Research
, Volume: 19( 9) DOI: 10.37532/0974-7486.23.21.001Interfacial Reactions of Cu-Sn-Ti Boundary Binary Systems
- *Correspondence:
- Guojun Zhou
Department of Mechanical Engineering
Hunan institute of Science and Technology
Hunan, Yueyang, China
Tel: 123456789
E-mail: 87973108@qq.com
Received: August 18, 2022, Manuscript No. tsms-22-72270; Editor assigned: August 22, 2022, PreQC No. tsms-22-72270; Reviewed: September 05, 2022, QC No. tsms-22-72270; Revised: January 03, 2023, Manuscript No. tsms-22-72270; Published: January 11, 2023, DOI:10.37532/0974-7486.23.21.001
Citation: Guojun Zhou, An H Cai, Y Luo, et al. Interfacial reactions of Cu-Sn-Ti boundary binary systems. Mater Sci Ind J. 2023;21(1):001
Abstract
The Cu-Sn-Ti ternary system and its three boundary binary systems are of critical importance in practice. The Ti/Sn and Cu/Sn solid/liquid diffusion couples and the Cu/Ti diffusion couples are made and examined. Only Sn3Ti2 forms if Ti/Sn solid/liquid diffusion couples are annealed at 873 K for 30~160 minutes. When annealed at 808 K for 10 minutes, only Cu3Sn forms in Cu/Sn solid/liquid diffusion couple. With the annealed time increasing, Cu41Sn11 layer forms between Cu and Cu3Sn in 30 mins and then Bcc_a2 layer forms between Cu and Cu41Sn11 in 60 mins. After annealing at 1023 K for 1000 hours, four compounds, CuTi2 , CuTi, Cu4Ti3 and Cu4Ti form in Cu/Ti diffusion couple while Cu3Ti2 is absent. The interfacial reaction process and phase formation sequence has been predicted with the maximum driving force model using the thermo calc software.
Keywords
Diffusion; Intermetallics; Phase diagram; Solid liquid reaction; Diffusion
Introduction
Brazing diamond grits onto a steel substrate in the form of a single layer configuration has been deemed as an effective route to the manufacturing of high performance diamond abrasive tools [1]. When compared with electroplated diamond tools, it is possible to expose higher protrusion heights of diamond grits above the level of the brazing alloy but still achieve stronger adhesion strength between the diamond grits and the brazing alloy. Such a unique characteristic can yield diamond abrasive tools exhibiting higher material removal rates as well as longer tool lives. The success of brazing the diamond grits onto the steel substrate depends on the adhesion strength between the diamond grits and the brazing alloy, which is usually enhanced by the incorporation of active elements, such as Ti, Cr, V and Zr into the brazing alloy [2,3]. These active elements can easily develop interracial compounds with the diamond grits during the brazing operation, which can alleviate the interfacial stress caused by the different crystallographic lattices and thermal expansion coefficients between the diamond grits and the braze matrix. Among various systems, alloys composed of Ag, Cu and a low concentration of active Ti (less than 5 wt pct) are commonly used as the brazing alloys for the diamond and the other ceramic materials [4-8].
In fact, segregation of Ti atoms to the interfacial area was widely recognized in several prior reports, not only in the brazing of diamond, but also in the brazing of various oxide carbide and nitride ceramic materials. Such interfacial layers were widely recognized to be beneficial to the adhesion strength between the braze matrix and the ceramic materials. Nevertheless, excessive development of the interfacial reaction layers can lead to the formation of defects, which inevitably impart weakening to the bond [9,10].
Alloys based on Cu-Sn-Ti are effective alternatives to Cu-Ag-Ti alloys for the processing of single layered brazed diamond tools and metal bonded diamond tool bits [11]. Both tool life and cutting speed have been increased. Up to now Cu-Sn-Ti filler metals show the best combination of properties, especially due to their relatively high strength and erosion resistance if compared to Ag-Cu alloys and their lower melting point if compared to Ni-base ones [12]. In the concentration range defined, the concentration of Ti dissolving in Cu-Ag-Sn-Ti alloys decreased with increasing Ag concentration, but increased with increasing Sn concentration. The addition of Sn not only could reduce the melting temperatures of these alloys, but also could promote the dissolution of a higher concentration of Ti into the liquid phase. The coexistence of high concentrations of Sn and Ti in the liquid phase can possibly cause precipitation of reinforcing Sn-Ti intermetallic phases in the braze matrix during cooling. Based on the previous analyses, the alloying concentration of Ti in the brazing alloy can be increased and the hardness of the braze matrix can be enhanced with the substitution of Sn for Ag.
