Original Article
, Volume: 7( 3)Innovative Green Thermosetting Composite of Embedding Calcium Sodium Alumino Silicate from Eggshells in Polyurethane
- *Correspondence:
- Tangboriboon N , Materials Engineering Department, Faculty of Engineering, Kasetsart University,Bangkok 10900, Thailand, Tel: 662 797-0999; E-mail: fengnnpt@ku.ac.th
Received: July 29, 2016; Accepted: August 13, 2016; Published: August 19, 2016
Citation: Tangboriboon N, Mulsow L, Sirivat A. Innovative Green Thermosetting Composite of Embedding Calcium Sodium Alumino Silicate from Eggshells in Polyurethane. Res Rev Polym. 2016;7(3):104.
Abstract
Calcium sodium aluminosilicate (CaNaAlSi2O7) powder was prepared from eggshells as a calcium carbonate (CaCO3) source to react with fumed silica (SiO2) and sodium aluminosilicate via the sol-gel process at room temperature. The obtained CaNaAlSi2O7 ceramic particles were used as a dispersed phase and loaded into the polyurethane (PU) foam matrix to make the thermosetting composites. The CaNaAlSi2O7 powder has high specific surface area 38.89 m2g-1 and fine particle size 13.27 μm. While PU foams are low specific surface area 1.00 m2g-1 and lightweight. When adding 10 vol% CaNaAlSi2O7 powder into PU, the thermosetting composite has high specific surface area 6.0 m2/g, lightweight, bulk density 0.213 gcm-3 ± 0.012 gcm-3, high decomposition temperature up to 790°C, and high compressive Young’s modulus 346.0 kPa ± 76.5 kPa. The CaNaAlSi2O7/PU thermosetting composites are good physical-thermal-mechanical properties and good microcellular interconnecting cells. The green CaNaAlSi2O7/PU thermosetting composites are potentially candidate materials suitable for using in various applications i.e., construction, catalysts, insulation, adsorbents, and friendly environmental materials.
Keywords
Polyurethane; Eggshell; Thermosetting composite; Sol-gel process; Calcium sodium aluminosilicate; Bio-materials; Composite foam
Introduction
At the present, the green industries and environmental consciousness are concerned gradually. Sustainable development and eco-efficiency are focused to the majority of every country [1-4]. Most of researchers pay attention to use the bio-materials, green materials, nontoxic materials, and recycle materials i.e., biological wastes, bio-fibers, industrial wastes, and biodegradable materials as a starting material to do researches [1-4].
Eggs are one of the most complete foods as they contain protein, lipid, and carbohydrates which are essential for a good diet. They also contain vitamins and mineral elements which are necessary for the development of young and elderly people. Egg and its derivative are one of important raw materials to make food, drug, bakery, and cosmetic industries i.e., for manufacturing bread, cakes, crackers, ice creams, and food additive. Eggshell as by products provides approximately 11 wt% of the total weight (65 g to 70 g per egg). The main composition of eggshell composed of more than 96 wt% calcium carbonate (CaCO3), 1 wt% magnesium carbonate (MgCO3), 1 wt% calcium phosphate (CaPO4), and 2 wt% other organic matters. Therefore, in order to maximize the recycling opportunities for eggshells, reduce eggshell wastes, conserve the environment without pre-treatment, and increase agricultural evaluation are considered to use as an effective biomaterial. It is estimated that eggshell waste amounts are many millions tons per day from hen, duck, and bird eggs. Thus, an efficient eggshell recycle or disposal method is required. Eggshells are porous ceramic materials because the developing embryos require gases both oxygen and carbon dioxide to breath in and out by diffusion through the pores. Mostly, the structure of eggshells composed of micro-pore functioned as catalysts or adsorbents [5-9].
