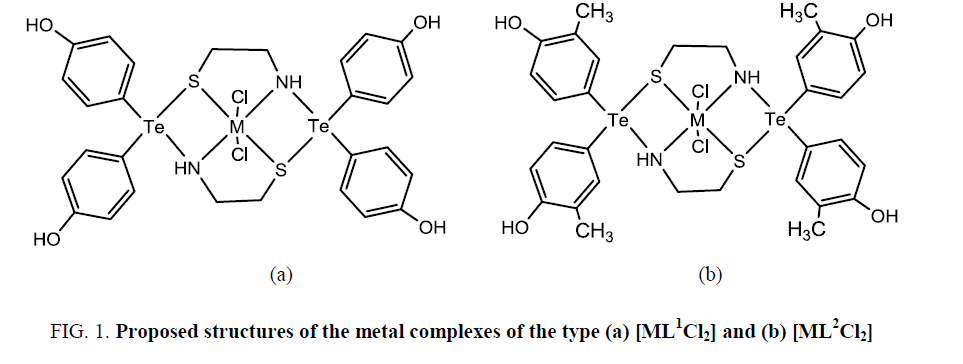
Based on elemental analyses, conductance, magnetic, infrared, electronic and proton magnetic resonance spectral studies, a distorted octahedral geometry involving two N and two S of the dithiadiaza macrocycle and 2 Cl may be proposed for these metal complexes (Figure 1).
Antimicrobial results
The results are compiled in Tables 5 and 6. MIC values indicate that the complexes possess moderate antimicrobial activity as compared to the standard bactericide (Cefadroxil) and fungicide (Fluconazole) but all the complexes show better antimicrobial activity than their precursors and thus indicated that complexation to metal enhances the activity of the ligand. The complex [MnL1Cl2] shows good activity against C. albicans (MIC=6.25) while [MnL2Cl2] is found to be most active against s (MIC=6.25) and C. albicans (MIC=6.25).
| Complex | MIC (µg ml-1) | ||||
|---|---|---|---|---|---|
| Salmonella typhi |
Bacillus subtilis |
Escherichia coli |
Bacillus cereus |
Staphylococcus aureus |
|
| NH2(CH2)2SH | 12.5 | 12.5 | 25 | 12.5 | 12.5 |
| R2TeCl2 (p-hydroxyphenyl) |
25 | 25 | 25 | 25 | 25 |
| R2TeCl2 (3-methyl- 4-hydroxyphenyl) |
25 | 25 | 12.5 | 25 | 25 |
| [CoL1Cl2] | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| [MnL1Cl2] | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| [CuL1Cl2] | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| [CoL2Cl2] | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| [MnL2Cl2] | 12.5 | 12.5 | 6.25 | 12.5 | 12.5 |
| [CuL2Cl2] | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Cefadroxil | 3.12 | 1.56 | 3.12 | 1.56 | 3.12 |
Table 5: Minimum Inhibitory Concentration (MIC) of the complexes against bacteria.
| Complex | MIC (µg ml-1) | ||
|---|---|---|---|
| Aspergillus niger | Aspergillus fumigatus | Candida albicans | |
| NH2(CH2)2SH | 12.5 | 12.5 | 25 |
| R2TeCl2 (p-hydroxyphenyl) |
25 | 25 | 25 |
| R2TeCl2 (3-methyl-4-hydroxyphenyl) |
25 | 25 | 12.5 |
| [CoL1Cl2] | 12.5 | 12.5 | 12.5 |
| [MnL1Cl2] | 12.5 | 12.5 | 6.25 |
| [CuL1Cl2] | 12.5 | 12.5 | 12.5 |
| [CoL2Cl2] | 12.5 | 12.5 | 12.5 |
| [MnL2Cl2] | 12.5 | 12.5 | 6.25 |
| [CuL2Cl2] | 12.5 | 12.5 | 12.5 |
| Fluconazole | 3.12 | 3.12 | 1.56 |
Table 6: Minimum Inhibitory Concentration (MIC) of the complexes against fungi.
