Original Article
, Volume: 11( 6)Innovative Green Bio-Filler and Bio-Flux Calcium Carbonate from Eggshells to Hard-Soft Porcelain Preparation
- *Correspondence:
- Tangboriboon N , Materials Engineering Department, Faculty of Engineering, Kasetsart University, Bangkok 10900, Thailand, Tel: +66 2 579 0113; E-mail: fengnnpt@ku.ac.th
Received: October 10, 2016; Accepted: November 11, 2016; Published: November 17, 2016
Citation: Tangboriboon N, Selarak P, Shamunee P, et al. Innovative Green Bio-Filler and Bio-Flux Calcium Carbonate from Eggshells to Hard-Soft Porcelain Preparation. Chem Technol Ind J. 2016;11(6):109.
Abstract
Calcium carbonate (CaCO3) powder was prepared from raw coturnix bird eggshells acted as a bio-ceramic material. Embedding raw bird eggshell powder in porcelain products can increase physical, thermal, and mechanical properties. The main composition of raw bird eggshell is calcium carbonate or calcite more than 97.44% wt and other oxide compounds. CaCO3 can react with silica (SiO2) and alumina (Al2O3) in porcelain clay via slip casting process at room temperature. When porcelain products were fired, the phase can transform to calcium feldspar or anorthite (CaAl2Si2O8). Anorthite is one kind of feldspars and can reduce firing temperature called sintered aid. However, the excess amount of eggshell added into porcelain and fired at very high firing temperature and long firing time affects to crack and brittle samples. In this study, the amount of 0, 5, 10, 15, and 20% vol raw bird eggshell powder was added, mixed with porcelain slip containing sodium silicate as a dispersing agent to prevent particles agglomeration, and fired at 600°, 700°, 800°, and 900°C for 1, 3, and 5 h at each firing temperature. The best porcelain product is the sample encoded 10-5-900. It means 10% vol raw bird eggshell adding, soaking time for 5 h, and firing temperature at 900°C. The XRD phase transformation, true density, thermal expansion coefficient, and mechanical properties are reported here.
Keywords
Calcium carbonate (CaCO3); Raw bird eggshell; Ceramic products; Bio-filler; Bio-flux; Phase formation; Bio-materials; Renewable resource
Introduction
At the present, the green industries and environmental consciousness are concerned gradually. Sustainable development and eco-efficiency are focused to the majority of every country [1-4]. Most of researchers pay attention to use the bio-materials, green materials, nontoxic materials, and recycle materials i.e. biological wastes, bio-fibers, industrial wastes, and biodegradable materials as a starting material to do researches [1-4].
Eggs are one of the most complete foods as they contain protein, lipid, and carbohydrates which are essential for a good diet. They also contain vitamins and mineral elements which are necessary for the development of young and elderly people. Egg and its derivative are one of important raw materials to make food, drug, bakery, and cosmetic industries i.e. for manufacturing bread, cakes, crackers, ice creams, and food additive. Hen eggshell as by products provides approximately 11% wt of the total weight (65 g to 70 g per egg). Duck eggshell provides approximately 12.78% wt of the total weight (65 g to 71 g per egg). Coturnix quail eggshell provides approximately 11.04% wt of the total weight (10.18 g to 13 g per egg). The main composition of all kinds of eggshell composed of more than 97%wt calcium carbonate (CaCO3), 1% wt magnesium carbonate (MgCO3), 1%wt calcium phosphate (CaPO4), and 2% wt other organic matters. Coturnix quail eggshell color varies from white to blue and green, including brown and reddish-brown [5-8]. Coturnix quail eggshell weight and thickness increased with age, and reached its peak in the 29th week of age, with a subsequent gradual decrease in eggshell weight until the end of the laying period [9]. The eggshell proportion tended to increase towards the end of the laying period. The objective in this research to maximize the recycling opportunities for eggshells, reduce eggshell wastes, conserve the environment without pre-treatment, and increase agricultural evaluation. It is estimated that eggshell waste amounts are many millions tons per day from hen, duck, and bird eggs. Thus, an efficient eggshell recycle or disposal method is required. Eggshells are porous ceramic materials because the developing embryos require gases both oxygen and carbon dioxide to breath in and out by diffusion through the pores. Mostly, the structure of eggshells composed of micro-pore functioned as catalysts or adsorbents [10-14].