Calcium sodium aluminosilicate (CaNaAl2Si2O7) is one kind of aluminosilicate compounds made from calcium carbonate (CaCO3) of eggshell to react with fumed silica (SiO2) and sodium aluminosilicate via the sol-gel process at room temperature. Calcium sodium aluminosilicate can be used as a starting material in many industries i.e., petroleum and petrochemical, pharmaceutical, agricultural, food, and beverage. Aluminosilicate compounds are obtained from both natural and synthesis process. CaNaAl2Si2O7 composed of hydrated aluminosilicate of alkaline and alkaline-earth metals, has been produced as a heterogeneous catalyst in a catalytic converter [10-13]. However, the aluminosilicate production process has a limitation due to the optimum stoichiometry. Thermosetting composites are one of polymer composites and a class of cellular materials that possess distinctive mechanical, physical, and thermal properties.
Thermosetting composites can be divided into 3 types: flexible, semi-rigid, and rigid foams depending on their chemical compositions and degree of crosslink [14,15]. Foams can be further classified into two categories: closed-cell walls and open-cell walls. The closed-cell walls are cell walls enclosed completely by a dispersed gas. The open-cell foams are unconfined and connected by open passages [15]. In general, polymer foams can be made from polyurethane (PU), polystyrene (PS), polyolefin-polyethylene (PE) and polypropylene (PP), poly vinyl chloride (PVC), and polycarbonate (PC). Polyurethane foams are thermosetting containing a urethane linkage (–NH–CO–O–) created by a reaction of di-isocyanates with compounds cooperate an active hydrogen, namely diol. The flexible PU foams have excellent elastic deformation and recovery characteristics that make them suitable for packaging materials, comfort cushions, bed mattresses, carpet backing and resilient floor covering. Another kind of PU foam is rigid PU foam. It has a complete combination having high strength, low weight, and good heat resistance. Rigid PU foams are mostly used in sandwich structures such as wind turbine blades, nautical structures, thermal insulation, and acoustic insulation [15-17]. Therefore, in this study needs to develop composite foams with light-weight, good mechanical- physical-thermal properties, a great deal of attention has been focused on PU polymer composites loaded with aluminosilicate oxide (CaNaAl2Si2O7) nanoparticles made from eggshells via sol-gel process. The obtained PU thermosetting composites are potentially useful for various applications.
In this work, the objective is to prepare calcium sodium aluminosilicate oxide (CaNaAl2Si2O7) nanopowder from eggshells via the sol-gel process with a molar ratio of CaO:Al2O3:Na2O:SiO2 equal to 1:1:2:8 embedded into PU matrix. The ceramic/polymer (CaNaAl2Si2O7/PU) composites are formed by the direct-foaming technique. The fabricated CaNaAl2Si2O7/PU composite foams were characterized microstructures by the scanning electron microscopy (SEM), specific surface area by Brunauer-Emmett-Teller (BET), true and bulk density according to the ASTM D3575 and D1056. Furthermore, compressive strength and thermal testing are also carried out and the results are reported here.
Experimental
Materials
Chicken eggshell was cleaned with tap water, dried, and fired at high temperature to transform phase and composition from CaCO3 to calcium oxide (CaO) via pyrolysis process. Hydrophilic fumed silica (SiO2) was supplied by WACKER Chemie AG, Germany, having high silica purity 99.8%, specific surface area 206 m2g-1, pH 3.9, and average particle size 40 μm. Precipitated sodium aluminosilicate was purchased from United Silica (Siam) Ltd., Thailand, composed of 82% silica, 10% alumina, 6% sodium oxide, and 0.04% iron oxide. Precipitated sodium aluminosilicate has specific surface area 75 m2g-1, drying loss 5.2%, pH 10.0, bulk density 200 kgm-3, and average particle size 45 μm. The 98% pure sodium hydroxide pellets were purchased from Molecule Co., Ltd., Thailand. Hydrochloric acid (HCl; Analytical Reagent grade, AR) was purchased from Lab-Scan Co. Ltd., Thailand. Di-isocyanate or methylene diphenyl diisocyanate (MDI)-based on pre-polymer type (SUPRASEC® 2749) has density 1.2 gcm-3, and polyether polyol consists of ethane–1–2–diol, diethylene glycol, extenders, catalysts, and additives (DALTOPED® FF 45084) obtained from Huntsman Belgium (BVBA), Belgium.