Conclusion
Co (II), Mn (II) and Cu (II) complexes have been synthesized by template condensation of diaryltellurium dichlorides with 2-aminoethanthiol in presence of metal chlorides and characterized by elemental analyses, molar conductance, electronic absorption, IR and 1H NMR spectral studies. Based on these studies, a distorted octahedral structure has been assigned to these complexes. Some Mn (II) complexes show good antimicrobial activity against some pathogenic bacteria and fungi while others are moderately active.
Acknowledgement
The authors are thankful to M. D. University, Rohtak for providing the necessary facilities. One of the authors (SK) is also thankful to M. D. University, Rohtak for providing University Research Scholarship. We also thank SAIF, Panjab University Chandigarh for providing the CHN analyses and spectral data.
References
- Jones PG, Carman RD. Arellano, Synthesis of aryl selenides using arylmercurials. Cyclopalladation of Se (R) Ph [R=C6H3(N=NC6H4Me-4′)-2, Me-5]. Crystal structures of Se2R2 and [Pd{C6H3[N=NC6H3(SePh)-2′,Me-4′]-2,Me-5}Cl]. J Chem Soc Dalton Trans. 1996;pp:2713.
- Kienitz CO, Thone C, Jones PG. Coordination chemistry of 2, 2’-dipyridyl diselenide: X-ray crystal structures of PySeSePy, [Zn(PySeSePy)Cl2], [(PySeSePy)Hg(C6F5)2], [Mo(SePy)2(CO)3], [W(SePy)2(CO)3] and [Fe(SePy)2(CO)2] (PySeSePy=C5H4NSeSeC5H4N; SePy=[C5H4N(2-Se)-N, Se]). Inorg Chem. 1996;35:3990.
- Chandra S, Gupta R, Gupta N, et al. Biologically relevant macrocyclic complexes of copper spectral, magnetic, thermal and antibacterial approach. Trans Met Chem. 2006;31:147.
- Chandra S, Gupta LK, Agrawal S. Synthesis spectroscopic and biological approach in the characterization of novel [N4] macrocyclic ligand and its transition metal complexes. Trans Met Chem. 2007;32:558.
- Seto J, Tamura S, Asai N, et al. Macrocyclic functional dyes: Applications to optical disk media, photochemical hole burning and non-linear optics. Pure Appl Chem. 1996;68:1429.
- Nishat N, Din RU, Dhyani S. Synthesis, characterization and antimicrobial activity of a new macrocycle and its transition metal complexes. J Coord Chem. 2009;62(6):996-1004.
- van Veggel FCJM, Verboom W, Reinhoudt DN. Metallomacrocycles: Supramolecular chemistry with hard and soft metal cations in action. Chem Rev. 1994;94:279.
- Steigerwald ML, Sprinkle CR. Organometallic synthesis of II-VI semiconductors. Formation and decomposition of bis (organotelluro) mercury and bis (organotelluro) cadmium compounds. J Am Chem Soc. 1987;109:7200.
- Hirpo W, Dhingra S, Sutorik AC, et al. Synthesis of mixed copper-indium chalcogenolates. Single-source precursors for the photovoltaic materials CuInQ2 (Q=S, Se). J Am Chem Soc. 1993;115:1597.
- Vander Pleog AFMJ, Vander Kolk CEM, Van Koten GJ. Arylmercury (II) compounds involving intramolecular coordination via 2-Me2NCH2- and chiral (S)-2-Me2NCHMe-ring substituents. J Organomet Chem. 1981;212:283.
- Gange RR, Alison JL, Gall S, et al. Models for copper-containing proteins: Structure and properties of novel five-coordinate copper (I) complexes. J Am Chem Soc. 1977;99(22):7170.
- Martin JWL, Johnston JH, Curtis NF. Complexes of 2, 4, 4-trimethyl-1, 5, 9-triazacyclododec-1-ene with cobalt (II), nickel (II) and copper (II); X-ray structure determination of di-isothiocyanato (2, 4, 4-trimethyl-1, 5, 9-triazacyclododec-1-ene) nickel (II). J Chem Soc. Dalton Trans. 1978;pp:68.