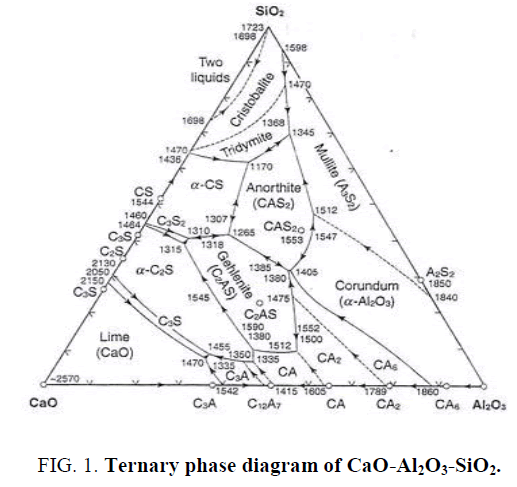
Calcium carbonate (CaCO3) is one kind of calcium sources that can be used as a filler, starting material, sintering aid or flux. Calcium carbonate (CaCO3) in eggshell can react to silica (SiO2) and alumina (Al2O3) within porcelain clay to form anorthite or calcium feldspar (CaAl2Si2O8), calcium silicate (CaO.SiO2), mullite (Al2O3.SiO2), and wollastonite (CaSiO3) after firing at high temperature consistent with eq.1 and the ternary phase diagram as shown in Figure 1 [15-16].
CaO +Al2O3.2SiO2 ------> CaO.Al2O3.2SiO2 or CaAl2Si2O8 or calcium feldspar (1)
Porcelain clay is an important kind of clays and can be used as a raw material to produce many kinds of products such as pottery, brick, tile, artificial tooth and bone, and filler for industries [16-19].
In this work, the objective is to prepare calcium feldspar or anorthite phase formation (CaAl2Si2O8) made from adding bird eggshells into porcelain via slip casting process by varying the percentage of volume 0, 5, 10, 15, and 20 and fired at 600°, 700°, 800°, and 900°C for 1, 3, and 5 hr each firing temperature. The raw materials (raw bird eggshell and porcelain clay) and porcelain products with/without raw coturnix bird eggshell powder adding were characterized physical-thermalmechanical properties and microstructures by Brunauer-Emmett-Teller (BET), particle size analyser, true and bulk density according to the ASTM D3575 and D1056, dilatometer, extensometer, vicker microhardness tester, XRD, and scanning electron microscopy (SEM), respectively, also carried out and the results are reported here.
Experimental
Materials
Raw Coturnix quail eggshell powder was obtained from the egg’s shop of the local cafeteria within the University. It was cleaned with tap water and dried in the air 1-2 days and ground by the rapid mill for 120 mins. Porcelain clay acted as a raw material for making clay products by slip casting process and purchased from Compound Clay Co., Ltd. Thailand. Sodium silicate was used as a dispersing agent or deflocculant of porcelain clay particles for the slip preparation purchased from Compound Clay Co., Ltd. Thailand. The sodium silicate solution was prepared at a weight ratio of sodium silicate to water equal to 20:1. The solution of sodium silicate was added to adjust the viscosity of the slip. The ratio between porcelain clay and sodium silicate solution is 100 Kg: 250 g.