Instrument
A laboratory muffle furnace (Linn High Thermo GmbH, LM 412.27, DC021032 with thermocouple type K, NiCr-Ni) was used to prepare CaO and CaNaAlSi2O7 powder. Cumulative mass percent finer (CMPF) and particle size were measured by a particle size analyzer (Mastersizer S, Model Polydisperse 2.19). The samples were dispersed in a water medium and vibrated in an ultrasonic cleaner for 20 min before being measured. Micrographs were characterized by using a SEM (JEOL-5200). The ceramic/polymer composite foams were mounted on stubs by using a carbon paste and sputter-coated to ~0.1 μm with gold to improve electrical conductivity. An acceleration voltage of 15 kV with magnification of 300X was used. The true density of the samples was measured by a gas pycnometer (Quantachrome, Ultra pycnometer 1000). Both bulk and true density values of samples were measured according to the ASTM D3575 and D1056, and then calculated by using Eq. 1:
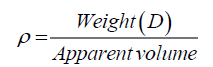 (1)
(1)
Where, ρ is the true and bulk density (g/cm3); D is the weight of the dry sample (g); and the ‘Apparent volume’ is the volume of the sample with/without open pores and closed pores within the sample (cm3). The thermal properties were measured by using a differential scanning calorimeter (DSC; NETZSCH 409) and a simultaneous thermal analyzer (STA; NETZSCH 409). The samples were heated with a heating rate 10°C min-1 under air atmosphere from room temperature to 1000°C. An AUTOSORB-1 (quantachrome) was used to characterize the specific surface area and pore size. The specific surface area, S, of the solid was calculated the total surface area according to Eq. 2 and 3:
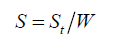 (2)
(2)
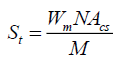 (3)
(3)
Where, S is the specific surface area of the solid, St is the total surface area, W is the sample weight, N is the Avogadro’s number (6.023 × 1023 molecules mol-1), M is the molecular weight of the adsorbate, and Acs is the area occupied by one adsorbate molecule (16.2 × 10-20 m2 for N2 and 19.5 × 10-20 m2 for Kr). Compression testing was measured with a mechanical testing machine (England RH 15 5DZ, Serial No R100/319 with the software QMat 5.44 M-Series-10KM).
CaNaAlSi2O7 powder preparation
Raw chicken eggshells were cleaned with tap water, dried at room temperature, and then crushed with a porcelain mortar. The fine eggshell particles were calcined to obtain CaO and then dissolved with HCl to be calcium chloride solution. The calcium chloride solution was added into the sodium aluminosilicate mixture solution. The solution mixture changed to gel within 5 h. The molar ratios of the CaO:Al2O3:Na2O:SiO2 are 1:1:2:8 via the sol-gel process at room temperature (25°C) for 24 h to get the complete gel. The gel samples were dried at 110°C for 24 h and then calcined at 300°C for 1 h. The obtained calcined ceramic powder is white, soft, and fine powder [12].
Polyurethane foams preparation
PU micro-cellular was carried out by mixing the mass ratio between DALTOPED® FF 45084 (the fully blended composition of polyether polyols, the chain extenders, the catalysts, and the additives) and SUPRASEC® 2749 acted as an MDI-based on pre-polymer with a weight ratio 100:94-98 for 3 to 5 min. The mixture of PU foam was casted into a paper cup mold and allowed to cure completely for 4 days at room temperature. The obtained white PU foam is lightweight, semi-rigid, and high porosity. In this study, the formula of the CaNaAlSi2O7/PU composite foams are prepared as tabulated in Table 1.