- Hughes MN. Inorganic Chemistry of Biological Processes, 2nd ed, Wiley, New York;1981.
- Casella L, Gullotti M, Gioia LD, et al. Synthesis, ligand binding and biomimetic oxidations of deuterohaemin modified with an undecapeptide residue. J Chem Soc Dalton Trans. 1991;pp:2945.
- James SR, Margerum DW. Stability and kinetics of a macrocyclic tetrapeptide complex, tetradeprotonated (cyclo-(beta.-alanylglycyl-.beta.-alanylglycyl)) cuprate (II). Inorg Chem. 1980;19:2784.
- Nasman OSM. N2S2-Donor macrocycles with some transition metal ions: Synthesis and characterization. Phosphorus, Sulfur and Silicon. 2008;183:1541-51.
- Nitu A, Verma KK. Synthesis and characterization of some divalent transition metal complexes with tellurium containing 10-membered tetraazamacrocyclic ligands. J Chem Pharm Res. 2010;2(4):793.
- Nitu A, Verma KK. Study on divalent transition metal complexes with tellurium containing 12-membered tetraazamacrocyclic ligands. Int J Chem Sci. 2011;9(1):229.
- Rathee N, Verma KK. Studies on nickel (II) and palladium (II) complexes with some tetraazamacrocycles containing tellurium. J Serb Chem Soc. 2012;77(3):325-33.
- Srivastava S, Kalam A. Synthesis and characterization of manganese (II), cobalt (II), copper (II), nickel (II), zinc (II) complexes of a tellurium containing tetraaza macrocycle: A photoelectron spectroscopic study. J Indian Chem Soc. 2006;83:563.
- Nitu A, Verma KK. Investigations on some d10 metal-ion complexes with 12-membered tellurium containing tetraazamacrocycles. Der Pharma Chemica. 2011;3(1):97.
- Kumari S, Verma KK, Garg S. Studies on some d10 metal-ion complexes with tellurium containing dithiadiaza macrocycles. Research J Pharma Biol Chem Sci. 2016;7(6):2526.
- Kumari S, Verma KK, Garg S.Synthesis, spectral and antimicrobial studies of some d10 metal-ions ditellura tetraazamacrocyclic complexes.Chem Sci Trans. 2017;6(1):77-86.
- Vogel AI. A Text Book of Organic Chemistry, 3rd ed, Longman, London. 1975.
- Weissberger A. Editor. ‘Technique of Organic Chemistry’, Vol. 7, 2nd ed, Interscience Publishers, Inc. New York. 1967.
- Khandelwal BL, Kumar K, Raina K. Synthesis and characterization of (methylhyinoxyphenyl)-tellurium (IV) halides. Synth React Inorg Met Org Chem. 1981;11(1):65.
- Khandelwal BL, Kumar K, Berry FJ. Hydroxyphenyltellurium (IV) halides. Inorg Chim Acta. 1981;47:135-7.
- Vogel AI. A Text Book of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis, 3rd ed, Longman, London. 1975;324:266.
- Cappuccino JC, Sherman N. Microbiology: A Laboratory Manual’, Addison Wesley, California. 1999;p:263.
- Pharmacopoeia of India. Volume 1, Controller of Publications, Ministry of Health Department, Government of India, New Delhi; 2007. P: 37.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev. 1971;7:81-122.
- Lever ABP. ‘Inorganic Electronic Spectroscopy’, Elsevier, Amsterdam; 1984.
- Figgis BN, Lewis J. The Magnetic Properties of Transition Metal Complexes. In: ‘Progress in Inorganic Chemistry’, John Wiley and Sons Inc. 1964;pp:37-239.
- Chandra S, Gupta K, Sharma S. Synthesis and spectral studies of transition metal complexes with 5, 7, 12, 14-tetramethyl-1, 4, 8, 11-tetraazacyclotetradeca-4, 7, 11, 14-tetraene, a fourteen membered tetradentate macrocyclic ligand. Synth React Inorg Met Org Chem. 2001;31:1205.
- Campbell MJ, Grzeskowiak R. Some Cu (II) complexes of thiosemicarbazide. J Chem Soc. 1967;pp:396.