Instrument
Cumulative mass percent finer (CMPF) and particle size were measured by a particle size analyzer (Mastersizer S, Model Polydisperse 2.19). The samples were dispersed in a water medium and vibrated in an ultrasonic cleaner for 20 min before being measured. X-ray fluorescence (XRF) was used to determine the chemical compositions. XRF (Philips, model PW 2400) was used with the tube current of 1000 mA and an acquisition lifetime of 30s. The true density of the samples was measured by a gas pycnometer (Quantachrome, Ultra pycnometer 1000). Both bulk and true density values of samples were measured according to the ASTM D3575 and D1056, and then calculated by using Eq. 2:
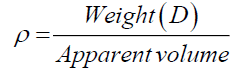 (2)
(2)
where ρ is the bulk and true density (g/cm3); D is the weight of the dry sample (g); and the apparent volume is the volume of the sample with/without open pores and closed pores within the sample (cm3). X-ray diffraction (XRD) data were taken and analyzed using an analyzer (Bruker, D8 Discover) with a VANTEC-1 detector and a double-crystal wide-angle goniometer. Scans were obtained from 10° to 80° 2θ at a scan speed of 2° 2θ/min in 0.02° 2θ increments using CuK α radiation (λ=0.154 nm). Peak positions were compared with standard JCPDS files to identify the crystalline phases. An AUTOSORB-1 (Quantachrome) was used to characterize the specific surface area and pore size. The specific surface area, S, of the solid was calculated the total surface area according to Eqs. (3) and (4):
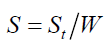 (3)
(3)
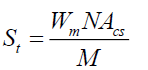 (4)
(4)
where S is the specific surface area of the solid, St is the total surface area, W is the sample weight, N is the Avogadro’s number (6.023 × 1023 molecules mol-1), M is the molecular weight of the adsorbate, and Acs is the area occupied by one adsorbate molecule (16.2 × 10-20 m2 for N2 and 19.5 × 10-20 m2 for Kr). Hardness of fired porcelain samples was measured by hardness testing machine (Mitutoyo, MVK-H1). Compressive strength values of fired porcelain samples were measured by universal testing machine (UTM, Hounsfield, H50KS).
Dilatometer (Netzsch, DIL402PC) was used to measure thermal expansion coefficient of fired porcelain clay products with dimension 5 mm × 5 mm × 25 mm.
Porcelain Clay Products Preparation
The weight ratio of porcelain clay: water: sodium silicate is equal to 10 kg:5 kg:0.001 kg. All raw materials were mixed by stirrer for 1 h to obtain slip or slurry. Then raw bird eggshell powder was added into slip by varying 0, 5, 10, 15, and 20% vol and continuously stirred for 45 min. After that, the porcelain slip was poured into dried plaster mold in rectangular shape. The porcelain samples were casted totally 60 formula (120 pieces) as data tabulated in Table 1. Porcelain samples were let to set solidification in plaster mold and cast to dry at room temperature. The dried porcelain samples were fired at 600°, 700°, 800°, and 900°C for 1, 3, and 5 h at each firing temperature, respectively. Porcelain clay products were characterized physical properties (appearance, bulk and true density, and phase formation), thermal properties (thermal appearance, crack-free, and thermal expansion coefficient), and mechanical properties (compressive strength and hardness).
| Samples a | Samples | Samples | Samples | Samples |
|---|---|---|---|---|
| 0-1-600 | 5-1-600 | 10-1-600 | 15-1-600 | 20-1-600 |
| 0-3-600 | 5-3-600 | 10-3-600 | 15-3-600 | 20-3-600 |
| 0-5-600 | 5-5-600 | 10-5-600 | 15-5-600 | 20-5-600 |
| 0-1-700 | 5-1-700 | 10-1-700 | 15-1-700 | 20-1-700 |
| 0-3-700 | 5-3-700 | 10-3-700 | 15-3-700 | 20-3-700 |
| 0-5-700 | 5-5-700 | 10-5-700 | 15-5-700 | 20-5-700 |
| 0-1-800 | 5-1-800 | 10-1-800 | 15-1-800 | 20-1-800 |
| 0-3-800 | 5-3-800 | 10-3-800 | 15-3-800 | 20-3-800 |
| 0-5-800 | 5-5-800 | 10-5-800 | 15-5-800 | 20-5-800 |
| 0-1-900 | 5-1-900 | 10-1-900 | 15-1-900 | 20-1-900 |
| 0-3-900 | 5-3-900 | 10-3-900 | 15-3-900 | 20-3-900 |
| 0-5-900 | 5-5-900 | 10-5-900 | 15-5-900 | 20-5-900 |
| a Encoded samples x-x1-xxx2 are the percentage of raw coturnix quail eggshell powder adding-firing time (h)-firing temperature (°C). | ||||
Table 1: Sample codes of porcelain products with/without raw bird eggshell powder adding.