| Samples | Weight of DALTOPED®FF 45084a(g) | Weight of SUPRASEC®2749b(g) | Weight of CaNaAlSi2O7 powderc(g) | Ratio of PUd:CaNaAlSi2O7(Vol:Vol) |
|---|---|---|---|---|
| Pure PU foam | 25.00 | 24.50 | 0.00 | 100:0 |
| 1vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 0.85 | 100:1 |
| 5vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 4.23 | 100:5 |
| 10vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 8.46 | 100:10 |
| 15vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 16.91 | 100:15 |
| 20vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 25.37 | 100:20 |
| 50vol% CaNaAlSi2O7/PU | 25.00 | 24.50 | 42.28 | 100:50 |
aDensity of DALTOPED®FF 45084aspolyether polyol is equal to 1.1 g/cm3. bDensity of SUPRASEC®2749 asMDI-based prepolymer is equal to 1.2 g/cm3. cDensity of CaNaAlSi2O7 as ceramic powder is equal to 1.96 g/cm3.dPolyurethane foam (PU) prepared from mixing DALTOPED®FF 45084 and SUPRASEC®2749 and stirring vigorously with high speed 1200 rpm for 1 min at room temperature.
Table 1: Formula of the green CaNaAlSi2O7/PU thermosetting composites prepared at room temperature and casted into a paper cup mold.
Results and Discussion
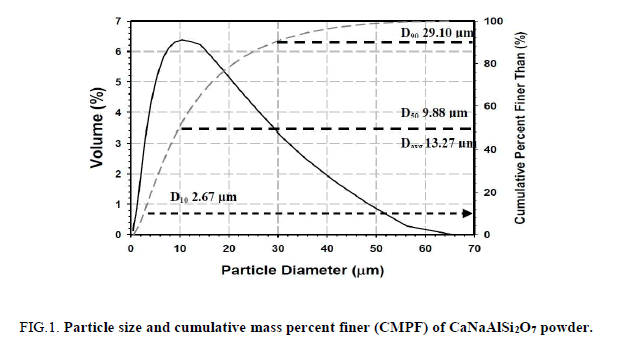
The obtained CaNaAlSi2O7 powder has high specific surface area 38.89 m2g-1. The particle size and CMPF of CaNaAlSi2O7 powder are shown in Figure. 1. The particle sizes of CaNaAlSi2O7 ceramic powder at 10% CMPF (d10), 50% CMPF (d50), and 90% CMPF (d90) are equal to 2.67, 9.88, 29.10 μm, respectively. The average particle size (davg) is equal to 13.27 μm.
The specific surface area and true density values of CaO, fumed silica, aluminosilicate, CaNaAlSi2O7 powder, PU foam, and 10 vol% CaNaAlSi2O7/PU composites are shown in Table 2. The specific surface area of pure PU foam and 10 vol% CaNaAlSi2O7/PU composite are 1.00 and 6.00 m2g-1, respectively. The true density values of PU foam and 10 vol% CaNaAlSi2O7/PU composite are 1.13 and 1.47 gcm-3, respectively.
| Samples | Specific surface area BET(m2/g) | True density(g/cm3) |
|---|---|---|
| CaO | 7.79 | 2.16 |
| Fumed silica (SiO2) | 206.00 | 2.12 |
| Aluminosilicate | 75.00 | 2.04 |
| CaNaAlSi2O7 powder | 37.91 | 1.96 |
| Polyurethane foam (PU foam) | 1.00 | 1.13 |
| 10 vol% CaNaAlSi2O7/PU composite foams | 6.00 | 1.47 |
?-? means not measured
Table 2: Specific surface area and true density of raw materials, CaNaAlSi2O7 powder, PU foams, and 10 vol% PU/CaNaAlSi2O7 composite foams.
Table 3 shows the bulk density values of pure PU foam and CaNaAlSi2O7/PU composite foams. Adding a small amount of CaNaAlSi2O7 powder into the PU foam affects to bulk density due to the interconnecting open and closed cells. On the other hand, adding 15 vol% and 20 vol% CaNaAlSi2O7 powder into the PU foams can increase the bulk density higher than that of pure PU foam. Because the agglomeration of particles during foam formation affects to large open cell and high pore sizes. Adding 50 vol% CaNaAlSi2O7 powder into PU foam causes the PU foam to brittle and fracture. Therefore, the bulk density value of 50 vol% CaNaAlSi2O7/PU composite sample cannot be measured.