In order to predict which compound will form first, the nucleation activation energy for each compound should be compared. The nucleation activation energy depends on the driving force and the interface energy, but information on the interface energy is lacking. Besides, the interface energy, which is experimentally determined based on the macroscopic concept, tends to be modified for a smaller cluster such as the critical nucleus. For practical purposes, the driving force can be an approximate criterion to predict the first forming compound.
Materials and Methods
Preparation of Sn/Ti liquid/solid diffusion couples: Ti plate (99.9 wt%) and Sn ingot (99.999 wt%), were used as starting, materials. Small pieces of Sn and Ti were cut off and the surfaces of each piece were polished metallographically and cleaned with ultrasonic. Each pair of Sn and Ti was sealed in an evacuated quartz tube filled with argon (to avoid floating of Ti in molten Sn, Ti pieces were fixed in the tubes with Mo wires).
Place the tubes into annealing furnace vertically, subsequently rise the temperature to 873 K and make Sn pieces molted. Shake the tubes to make Ti pieces merged into molten Sn and Sn/Ti liquid/solid diffusion couples were obtained. The tubes were kept at 873 K for 30 and 160 mins.
Preparation of Cu/Sn solid/liquid diffusion couples: Cu plate (99.97 wt%) and Sn ingot (99.999 wt%), were used as starting materials. The diffusion couples were made with the similar progress as making Sn/Ti diffusion couples. The annealing temperature was 808 K and the annealing time were 10, 30 and 60 mins respectively.
Preparation of Cu/Ti diffusion couple: Cu and Ti pieces of 5 mm × 8 mm were cut off and diffusion welded at 1023 K, 4 MPa pressure for 15 mins, sealed in an evacuated quartz tube filled with argon and annealed at 1023 K for 1000 h.
The tubes were quenched in water and broken. The taken out samples were mounted, grounded and polished metallographically. Then the samples were analyzed with Scanning Electronically Microscope (SEM) and EPMA (JEOL JXA-8800R), in order to determine the formed intermetallic compounds.
Results and Discussion
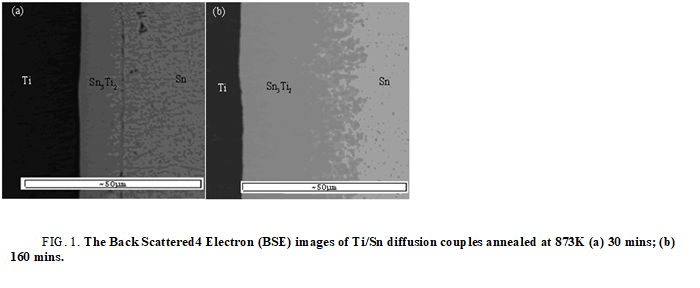
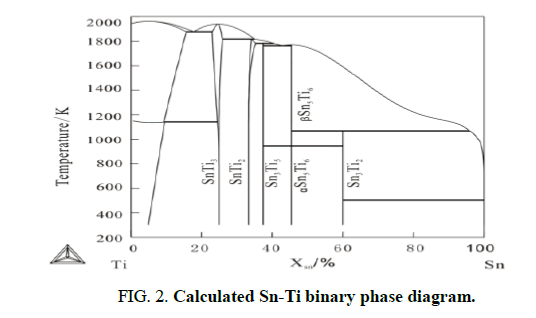
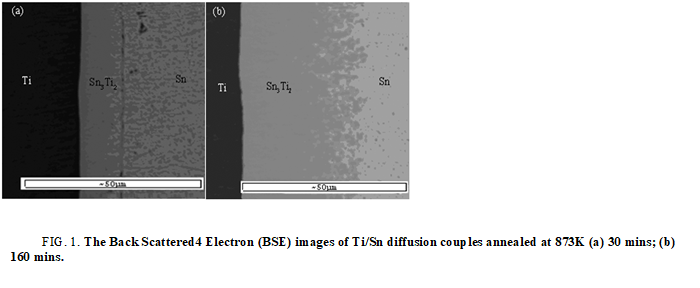
The first phase forms in Ti/Sn diffusion couple: Based on the former literature, Kuper found a new binary phase Sn3Ti2. Based on work of workgroup member Liu Chunlei’s work, the author reevaluated the Ti-Sn binary phase diagram, as shown in Figure 2. From Ti-Sn binary phase diagram, there are five binary compounds, SnTi3, SnTi2, Sn3Ti5, Sn5Ti6 and Sn3Ti2, exist stably at 873 K. Figure 1 shows the Backscattered Electron (BSE) images of Ti/Sn diffusion couples annealed at 873 K. The EPMA result shows there is only Sn3Ti2 a form at the interface (Figure 2 and Table 1).