Results and Discussion
The chemical composition comparison between raw hen-, duck-, and coturnix bird eggshell powder and porcelain clay measured by XRF is shown in Table 2. The main composition of three kinds (hen, duck, and coturnix quail eggshell powder) is calcium carbonate equal to 96.46, 99.39, and 97.44% wt, respectively, and the other oxide compounds. Therefore, raw hen, raw duck, and raw coturnix bird eggshell powder can be used as a calcium source to prepare porcelain products, acted as a bio-filler and bio-flux of the process. The main composition of porcelain clay is 71.089% wt SiO2, 26.195% wt Al2O3, 1.747% wt K2O, and 0.969% wt other oxide compounds. When raw bird eggshell powder was added into porcelain clay and fired at high temperature, it can react to porcelain to form calcium feldspar or anorthite (CaAl2Si2O8) phase formation as shown in the Eq.1.
| Chemical composition | Raw coturnix quail eggshell (% wt) | Raw hen eggshell (% wt) | Raw duck eggshell (% wt) | Porcelain clay (% wt) |
|---|---|---|---|---|
| Al2O3 | - | - | - | 26.195 |
| SiO2 | 0.04 | - | 0.01 | 71.089 |
| K2O | 0.11 | 0.21 | 0.02 | 1.747 |
| CaO | - | - | - | 0.820 |
| CaCO3 | 97.44 | 96.46 | 99.39 | - |
| Fe2O3 | - | - | - | 0.149 |
| Na2O | 0.23 | - | <0.01 | - |
| MgO | 1.12 | - | 0.10 | - |
| P2O5 | 1.19 | 0.82 | 0.27 | - |
| SO3 | 0.98 | - | 0.16 | - |
| Cl | 0.06 | - | - | - |
| SrO | 0.02 | 779 ppm | 0.02 | - |
| ZrO2 | - | - | 0.02 | - |
Table 2: Comparison of chemical composition of raw bird, raw hen, and raw duck eggshell powder and porcelain clay by XRF.
Raw hen-, duck-, and coturnix quail eggshell powder was characterized physical properties compare to commercial calcium carbonate and porcelain clay on true density, bulk density, specific surface area, and pore diameter are tabulated in Table 3. The true density of raw bird eggshell powder is close to true density value of porcelain clay equal to 1.91 and 1.70 g/cm3, respectively. Furthermore, all true density values of raw hen, raw duck, and raw bird eggshell are lower than that of commercial calcium carbonate (2.73 g/cm3). The particle size of raw hen, raw duck, and raw bird eggshell powder is bigger and more roughness than porcelain clay. True density, average particle size, specific surface area, and pore diameter of bird eggshell powder are equal to 1.91 g/cm3, 313.28 Å, 0.68 m2/g, 267.90 Å, respectively. While true density, average particle size, specific surface area, and pore diameter of porcelain clay are equal to 1.70 g/cm3, 39.98 Å, 19.92 m2/g, 183.30 Å, respectively.
| Samples | True density (g/cm3) | Avg. particle size (Å) | Specific surface area (m2/g) | Avg. pore diameter (Å) |
|---|---|---|---|---|
| Raw Coturnaix quail eggshell | 1.91 | 313.28 | 0.68 | 267.90 |
| Raw hen eggshell | 2.30 | 153.78 | 4.06 | 196.90 |
| Raw duck eggshell | 2.25 | 100.07 | 7.79 | 176.90 |
| Porcelain clay | 1.70 | 39.98 | 19.92 | 183.30 |
| Commercial CaCO3 | 2.73 | 20.63 | - | - |
Table 3: Physical properties of raw coturnix quail eggshell and porcelain clay.
The comparison study of particle size distribution between raw bird eggshell powder and porcelain clay is found the d10, d50, d90, and davg of raw bird eggshell powder ground 120 min equal to 68.21, 286.03, 596.99, and 313.28 μm, respectively. While the d10, d50, d90, and davg of porcelain clay are 0.94, 16.92, 109.07, and 39.98 μm, respectively. The particle size of porcelain clay is smaller than that of raw bird eggshell powder. Therefore, to prevent the agglomeration need to add small amount of sodium silicate acted as the deflocculant to disperse the porcelain and raw quail eggshell particles within the medium.