| Samples | PU foams(vol%) | CaNaAlSi2O7 ceramic powdera(vol%) | Bulk density of composite foams with pores(g/cm3) |
|---|---|---|---|
| Pure PU foam | 100 | 0 | 0.213 ± 0.012 |
| 1 vol% CaNaAlSi2O7/PU | 100 | 1 | 0.141 ± 0.009 |
| 5 vol% CaNaAlSi2O7/PU | 100 | 5 | 0.152± 0.020 |
| 10 vol% CaNaAlSi2O7/PU | 100 | 10 | 0.213± 0.012 |
| 15 vol% CaNaAlSi2O7/PU | 100 | 15 | 0.290 ± 0.031 |
| 20 vol% CaNaAlSi2O7/PU | 100 | 20 | 0.292 ± 0.015 |
| 50 vol% CaNaAlSi2O7/PU | 100 | 50 | N/A |
aTrue density of CaNaAlSi2O7 is equal to 1.96 g/cm3.
Table 3: Bulk density of pure PU foams and CaNaAlSi2O7/PU composite foams.
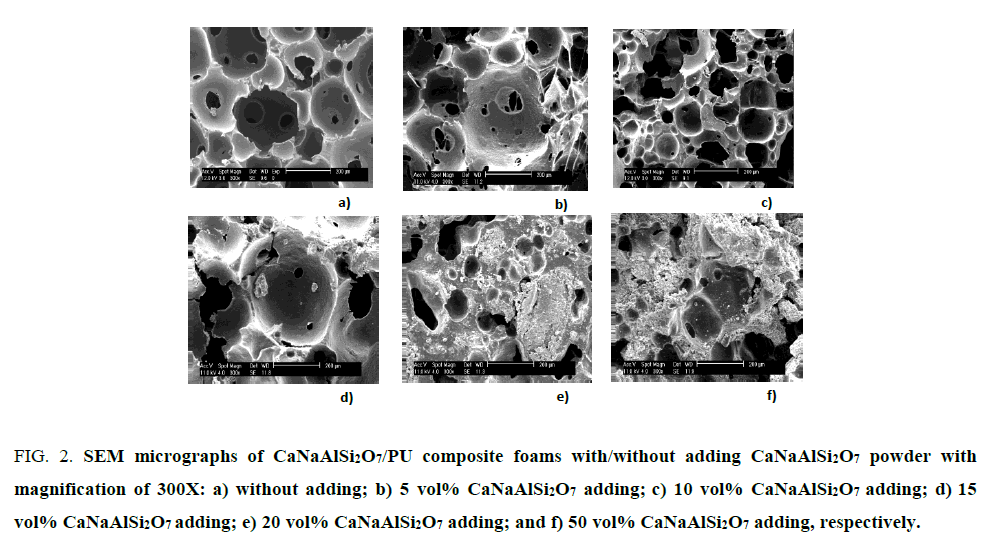
The SEM micrographs show microstructures of the CaNaAlSi2O7 powder and CaNaAlSi2O7/PU thermosetting composites with various volume percentage loadings: 1, 5, 10, 15, 20, and 50 vol% as shown in Figure. 2. The CaNaAlSi2O7 powder addition into the PU foam affects to the configuration of the closed cells and open-interconnecting cells. When the percentage of the CaNaAlSi2O7 powder increases, the size of the micro-pores of the CaNaAlSi2O7/PU composite foam tends to decrease.However, the addition of CaNaAlSi2O7 powder into PU foams greater than 20 vol% caused poor composite foam, brittleness, and low compressive strength. Adding the CaNaAlSi2O7 powder close to 50 vol% affects to particle agglomeration and fracture. The SEM micrographs show an agglomeration of the CaNaAlSi2O7 powder as shown in Figure. 2f. In general, polymer foams can be classified cellular structures into four types: macrocellular (>100 μm), microcellular (1 μm to 100 μm), ultramicro cellular (0.1 μm to 1.0 μm), and nanocellular (0.1 nm to 100 nm) [16]. In this study, the 10 vol% CaNaAlSi2O7/PU composite foam is the best condition and its porosity is in the range of micro- and macro-cellular (15 μm to 200 μm) as shown in Figure. 2c.