FIG 1: The Back Scattered4 Electron (BSE) images of Ti/Sn diffusion couples annealed at 873K (a) 30 mins; (b) 160 mins.
| Metastable equilibrium | Hcp (Ti)+liquid (Sn) | ||||
|---|---|---|---|---|---|
| Phases stable at 873 K | Sn3Ti2 | Sn5Ti6 | Sn3Ti5 | SnTi2 | SnTi3 |
| Driving force (KJ/mol) | 4996.27 | 3242.93 | 1847.19 | 211.21 | -897.10 |
Table 1. The formation driving force of the intermetallic compounds under the metastable equilibrium of hcp (Ti)+liq (Sn) at 873 K.
The formation driving force of the intermetallic compounds under the metastable equilibrium of hcp (Ti)+liq (Sn) at 873 K are listed in Table 1. As can be seen in table, at 873 K, under the metastable equilibrium state of hcp (Ti) and liquid, the phase with the highest formation driving force is Sn3Ti2. According to largest driving force criteria, the first forming phase should be Sn3Ti2 and the theoretical calculated results agree well the experiment results (Figure 3).
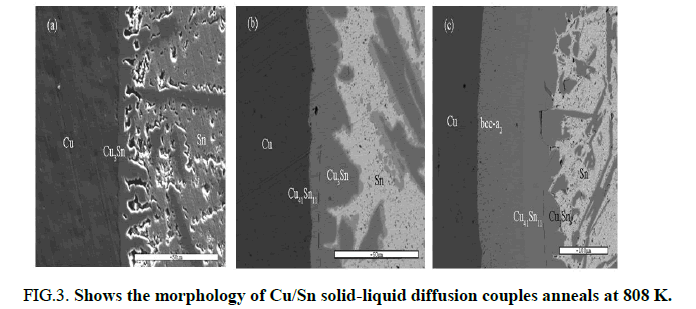
FIG 3:Shows the morphology of Cu/Sn solid liquid diffusion couples anneals at 808 K.
The formation sequence in Cu/Sn diffusion couples
From Figure 3, it can be seen that after annealing for 10 mins, there is only Cu3Sn forms in the couple. With the annealing time increasing to 30 mins, Cu41Sn11 appears in the diffusion layer between Cu and Cu3Sn. After annealing for 60 mins, another phase bcc a2 between Cu and Cu3Sn can be detected.
BSE image of Cu/Sn diffusion couple annealed at 808 K. The types of phase are detected with EPMA. The interface between Cu41Sn11 and Cu3Sn are indicated in dashed lines (a) -10 mins (b) -30 mins (c) -60 mins.
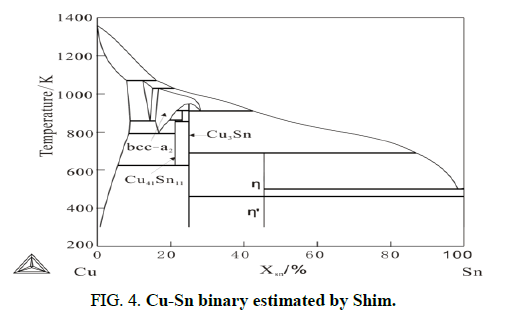
In this work, the thermodynamic parameters estimated by shim are applied for calculating. The calculated Cu-Sn binary phase diagram is shown in Figure 4. From Cu-Sn binary phase diagram, the phases exist stably at 808 K are Cu3Sn, Cu41Sn11 and Bcc_a2. There formation driving forces are listed in Table 2.