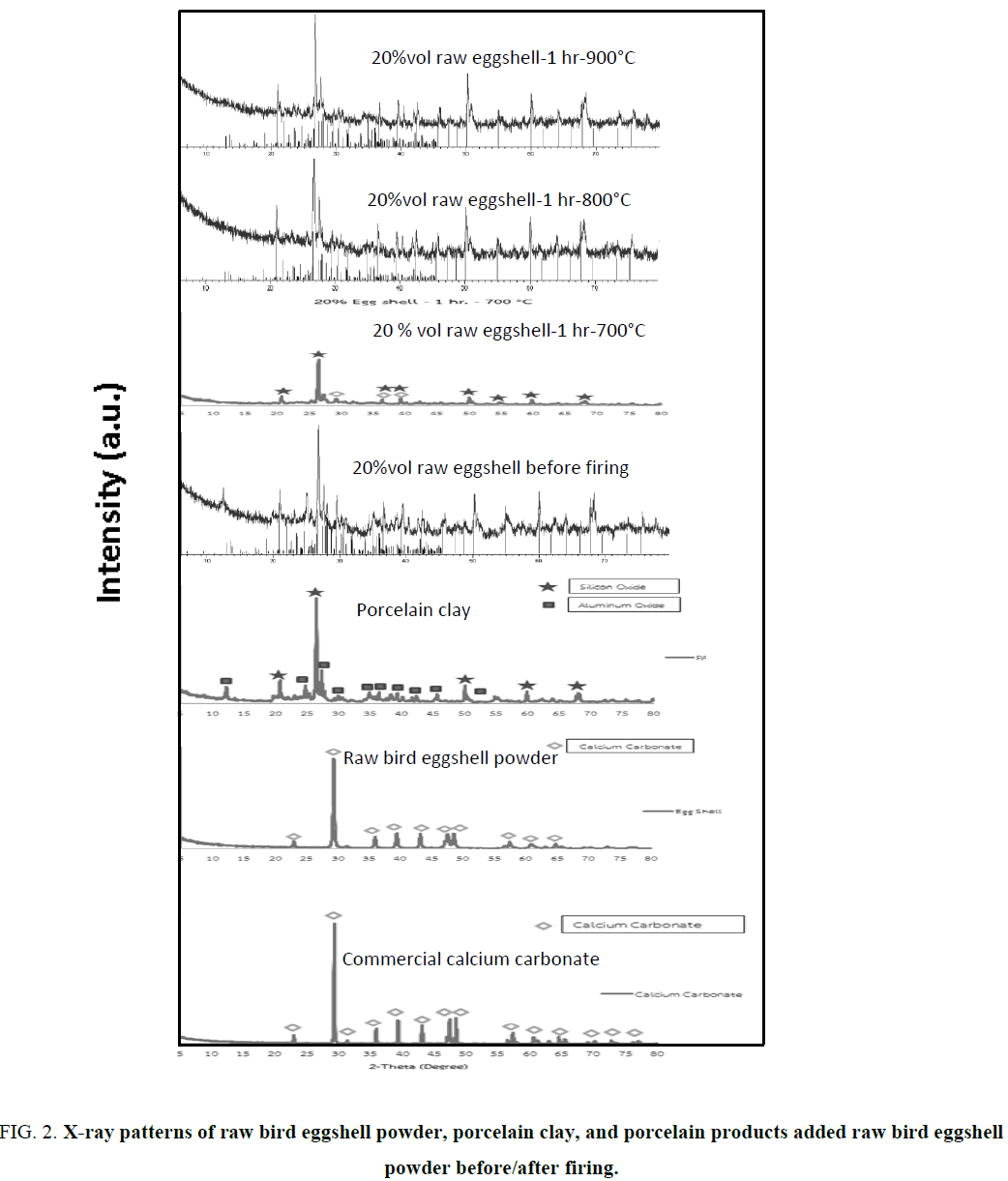
The comparison of XRD peak patterns of raw materials (commercial calcium carbonate, raw bird eggshell, and porcelain clay) and porcelain products added the highest percentage of raw bird eggshell quantity (20%vol) before and after firing at 800° and 900°C are shown in Figure 2. XRD peak pattern of raw bird eggshell is similar to that of commercial calcium carbonate consistent with the JCPDS file nos. 01-072-1937 and 01-085-1108 in terms of calcite or rhombohedral phase formation at 2θ 29.369° (104), 39.370° (113), and 35.937° (110). While the XRD peak pattern of porcelain clay is consistent with the JCPDS file no. of 01-089-8936 belong to silicon dioxide or quartz (SiO2, hexagonal phase formation) at 2θ: 26.566° (011) and 20.790° (100), and JCPDS file no.00-031-0026 of aluminum oxide (Al2O3) at 2θ: 20.935°, 10.996°, and 12.266°, respectively. The XRD peak pattern of porcelain sample added 20% vol raw bird eggshell powder before firing is consistent with calcium carbonate (CaCO3), silicon dioxide (SiO2), and alumina (Al2O3) of JCPDS file no. 00-003-0418 in terms of calcium magnesium aluminum silicate (CaMgAlSiO). While XRD peak patterns of porcelain products added 20% vol raw bird eggshell powder and fired at 800° and 900°C for 1 h which are encoded 20-1-800 and 20-1-900 shown the same peak position of JCPDS file no. 01-089-1459 namely anorthite or calcium aluminum silicate or calcium feldspar (Al2CaO8Si2). The porcelain sample added 20%vol raw bird eggshell powder and fired 700°C for 1 h (20-1-700) shows small amount of anorthite or calcium aluminum silicate or calcium feldspar (Al2CaO8Si2) mixed with quartz and corundum phase formation due to low firing temperature and short firing time. Therefore, the sample encodes 20-1-700 is incomplete densification.