Figure 2: SEM micrographs of CaNaAlSi2O7/PU composite foams with/without adding CaNaAlSi2O7 powder with magnification of 300X: a) without adding; b) 5 vol% CaNaAlSi2O7 adding; c) 10 vol% CaNaAlSi2O7 adding; d) 15 vol% CaNaAlSi2O7 adding; e) 20 vol% CaNaAlSi2O7 adding; and f) 50 vol% CaNaAlSi2O7 adding, respectively.
Furthermore, the compression testing of pure polyurethane foams (PU foam) and 10 vol% CaNaAlSi2O7/PU composite foams are tabulated in Table 4. The compressive strength and compressive Young’s modulus of pure PU foams are 268.1 kPa ± 65.7 kPa and 841.3 kPa ± 40.9 kPa, respectively. While the compressive strength and the compressive Young’s modulus of 10 vol% CaNaAlSi2O7/PU composite foams are 184.7 kPa ± 16.7 kPa and 346.0 kPa ± 76.5 kPa, respectively. Adding CaNaAlSi2O7 powder into PU foam causes the compressive strength and compressive Young’s modulus decrease less than those of pure PU foam due to bonding interaction between PU foam and CaNaAlSi2O7 filler particles. In addition, adding excess CaNaAlSi2O7 ceramic powder into PU foam matrix affects to compressive strength and compressive Young’s modulus decrease due to particle agglomeration, especially adding CaNaAlSi2O7 particles with 20 vol% and 50 vol%. When the amount of filler increases, the bulk and true density values increase whereas the compressive strength and compressive Young’s modulus of composite foams decrease. The comparison of mechanical properties between pure PU foam and 10 vol% CaNaAlSi2O7/PU composite foam are consistent with the obtained SEM micrographs. The CaNaAlSi2O7 filler particles composed of Ca2+, Na+, Al3+, Si4+, and O2– ions in their structure. They can react and intercalate within the CaNaAlSi2O7/PU composite foam.
| Samples | Diameter(mm) | Yield strength (kPa) | Compressive strength(kPa) | Compressive Young’s Modulus (kPa) |
|---|---|---|---|---|
| Pure polyurethane foams | 28.44 ± 0.21 | 268.1 ± 65.7 | 268.1 ± 65.7 | 841.3 ± 40.9 |
| 10 vol% CaNaAlSi2O7/PUcomposite foams | 29.31 ± 0.12 | 184.7 ± 16.7 | 184.7 ± 16.7 | 346.0 ± 76.5 |
Table 4: Compression testing results of pure polyurethane foams and 10 vol% CaNaAlSi2O7/PU composite foams.
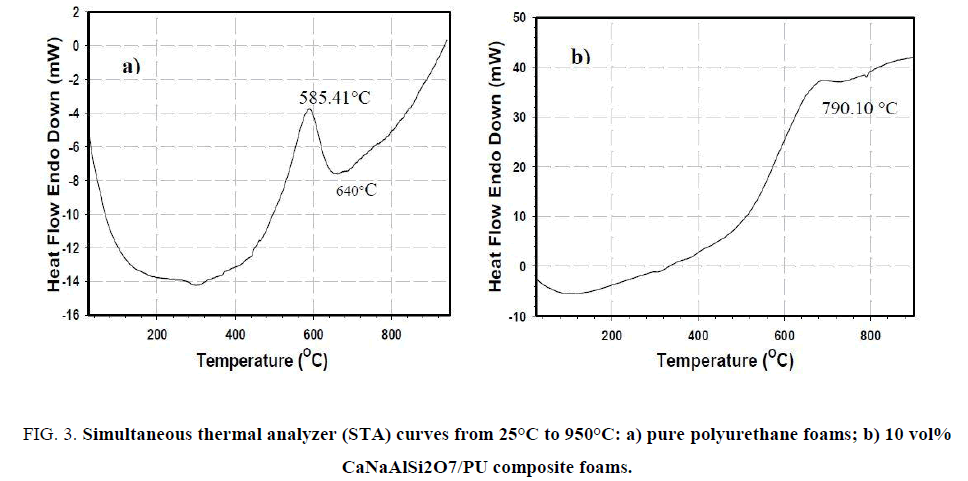
In addition, the STA measurement was found the endothermic reaction of 10 vol% CaNaAlSi2O7/PU composite foams decomposed at 790.10°C higher than that of pure PU foams at 640°C as shown in Figure. 3. The obtained results show that 10 vol% CaNaAlSi2O7/PU composite foams are good thermal resistance better than pure PU foams.