| Phase | Driving force (J/mol) | ||
|---|---|---|---|
| Cu+ liquid (Sn) | Cu+ Cu3Sn | Cu+ Cu41Sn11 | |
| Cu3Sn | 105.01 | 0 | -151.22 |
| Cu41Sn11 | -977.44 | 114.12 | 0 |
| Bcc_a2 | -202.32 | 104.51 | 31.69 |
Table 2. The formation driving force of the intermetallic compounds in Cu-Sn system at 808 K.
As can be seen in this table, under the metastable equilibrium state of liquid and fcc-Cu, the phase with the highest driving force of formation is Cu3Sn. According to largest driving force criteria, the first formatting phase should be Cu3Sn and this can be seen in Figure 3. According to essential rules of thermodynamics and diffusion couple approach, formation of any phase must combining with decrease of Gibbs Energy, it means phases with negative driving force cannot form. However, after Cu3Sn appearing, diffusion couple made of Cu and Cu3Sn are obtained. According to the driving force of other phases under metastable equilibrium Cu+Cu3Sn, which are listed in table, both formation driving forces of Cu41Sn11 and Bcc_a2 are positive and that Cu41Sn11 is larger. Therefore, after Cu3Sn, the second phase forms in diffusion couple should be Cu41Sn11 and the phase appears at last should be Bcc_a2. As illustrated in Figure 3, the theoretical calculated results agree well with the experiment results (Figures 5 and 6).
FIG 5:BSE image of Cu/Ti diffusion couple annealed at 1023 K for 1000 hours.
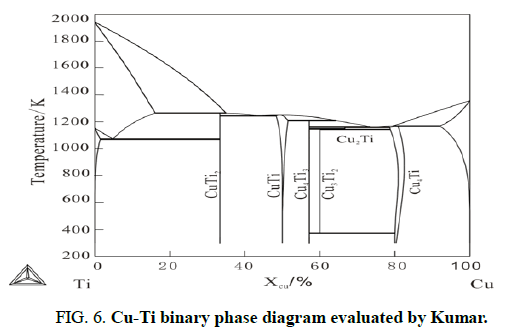
Figure 6 shows the Cu-Ti binary phase diagram evaluated by Kumar. From the phase diagram, it can be seen there are 5 stable binary compounds in this system at 1023 K. However, Figure 5 shows the morphology of Cu/Ti diffusion couple annealing at 1023 K for 1000 h, there are four compounds CuTi2, CuTi, Cu4Ti3, Cu4Ti3, appears in the sample, while Cu3Ti2 is absent.
To explain the reason that Cu3Ti2 does not appear when all other stable phase exist, the formation driving force of intermetallic compounds are calculated and listed in Table 3. Meanwhile, the metastable have no relations with this experiment result are not given.
| Phase | Driving force (KJ/mol) | ||
|---|---|---|---|
| Fcc (Cu)+hcp (Ti) | Fcc (Cu)+CuTi2 | Fcc (Cu)+Cu4Ti3 | |
| CuTi2 | 5528.09 | 0 | -4889.85 |
| CuTi | 3553.86 | 2834.4 | -699.41 |
| Cu4Ti3 | 1725.91 | 3069.65 | 0 |
| Cu3Ti2 | 809.34 | 2977.70 | 126.48 |
| Cu4Ti | -2608.37 | 1998.27 | 724.38 |
Table 3. The forming driving force of the intermetallic compounds in Cu-Ti system at 1023 K.