Figure 2: X-ray patterns of raw bird eggshell powder, porcelain clay, and porcelain products added raw bird eggshell powder before/after firing.
Thermal expansion coefficient values of fired porcelain products are tabulated in Table 4. The thermal expansion coefficient values of porcelain samples without adding raw bird eggshell powder encoded 0-3-600 and 0-3-900 are equal to 6.3015 × 10-6 and 6.6396 × 10-6 (°C)-1, respectively. While the sample encoded 15-3-600 has thermal expansion coefficient equal to 6.1435 × 10-6 (°C)-1 which is less than sample encoded 0-3-600 due to amount of raw bird eggshell powder added in porcelain products effect to increase thermal resistance. Porcelain samples encoded 10-5-900 and 15-1-800 have low thermal expansion coefficient values equal to 1.0515 × 10-6 and 1.1200 × 10-6 (°C)-1, respectively. Thermal expansion coefficient value decreases effect to thermal resistance increases. As a result of this, because many factors affect to thermal properties i.e. amount of raw bird eggshell powder adding in porcelain products, firing temperature, and time of firing temperature. The porcelain samples added excess amount of raw bird eggshell powder, fired at high firing temperature, and long firing time affect to brittle and fragile i.e. sample encoded 15-3-900, 20-y-800 (y=firing time for 1, 3, and 5 hr), and 20-y-900 (y=firing time for 1, 3, and 5 h).
| Samples a | Thermal expansion coefficient × 10-6 (°C)-1 | True density (g/cm3) |
|---|---|---|
| 0-3-600 | 6.3015 | 2.3295 ± 0.2539 |
| 0-3-900 | 6.6396 | 2.2700 ± 0.1562 |
| 10-5-900 | 1.0515 | 2.4180 ± 0.1047 |
| 15-3-600 | 6.1435 | 2.3644 ± 0.0851 |
| 15-1-800 | 1.1200 | 2.6280 ± 0.1033 |
| a Encoded samples x-x1-xxx2 are the percentage of raw bird eggshell powder adding-firing time (hr)-firing temperature (°C). | ||
Table 4: Thermal expansion coefficient values of fired samples.