Figure 3: Simultaneous thermal analyzer (STA) curves from 25°C to 950°C: a) pure polyurethane foams; b) 10 vol% CaNaAlSi2O7/PU composite foams.
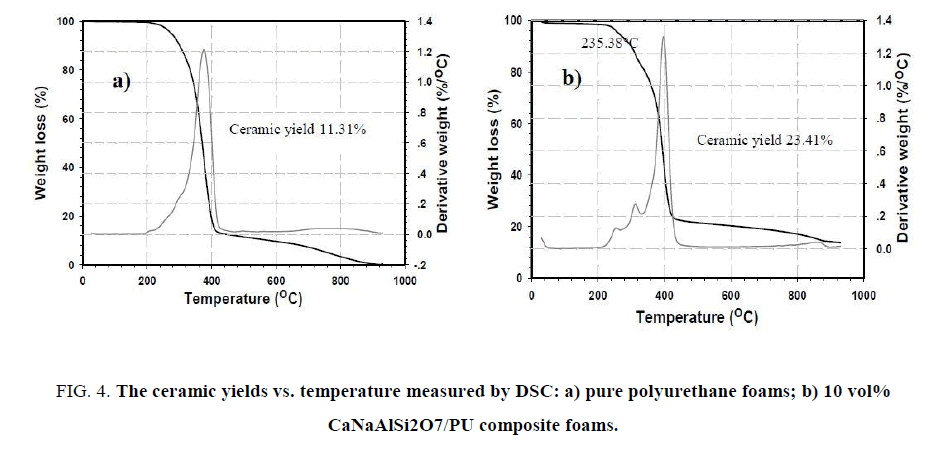
The percentage of ceramic yield of pure polyurethane foams and 10 vol% CaNaAlSi2O7/PU composite foams was measured by DSC at room temperature to 1000°C as shown in Figure 4 . The ceramic yields of pure polyurethane foams and 10 vol% CaNaAlSi2O7/PU composite foams are equal to 11.31 and 23.41 wt%, respectively. The obtained results suggest that 10 vol% CaNaAlSi2O7/PU composite foams possess thermal resistance greater than pure PU foams.
Figure 4: The ceramic yields vs. temperature measured by DSC: a) pure polyurethane foams; b) 10 vol% CaNaAlSi2O7/PU composite foams.
Conclusion
The green calcium sodium aluminosilicate (CaNaAlSi2O7)/PU composite foams are thermosetting composites. The CaNaAlSi2O7 powder can be made from eggshells via sol-gel process. The CaNaAlSi2O7/PU thermosetting composites were prepared by the foaming process called the direct-foaming technique. The foaming process generates high density of bubbles in the foam slurry and after setting to form high porosity within the composite foams. Adding 10 vol% CaNaAlSi2O7 powder affects to obtain good physical and thermal properties of composite foams. This is the best condition to obtain micro-and macro-cellular structures, high elastic modulus, lightweight, good thermal resistance, easily forming through the direct-foaming process. The obtained specific surface area, true density, and bulk density of 10 vol% CaNaAlSi2O7/PU composites are 6.00 m2g-1, 1.47 gcm-3, and 0.2126 gcm-3 ± 0.0116 gcm-3, respectively. The SEM micrographs indicate that the 10 vol% CaNaAlSi2O7 powder added into PU foam affects to the open and closed cell morphology, bridge bonding, and interconnecting cells in the range of micro- and macro-cellular structures. The compressive strength and compressive Young’s modulus of 10 vol% CaNaAlSi2O7/PU composite foams are 184.7 kPa ± 16.7 kPa and 346.0 kPa ± 76.5 kPa, respectively. Furthermore, 10 vol% CaNaAlSi2O7/PU composite foam is also good thermal resistance better than pure PU foam. Therefore, the CaNaAlSi2O7/PU thermosetting composites are potentially useful for various applications i.e., building construction, energy and sound absorption, insulation, and catalysts [15-18].