The phase absent in Cu/Ti diffusion couple
Combining Cu-Ti binary phase diagram and table, we can deduce the reacting process of Cu/Ti diffusion couple is in this way. At 1023 K, under the metastable equilibrium of fcc (Cu)+hcp (Ti), the phase CuTi2 with maximum formation driving force forms firstly. According the binary phase diagram, the equilibrium between Ti and Cu3Ti2 is stable (there is no other stable phase between them), so the equilibrium will no longer being considered. The next step should be considering the metastable equilibrium Cu+Cu3Ti2 and the intermetallic phase with largest driving force is Cu4Ti3. Once Cu4Ti3 forms between Cu and Cu4Ti3, other two metastable equilibria appear. Because the composition of Cu3Ti2 locates between Cu and Cu4Ti3 and the compound, Cu3Ti2, between CuTi2 and Cu4Ti3 has formed in the sample, there is only one metastable equilibrium, Cu+Cu4Ti3, needs further study. As indicated in this table, the two phases between Cu and Cu4Ti3 are Cu3Ti2 and Cu4Ti and both of them have positive driving force. However, the formation driving force of Cu4Ti is larger than that of Cu3Ti2. Until now, it is explain theoretically that Cu3Ti2 is the phase forms at last in Cu-Ti binary system. In addition, it is the very reason for the absence of Cu3Ti2 when all of CuTi2, CuTi, Cu4Ti3 and CuTi appear.
Conclusion
Only Sn3Ti2 forms if Ti/Sn solid/liquid diffusion couples are annealed at 873 K for 30~160 mins. When annealed at 808 K for 10 mins, only Cu3Sn forms in Cu/Sn solid/liquid diffusion couple. With the annealed time increasing, Cu41Sn11 layer forms between Cu and Cu3Sn in 30 mins and then bcc-a2 layer forms between Cu and Cu41Sn11 in 60 mins. After annealing at 1023 K for 1000 hours, four compounds, CuTi2, CuTi, Cu4Ti3 and Cu4Ti3 form in Cu/Ti diffusion couple while Cu3Ti2 is absent.
The interfacial reaction process and phase formation sequence has been predicted with the maximum driving force model using thermo calc software.
Acknowledgement
This work was supported by the science foundation of Hunan province (Grant No. 2020JJ4335).
References
- Yamazaki T, Suzumura A. Role of the reaction product in the solidification of Ag-Cu-Ti filler for brazing diamond. J Mater Sci. 1998;33(5):1379-1384. [Google Scholar]
- Scott PM, Nicholas M, Dewar B, et al. The wetting and bonding of diamonds by copper base binary alloys. J Mater Sci. 1975;10(11):1833-1840.
- Evens, M. Nicholas PM. The wetting and bonding of diamonds by Copper-Tin-Titanium Alloys. Ind Dia Rev. 1977;37:306. [Google Scholar]
- Suzumura A. Yamazaki T. Solidification Phenomena and bonding strength at the interface of diamond and active metal brazing filler: study on the bonding of diamond to metals. J Jpn Weld Soc. 1994;12(4):509-514. [Crossref]
- Chattopadhyay AK, Chollet L, Hintermann HE, et al. On performance of brazed bonded monolayer diamond grinding wheel. CIRP Annals. 1991;40(1):347-350. [Crossref] [Google Scholar]
- Loehman RE, Tomsia AP. Wetting and joining of mullite ceramics by active metal braze alloys. J Am Ceram Soc. 1994;77(1):271-274. [Crossref] [Google Scholar]
- Paulasto M, Kivilahti JK. Formation of interfacial microstructure in brazing of Si3N4 with Ti activated Ag-Cu filler alloys. Scripta Metall Mater. 1995;32(8):1209-1214. [Crossref]
- Lee HK, Hwang SH, Lee JY, et al. Effects of the relative contents of silver and copper on the interfacial reactions and bond strength in the active brazing of SiC. J Mater Sci. 1993;28(7):1765-1774.[Google Scholar]
- Hsieh YZ, Chen JF, Lin ST, et al. Pressure less sintering of metal bonded diamond particle composite blocks. J Mater Sci. 2000;35(21):5383-5387.[Google Scholar]
- Li WC, Lin ST, Liang C, et al. Interfacial segregation of Ti in the brazing of diamond grits onto a steel substrate using a Cu-Sn-Ti brazing alloy. Metall Mater Trans. 2002;33(7):2163-2172. [Google Scholar]
- Massalski TB. Binary alloy phase diagrams. ASM Int. 1990;12. [Crossref]
- Shim JH, Oh CS, Lee BJ, et al. Thermodynamic assessment of the Cu-Sn system. Int J Mater Res. 1996;87(3):205-212. [Crossref] [Google Scholar]