Mechanical properties (compressive strength and hardness) of fired porcelain products are tabulated in Table 5. When amount of raw bird eggshell powder added in porcelain samples increases, the compressive strength and hardness of samples have trend to increase also. However, the amount of raw bird eggshell powder was added excessively effect to both compressive strength and hardness values decrease gradually due to high rate of CO2 decomposition at high temperature out of porcelain products. Therefore, the best porcelain sample obtained has the highest compressive strength and high hardness encoded 10-5-900 namely hard porcelain.
| Samples a | Compressive strength (N/mm2) | Hardness (HV) | True density (g/cm3) |
|---|---|---|---|
| 0-3-600 | 2320 | 7.1 ± 0.58 | 2.3295 ± 0.2539 |
| 0-3-900 | 2768 | 10.4 ± 0.40 | 2.2700 ± 0.1562 |
| 5-1-600 | 2063 | 6.8 ± 0.35 | 2.2962 ± 0.0620 |
| 5-3-800 | 3160 | 11.8 ± 0.50 | 2.6400 ± 0.2376 |
| 10-5-600 | 6255 | 7.6 ± 0.31 | 2.4409 ± 0.2060 |
| 10-5-900 | 6200 | 12.2 ± 0.30 | 2.4180 ± 0.1047 |
| 15-3-600 | 3444 | 9.1 ± 0.20 | 2.3644 ± 0.0851 |
| 15-5-600 | 3844 | 10.2 ± 0.55 | 2.3912 ± 0.0528 |
| 15-1-800 | 2012 | 16.6 ± 0.30 | 2.6280 ± 0.1033 |
| 20-5-600 | 3750 | 9.6 ± 0.32 | 2.3338 ± 0.2032 |
| 20-1-700 | 839 | 9.7 ± 0.06 | 2.3555 ± 0.0725 |
| a Encoded samples x-x1-xxx2 are the percentage of raw bird eggshell powder adding-firing time (hr)-firing temperature (°C). | |||
Table 5: Mechanical properties of fired porcelain products.
Conclusion
Raw coturnix quail eggshell is one kind of calcium carbonate source which mainly composed of 97.44% wt CaCO3 and other oxide compounds. There are many advantages to use CaCO3 as a bio-ceramic filler i.e. low price, abundance, renewable resource and as processing aid to increase mechanical and thermal properties of many kinds of products i.e. rubber, ceramic, paper, paint, toothpaste, and artificial tooth and bone. When the amount of raw bird eggshell powder added in porcelain products affects to increase true density value, thermal resistance, hardness, and compressive strength. However, the amount of raw bird eggshell powder adding is excess effect to fracture and brittle due to high CO2 releasing from chemical reaction of CaCO3 during firing process. In addition, there are other factors to control physical-thermal-mechanical properties of porcelain products i.e. firing temperature and firing time to do the process. The best condition to obtain good porcelain products in this study is the sample encoded 10-5-900. Adding 10% vol of raw bird eggshell powder and firing at 900°C for 5 hr is the best condition to obtain high strength porcelain products. The true density value, thermal expansion coefficient, compressive strength, and hardness of sample encoded 10-5-900 are 2.4180 ± 0.1047 g/cm3, 1.0515 × 10-6 (°C)-1, 6200 N/mm2, and 12.2 ± 0.30 HV, respectively. Because calcium carbonate (CaCO3) from raw bird eggshell powder can react to silica (SiO2) and alumina (Al2O3) particles within porcelain clay structure to form calcium feldspar or anorthite (Al2CaO8Si2) with optimum amount of raw bird eggshell powder, firing temperature, and firing time. While porcelain samples without adding raw bird eggshell powder i.e. samples encoded 0-3-900 and 0-5-900 are low true density 2.2880 ± 0.0683 g/cm3, low thermal resistance, and low mechanical properties (2768 N/mm2 and 10.4 ± 0.4 HV) due to incomplete densification. Furthermore, the incomplete densification samples cause high thermal expansion coefficient values approximately 6.6396 × 10-6 (°C)-1 which is not good for ceramic products. In this study, we obtained both soft and hard porcelain products. The samples encoded 15-1-800 and 10-5-900 are hard porcelain products useful for various applications i.e. dental and medical applications (tooth and artificial bone), household wares, adsorbent and catalysts, and thermal insulation.