Acknowledgement
The authors would like to thank the Petroleum and Petrochemical College and the Scientific and Technological Research Equipment Centre, at Chulalongkorn University and the Department of Materials Engineering, at Kasetsart University for the use of analytical equipment. We are also grateful for grant support from the Kasetsart University Research and Development, Conductive and Electroactive Polymers Research Unit of Chulalongkorn University, Thailand Research Fund (TRF-RTA), the Royal Thai Government, and the Center for Advanced Studies in Industrial Technology, Kasetsart University.
References
- Thakur VK, Singha AS, Thakur MK. Graft Copolymerization of Methyl Acrylate onto Cellulosic Biofibers: Synthesis, characterization and Application.J Polym Environ. 2012;20:164-74.
- Singha AS, Thakur VK. Synthesis and Characterization of Grewia Optiva Fiber-reinforced PF-based Composites. Int J PolymMater. 2008;57(12):1059-74.
- Singha AS, Thakur VK. Physical Chemical and Mechanical Properties of Hibiscus sabdariffa Fiber/Polymer Composite.IntJ PolymMater. 2009;58:217-28.
- Thakur VK, Singha AS, Kaur I, et al. Silane Functionalization of Saccaharum Cilliare Fibers: Thermal, Morphological and Physicochemical Study. Int J Polym Anal Charact. 2010;15:397-414.
- Tullett SG.Eggshell Conductance and other Functional Qualities of Ostrich Egg.Comp Biochem Physiol. 1984;78A(1):5-13.
- NysY, Gautron J, Garcia-Ruiz JM, et al. Avian Eggshell Mineralization: Biochemical and Functional Characterization of Matrix proteins. CR Palevol. 2004;3:549-62.
- Tsai WT, Yang JM, Lai CW, et al. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour Technol. 2006;97:488-93.
- Toro P, Quijada R, Yazdani-Pedram M, et al. Eggshell a new bio-filler for polypropylene composite. Mater Lett. 2007;61:4347-50.
- Adeyeye EI. Comparative Study on the Characteristics of Egg Shell of Some Bird Spicies.Bull Chem Soc Ethiopia. 2009;23(2):159-66.
- Kim JE, Kim DJ, Han JU, et al. Preparation and characterization of zeolite catalysts for etherification reaction.Catal Today. 2003;87:195-203.
- http://minerals.usgs.gov/minerals/pubs/commodity/zeolites/zeolimyb04.pdf
- Tangboriboon N, Wongkasemjit S, Kunanuraksapong R, et al. An Innovative Synthesis of Calcium Zeolite Type a Catalysts from Eggshells Via the Sol-Gel Process. J Inorg Organomet Polym. 2011;21:50-60.
- Valtchev, Mintova, Tsapatsis, editors. Ordered Porous Solids.1st ed. London: Elsevier; 2009.
- Xu H, Guan J, Wu S, et al. Mesoporous Zeolites: Preparation, Characterization and Applications.J Colloid Interface Sci.2009;329:346-50.
- DiasCJ, Das-Gupta DK. Inorganic Ceramic Polymer Ferroelectric Composite Electrets.IEEE TransDielectrElectrInsul. 1996;3(5):706-34.
- Kabir ME, Saha MC, Jeelani S. Tensile and Fracture Behavior of Polymer foams.Mater Sci Eng A. 2006;429(1-2):225-35.
- Richardson JT, Peng Y, Remue D. Properties of ceramic foam catalyst supports: pressure drop.Appl Catal A: General.2000;204:19-32.
- Parthasarathyand M, Klingenberg DJ.Electrorheology: Mechanisms and Models. Mater Sci Eng. 1996;R17:57-103.