- Chandra S, Verma S, Meera P. Synthesis and spectral studies of nitrogenoxygen donor macrocyclic metal complexes of Mn (II), Cu (II), Zn (II), Pd (II) and Pt (II). J Indian Chem Soc. 2008;85:896.
- Chandra S, Qanungo K, Sharma SK. New hexadentate macrocyclic ligand and their copper (II) and nickel (II) complexes: Spectral, magnetic, electrochemical, thermal, molecular modelling and antimicrobial studies. Spect Chim Acta part A: Molecular and Biomolecular Spectroscopy. 2012;94:312-7.
- Kumar U, Chandra S. Synthesis, characterization and in vitro anti-fungal screening of manganese (II) and copper (II) complexes of hexaaza [N6] macrocyclic ligand. J Nepal Chem Soc. 2010;pp:25.
- Chandra S. Synthesis, spectroscopic characterization, molecular modelling and antimicrobial activities of Mn (II), Co (II), Ni (II), Cu (II) complexes containing tetradentate aza Schiff base ligand. Spect Chim Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;103:338-48.
- Shakir M, Varkey SP. Synthesis and structural characterization of cobalt (II), nickel (II) and copper (II) complexes of 18-membered mixed donor macrocycles. Polyhedron. 1994;13:791-7.
- Rana VB, Singh P, Singh DP, et al. Divalent nickel, cobalt and copper complexes of tetra-dentate macrocycle, dibenzo (f, n) 2, 4, 10, 12-tetramethyl-1, 5, 9, 13-tetraazacyclohexadeca 1, 3, 9, 11-tetraene. Polyhedron. 1982;1:377.
- Srivastava S, Kalam A. Template synthesis and characterization of 12 and 14-membered pendant-armed tetraazamacrocyclic transition metal complexes: A photoelectron spectroscopic study. Synth React Inorg Met Org Chem. 2004;34:1529.
- Panda AK, Panda A, Sutar S, et al. Synthesis and characterization of transition metal complexes of a 16-membered (N4) macrocycle with tetrapeptide features. J Indian Chem Soc. 2009;86:908.
- Chandra S, Kumar R. Synthesis and spectral studies on mononuclear complexes of chromium (III) and manganese (II) with 12-membered tetradentate N2O2, N2S2 and N4 donor macrocyclic ligands. Trans Met Chem. 2004;29:269-75.
- Reddy PM, Rohini R, Krishna ER, et al. Synthesis, spectral and antibacterial studies of copper (II) tetraaza macrocyclic complexes. Int J Mol Sci. 2012;13:4982-92.
- Kulkarni YD, Srivastava S. Synthesis and characterization of some stable dibenzyltellurium (II and IV) derivatives and their antibacterial activity. Ind J Chem. 1985;22:710.
- Shakir M, Kumar D, Varkey SP. 3-Mercapto-1, 2-propanediol and its 1-methoxy and 1-ethoxy derivatives, HSCH2CH(OH)CH2OR’(R’=H, CH3, C2H5), as uninegative bidentate bridging ligands: synthesis and characterization of dimeric complexes with cobalt, copper and silver. Polyhedron. 1992;11(22):2831.
- Khan S, Nami SAA, Siddiqi KS. Synthesis, spectroscopic and thermal studies of transition metal complexes derived from benzyl and diethylenetriamine. Spect Chim Acta Part A: Mol Biomol Spectroscopy. 2007;68:269-74.
- Patil SA, Kamble UV, Badami PS. Template synthesis, characterization, in vitro antimicrobial and DNA cleavage studies of Co (II), Ni (II), Cu (II) and Zn (II) complexes with 15-membered N2O2 diazadioxa macrocycles. Main Group Chem. 2009;8:189-206.
- Shakir M, Islam KS, Mohamed AK, et al. Macrocyclic complexes of transition metals with divalent polyaza units. Trans Met Chem. 1999;24:577-80.
- Silverstein RM. Spectrometric Identification of Organic Compounds, 4th ed, John Wiley, New York. 1981.
- Pavia DL, Lampman GM, Kriz GS, et al. Spectroscopy. 2007.