Acknowledgment
The authors would like to thank the following: The Petroleum and Petrochemical College, and the Scientific and Technological Research Equipment Centre, at Chulalongkorn University, Thailand; the Department of Materials Engineering, at Kasetsart University for the use of their analytical equipment. We are grateful for the grant support from Agricultural Research Development Agency (Public Organization) or ARDA of Ministry of Agricultural and Cooperatives encoded PRP5605010080. We are also grateful for the grant support from center for advanced studies in industrial technology and the Kasetsart university research and development. We also would like to acknowledge the financial support from conductive and electroactive polymers research unit of Chulalongkorn University, the Thailand Research Fund (TRF-BRG), and the Royal Thai Government.
References
- Thakur VK, Singha AS, Thakur MK. Graft copolymerization of methyl acrylate onto cellulosic biofibers: synthesis, characterization and application. J Polym Environ. 2012;20:164-174.
- Singha AS, Thakur VK. Synthesis and characterization of grewia optiva fiber-reinforced pf-based composites. Int J Polymer Mater. 2008;57:1059-74.
- Singha AS, Thakur VK. Physical, chemical and mechanical properties of Hibiscus sabdariffa fiber/polymer composite. Int J Polymer Mater. 2009;58:217-28.
- Thakur VK, Singha AS, Kaur I, et al. Silane functionalization of saccaharum cilliare fibers: thermal, morphological, and physicochemical Study. Int J Polym Anal Charact. 2010;15:397-414.
- Narinc D, Aygun A, Karaman E, et al. Egg shell quality in Japanese quail: characteristics, heritabilities and genetic and phenotypic relationships. Animal. 2015;9/7:1091-6.
- Metin S, Oguz T. Quantification of Japanese quail eggshell colour by image analysis. Biol Res. 2009;42:99-105.
- Genchev A. Quality and composition of Iapanese quail eggs (Coturnix Japonica). Trakia J Sci. 2012;10/2:91-101.
- Nazligul A, Turkyilmaz AK, Bardakçioglu HE. A study on some production traits and egg quality characteristics of Japanese quail. Turk J Vet Anim Sci. 2001;25:1007-13.
- Lukáš Z, Zdenek L, Ludmila K. The effect of the age of Japanese quails on certain egg quality traits and their relationships. Veterinaeski Arhiv. 2013;83/2:223-32.
- Tullett SG. Eggshell conductance and other functional qualities of ostrich egg. Comp Biochem Physiol. 1984;78A/1:5-13.
- Nys Y, Gautron J, Garcia-Ruiz JM, et al. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. CR Palevol. 2004;3:549-62.
- Tsai WT, Yang JM, Lai CW, et al. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol. 2006;97:488-93.
- Toro P, Quijada R, Yazdani-Pedram M, et al. Eggshell, a new bio-filler for polypropylene composite. Mater Lett. 2007;61:4347-50.
- Adeyeye EI. Comparative study on the characteristics of egg shell of some bird species. Bull Chem Soc Ethiop. 2009;23/2:159-66.
- Ahmad S, Iqbal Y, Ghani F. Phase and microstructure of brick-clay soil and fired clay-bricks from some areas in Peshawar Pakistan. J Pak Mater Soc. 2008;2/1:33-9.
- Zhang L. Production of bricks from waste materials: A review. Constr Build Mater. 2013;47:643-55.
- Dondi M, Ercolani G, Guarini G, et al. The role of surface microstructure on the resistance to stains of porcelain stoneware tiles. J Eur Ceram Soc. 2005;25:357-65.
- Galos K. Composition and ceramic properties of ball clays for porcelain stoneware tiles manufacture in Poland. Appl Clay Sci. 2011;51:74-85.
- Martín-Márquez J, Rincón J Ma, Romero M. Effect of firing temperature on sintering of porcelain stoneware tiles. Ceram Int. 2008;34:1867-73.